Abstract
In Tunisia, the water resources are limited, partially renewable and unequally distributed between the wet north and the dry south of the country. The Sminja aquifer in Zaghouan city is located in north-east of Tunisia, between latitudes 36°38′ and 36°47′ and longitudes 9°95′ and 10°12′. This aquifer is used to satisfy the population needs for their domestic purposes and agricultural activities. Water analyses results are expressed by many methods, among which are geochemical methods combined with the geographic information system (GIS) (all schematic presentations of the diagram software (Piper, Riverside, Wilcox…), which can be used to assess the suitability of the Sminja aquifer groundwater for human consumption and irrigation purposes. A total of 23 wells were sampled in January 2013, and the concentrations of major cations (Na+, Ca2+, Mg2+ and K+), major anions (Cl−, SO4 2− and HCO3 −), electrical conductivity and total dissolved solids were analysed. In the Sminja groundwater, the order of the cations dominance was Na > Ca > Mg > K and that of the anions was Cl > HCO3 > SO4. All of the analysed samples of the study area exceed chemical values recommended by the World Health Organisation guidelines and Tunisian Standards (NT.09.14) for potability but with different percentages. The aquifer spatial distribution of saturation indices reveals that all groundwater samples are under-saturated with gypsum, halite and anhydrite and are over-saturated with respect to calcite and dolomite based on water quality evaluation parameters for irrigation purposes; here, 87 % of samples in Sminja aquifer groundwater are suitable, whereas 13 % are unsuitable for irrigation uses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water is life and without this precious resource all organisms on the earth (humans, livestock and plants) will die and disappear (Deshpande and Aher 2012). Access to safe and healthy water is a human right, but 800 million people are without access to safe drinking water worldwide (WHO 2011).
Groundwater represents an important freshwater reserve, constituting about half of our drinking water (BRGM 2012). The quality of groundwater is often better than surface water because they are protected by the soil.
Groundwater is sensitive and not free from pollution; this can be affected and become a disease vector and even affect human mortality (Hassan et al. 2008). Unfortunately, the contamination of groundwater persists and its remediation is more difficult (Bussard 2005).
In Tunisia, the demand for groundwater has increased in relation to the growth of the population and its socio-economic development.
The Zaghouan governorate is located in the north-east of Tunisia. It is known for its rich water resources, as it includes two dams (Bir Mcherga and Oued Rmal), nine mountain dams and 104 hill lakes (Ayedi 1995). The total water resources of Zaghouan (groundwater and shallow) were estimated at 23 Mm3 equivalents to 20 % of global resources of the governorate (PNUD 2006). The Sminja aquifer is one of the most important hydrogeological entities in this region after Oued Rmal aquifer with an estimated resource of 2.5 Mm3 (DGRE 2008). The principal objective of this study is to identify the purposes of water use of the Sminja aquifer either for human consumption, irrigation or for both combined through geographic information system and geochemical methods.
Study area
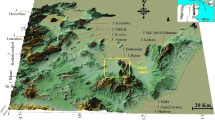
The Sminja aquifer, which belongs to Oued Meliane watershed, is located in north-east of Tunisia. The study area is limited by Rihane mountain from the north, Maouine and Jahfa mountains from the west, the reliefs of Bled Tella from the east and Zaghouan mountain from the south (Fig. 1).
a Geologic map of the study area and b schematic cross section along A–B (Berthon 1911, modified)
The watershed of the study area has an extension of 353 km2 and hosts an important water spring flowing from the mountains of the region, especially from Zaghouan Mountain. The Meliane valley is the main valley of the study area, which passes through Sminja aquifer. This valley runs through the study area from the south to the north.
In order to protect the southern suburbs of Tunis, two dams have been built: the dam of wadi el Kebir located in upstream of the plain of Sminja and the Bir Mcherga dam located in downstream (Kanfir 1988). The highest topographic elevation in the study area is about 1295 m (top of mountain Zaghouan) and the lowest one is around 130 m (output of watershed of Meliane valley). The climate of the region is continental Mediterranean. It is cold and wet in winter and hot and dry in summer.
The rainfall is highly variable in space and in time. The wet period lasts for 9 months from September to May. The dry period lasts 3 months. In the study area, the annual average temperature is 18.5 °C (Attia et al. 2005). The average rainfall is about 449 mm, with a minimum of 220 mm and a maximum of 861 mm (IAO 2004).
The geological formations in the study area range from Triassic age to Quaternary (Arnould 1946). The Quaternary cover the entire plain of Sminja (Fig. 1). This plain is a collapsed area limited from the north by the Triassic fault of the mountain of Aziz, from the south by the fault of Zaghouan and from the west by the Rouass and Rouissat mountains accident (Fig. 1).
The Quaternary deposits with a wide variety of lithology fulfil the plain of Sminja. The aquifer is a rifted valley covered by thick Quaternary sediments (few hundred metres). The lithological sections of boreholes of this aquifer are formed by alternations of sand, clay and gravel and with sand-dominated sequences and others with clay-dominated sequences (Kanfir 1988).
The feeding of the Sminja aquifer occurs mainly by direct infiltration of precipitation and infiltration of flooded valleys waters, essentially through Meliane River (Fig. 1). Sminja aquifer is especially recharged upstream of the aquifer, in the cities of Bir Halima and Bir Mcherga, where groundwater levels are lower than valley beds (Kanfir 1988). The transmissivity was calculated from flow tests of some Sminja aquifer boreholes. The highest transmissivity value was 7.5 × 10−3 m2/s, which corresponds to the most permeable zone in the centre and north-east of Sminja aquifer. The average value of the entire study area is 10−3 m2/s (Khanfir 1988).
Materials and methods
Sampling area and chemical analysis techniques
A total of twenty-three water samples used to satisfy the needs of the population for drinking water and irrigation were collected in January 2013. The selection of samples was done to cover the maximum of Sminja aquifer surface. The water samples were collected in two clean 500-ml polythene bottles. The sampling water was taken from wells that were fitted with electric motors after a 30-minute pumping.
In situ, the samples were measured for pH, EC, TDS, dissolved oxygen (DO) and temperature which were determined by portable multi-parameter water quality meter (Alaya et al. 2014).
At laboratory, all samples were filtered using Millipore 0.45-μm filter paper. A 500-ml bottle of each sample was acidified, without disturbing the sample volume, with 35 % of nitric acid (Ultrapure Merck). Acidification was preceded until pH of the samples attained 1. The purpose was to use them in cations analysis (Na+, Ca2+, Mg2+ and K+). The other bottles were reserved for the major anions analysis (Cl−, SO4 2− and HCO3 −). All these samples are stored below 4 °C (Hamzaoui et al. 2009).
The water samples were analysed by using the protocol for standard methods for the examination of water and wastewater (APHA 1995).
The cations Na+, Ca2+, Mg2+ and K+ were measured by the spectrometry of atomic absorption. The anions were determined by many techniques. Chloride was dosed by argentometric titration using standard AgNO3. Bicarbonate concentrations were determined by potentiometric method. The gravimeter method using BaCl2 was used to measure the sulphates concentrations.
The validation of chemical analyses results (Table 1) was performed by the verification of the percentage of error in the ionic balance (Vesper 2009).
∑ [cations] Zc = ∑ [anions] Za, with Z is the absolute value of ion charge. The per cent charge balance error was verified using the following equation (Freeze and Cherry 1979):
The results of analysis are judged acceptable when the percentage of error is less than or equal to 5 % (Appelo and Postma 2005).
If the per cent balance error is above 5 %, the analyses will not be reliable and accurate. We can deduce that the balance did not take into account, when calculating other unknown species present in water, or that the water is very acid and H+ ions are not listed and also that significant quantities of organic ions are here and may appear by coloured water (Hounslow 1995). The average balance error of Sminja aquifer water samples analyses is 1.95 %.
The evaluation of water quality of Sminja aquifer was determined using different software and several methods such as geochemistry methods combined with GIS and statistical approach.
Geographic information system (GIS)
A GIS was developed to make useful information from available data to better understand the functioning of Sminja aquifer and to adopt the adequate decision. The thematic maps of study area were obtained from 1:50000 scale with extended geo referencing of ArcGis9.3 to the UTM coordinate system and WGS 84 datum projections. The position of each borehole was determined by using a global positioning system (GPS). The spatial interpolation technique of chemical parameters such as TDS and the saturation indices were represented through inverse distance weighted (IDW) (Ahmadi and Sedghamiz 2007).
Hydrochemical methods
The identification of hydrochemical processes of Sminja aquifer was obtained by constructing several diagrams such as box plot, Piper diagram, many correlations (Pearson correlation matrix) and geochemical modelling.
Multivariate statistical analysis
Multivariate statistical techniques, such as principal component analysis (PCA) and cluster analysis (CA), are very important approaches which lead to a better interpreting of major sources of solutes in groundwater samples (Hamzaoui-Azaza et al. 2012). The statistical analysis was carried out using the software program XLSTAT and all of the graphics were plotted.
Principal components analysis (PCA)
Since long ago, the PCA has been a multivariate technique with wide uses in different fields like Geology (Duan et al. 2007). The PCA is a statistics analysing technique covering over at least two variables, allowing a graphical representation of links between variables n and individual positions relative to the vectors of these variables. The graphics were constructed from the matrix correlations, and PCA represents graphically the links between the different variables and selected factor. These graphs are projected in a circle of radius 1. The more significant is the relationship between two parameters, the closer to the extreme values −1 and 1 is the correlation coefficient (Hamzaoui-Azaza et al. 2013).
Hierarchical cluster analysis (HCA)
Hierarchical cluster analysis (HCA) is a part of cluster analysis, whereabouts the samples are grouped into hierarchical classes based on similarities and dissimilarities discerned from the classification of the datasets (Yidana et al. 2012).
HCA defined the type of likeness measure between groups and the linkage technique (Bratchell 1989). The important way to determine the similarity between groups is by using the Euclidean distance (Kellner et al. 2004; Brereton 2007). The benefit of HCA is that many variances can be used to classify waters (Güler et al. 2002).
Results and discussion
Groundwater chemistry
The physical and chemical analyses for 23 groundwater water samples of Sminja aquifer are mentioned below in Table 1. Sminja aquifer samples have pH values varying from 6.70 to 9.83 with an average value of 7.34 and a standard deviation SD of 0.58. The EC (µS/cm) varied from 154 to 1263 with mean and SD, respectively, 377.39 and 239.3.
The temperature values range from 11.8 to 23 °C with a median of 18.32 and a standard deviation of 2.99.
Figure 2 presents the box plots for the major ionic (meq/l) constituents of water samples. It shows that the major ions order of abundance is Na > Ca > Mg > K = Cl > HCO3 > SO4. Na+ which ranges from 7.35 to 122.83 meq/l is the dominant cation of Sminja aquifer, Cl− which is varying from 8.99 to 122.28 meq/l is the dominant anion. Ca2+ values range from 2.83 to 22.90 meq/l and Mg2+ varies from 2.66 to 12.97 meq/l. In addition, the hardness in the water samples ranged from 4.45 to 8.81 meq/l and K+ varied from 0.01 to 1.11 meq/l (Table 1).
The spatial distribution map of total dissolved solid (TDS) of Sminja aquifer (Fig. 3) shows that the concentration varied from 1170 to 9570 mg/l. The lowest values of TDS were observed in the south of the aquifer in the region of Bir Halima, and the highest concentrations were found close to the borough of Sminja. The principal cause of salination is related to many factors like the dissolution of gypsum and the type of rock linked with geochemical and hydrogeological settings (Hamzaoui et al. 2012).
Statistical analyses
Pearson correlation matrix
In the study area, the correlation between TDS and all other variables is significantly positive, with an excellent observed correlations with sodium (r = 0.93) and chloride (r = 0.98). The highly significant correlation between Na and Cl indicates that they have similar geochemical behaviours and come from the same external origin, which is the dissolution of halite (Hamzaoui-Azaza 2011). The higher positive correlation between pH and calcium (r = 0.56) showed that the pH of the borehole is caused by calcium ions. The positive correlation was shown between Ca and Mg, with a coefficient of correlation of 0.73 (Table 2), and this indicates that these two elements included, from the beginning, in addition to the alteration of the dolomite and magnesium calcite (common origin for Ca and Mg), the dissolution of the gypsum and calcite.
Principal component analysis
This method has been used to determinate the major variables responsible for the geochemistry of all aquifer water samples (Acero et al. 2013). The PCA was performed with 10 variables (pH, temperature, salinity and major elements) for 23 water samples of Sminja aquifer. The study of the spectrum of eigenvalues shows that three factors F1, F2 and F3 were extracted to explain the total variance.
The F1 axis represents 46.2 % of the total variance (Fig. 4) and is considered as the principal component of mineralisation, because it has high positive correlations with most major hydrochemical variables, whereas the axis F2 and axis F3 are 18 and 15.32 %, respectively, a total of 80 % (Table 3), which is very significant (Kaiser 1960). The main components F1 and F2 with 64 % are provided by the combination of TDS and concentrations of Na+, Cl−, Ca2+ and SO4 2−. The F3 axis is defined by both bicarbonate (HCO3−) and the potassium (K+) contents, and it represents 15 % of the total variance.
Hierarchical cluster analysis (HCA)
Figure 5, through the HCA and precisely ward’s methods, has just confirmed the results of ACP graphic (Fig. 4) and shows four sub-branches of Sminja aquifer groundwater samples:
A dendrogram showing the four hierarchical classification of waters on the Sminja aquifer (Ward 1963)
-
The first group is formed by FS1 water sample, which is located in the western part of the aquifer and which water is the most concentrated (salinity about 10,000 mg/l).
-
The second sub-branch, which is located in north-east part of Sminja aquifer and which water is less mineralised than the first group (salinity from 3260 to 5290 mg/l), is formed by six groundwater samples: PS10, PS11, FS9, PS3, PS5 and PS6.
-
The third class represents 17 % of total Sminja aquifer water samples whose water is moderately concentrated (salinity from 2350 to 3160/l).
-
The fourth group is consisted of 52 % of all study area boreholes whose water is the lowest diluted (salinity from 1670 to 2110/l).
Hydrogeochemical water types
Piper trilinear diagram (Piper 1944) is used to deduce the hydrochemical facies. It has the same shape as the graph produced by Hill (1940, 1942). The Piper trilinear diagram shows (Fig. 6) that the waters of Sminja aquifer are characterised by the predominance of two hydrochemical facies:
Piper diagram of groundwater samples from Sminja aquifer (Piper 1944)
-
a mixed facies Na–Ca–Cl–SO4, which is located in the upstream part of the Sminja aquifer (the recharge area);
-
Na–Cl facies, which characterises the wells FS1, PS1, PS2, PS3, PS5, PS6, PS10 and PS11 in the downstream of Sminja aquifer (to discharge zone).
The evolution of hydrogeochemical facies (Fig. 7), from upstream (south part of Sminja aquifer) to downstream (situated in the north of the aquifer), is explained by water–rock interaction (the origin of the acquisition solutes), the lithology of the aquifer, the residence time and the direction of flow of waters (Hamzaoui et al. Hamzaoui-Azaza et al. 2012).
Identification of water–rock interaction
The main objective of this part is to understand the origins and factors of Sminja aquifer geochemical mineralisation processes and the reaction between aquifer minerals and groundwater through many correlations and graphical plots (Hamzaoui-Azaza et al. 2013). Many rations are calculated to identify the common natural source and to control Sminja aquifer groundwater chemical evolution (Table 4). The source of sodium concentration in the groundwater can release from different chemical processes principally the dissolution of rock salts (Kumar et al. 2009). Based on the Na+/Cl− ratio, the majority of water samples (52 %) showed a report approximately equal to one which indicates that the principal origin of sodium and chloride in the water samples of Sminja aquifer is halite dissolution (Table 4).
Identification of mechanisms controlling the chemistry of Sminja aquifer using Gibbs diagrams (Gibbs 1970)
In the semiarid and arid regions like Tunisia, the dissolution of the halite increases in soil (Narany et al. 2014) and it is the dominant source of Cl− and Na+ in Sminja water samples. 35 % of groundwater samples of Sminja aquifer are less than 1 (Table 4) indicating that Na+, in study area, occurred essentially from silicate weathering (Deutsch 1997). However, just 13 % have a Na+/Cl− greater than 1, suggesting that water samples are enriched with Na+ against Cl− concentrations and that the source of Na+ is presumably attributed to silicate dissolution (Na-plagioclase). It is also suggesting that the geochemical evolution processes of groundwater samples can be related to ion exchange reaction between clay fraction, in fractured area of aquifer, and groundwater (Hamzaoui-Azaza et al. 2013). In study area, the strong correlation which exists between Ca2+ and Mg2+ may have derived from dissolution of carbonate minerals (dolomite and calcite) (Belkhiri et al. 2012). 83 % of water samples of Sminja aquifer having a Ca2+/Mg2+ molar ratio are higher than one and they also reflected the rock dissolution of more abundant calcite, while 13 % having a Ca2+/Mg2+ ratio equal to one can be derived from dolomite rocks dissolution (Narany et al. 2014). About 90 % of Sminja aquifer water samples illustrated Mg2+/Mg2++ Ca2+ ratios lower than 0.5, which means that the two ions had been derived from a similar common natural origin that may be from limestone weathering and dolomite (Hounslow 1995).
The Gibbs plot (Fig. 8) represents the TDS as a function of the weight ratios of Cl−/(Cl−+HCO3 −). Moreover, Na+/(Na++Ca2+) could indicate the principal natural processes governing groundwater chemistry, which comprises rock weathering, atmospheric precipitation and evaporation. These plots of data from Sminja aquifer show that evaporation is the major mechanism controlling the groundwater chemistry.
In the present study area, the indicator of silicate and carbonate weathering (Fig. 9a) is attested from the scatter diagram (Ca + Mg) against (HCO3 + SO4) in meq/l. The abundance of SO4 + HCO3 by 52 % over Ca + Mg by 48 % is a sign of silicate weathering, while the dominance of Ca2+ and Mg2+, in Fig. 9b, is a mark of reverse ion exchange (Elango and Kannan 2007). On the other hand, the presence of Ca2+ and Mg2+ ions in this aquifer water samples can be related to carbonate rock, whereas weathering of silicates represents the major source of bicarbonate ion in study area (Fig. 9a).
Geochemical modelling
SI evaluates the level of equilibrium between minerals of water and rocks (Hamzaoui-Azaza et al. 2012). The SI of Sminja aquifers for 23 samples is calculated using the geochemical software PHREEQ C (Parkhurst and Appelo. 1999).
If SI is greater than zero (SI > 0), the solution is saturated with the particular mineral, and then, precipitation from the groundwater is theoretically possible; when SI is below zero (SI < 0), the solution is under-saturated with this mineral (indicated by a negative SI values) and dissolving continues; and if SI is equal to zero (SI = 0), the mineral would be precipitating or dissolving (Hassan 2007). As shown in Fig. 10, the spatial distribution of SI of Sminja aquifer reveals that all samples are under-saturated with gypsum, halite and anhydrite and are supersaturated with respect to calcite and dolomite except for a few boreholes, respectively, FS6, FS7 and FS9; FS1, FS2, FS5, FS6, FS7, FS9 and PS1; FS6, FS7, PS1 and PS13. Not only calcite precipitation, but also gypsum and halite dissolution are the principal reactions that determine the chemical evolution in Sminja aquifer groundwater.
Drinking water quality of Sminja aquifer and its effects on human health
To judge that water is drinkable, it must be free of pathogens (bacteria, viruses), parasitic organisms and chemicals in excess. The World Health Organisation (WHO) has set limit values not to be exceeded if we want to respect international standards for human consumption. All countries of the world do not follow the same standards. Some adopt their own standards and others choose those recommended by WHO. Tunisia has fixed national standards (NT.09.14) for the potability of the water. The Tunisian standards differ from WHO guidelines for certain reference values as required for the management of water resources in Tunisia. The physical and chemical analyses for 23 groundwater water samples of the Sminja aquifer are mentioned in Table 5. The pH values of Sminja aquifer are in accordance with the guidelines for drinking water quality recommended by the WHO (as 6.5–9.2) and by Tunisian standards NT.09.14 (6.5–8.5), except for well PS11 (Table 5). According to WHO and NT.09.14 standards for the EC, all water samples respect the maximum permissible limit of 1500 (µS/cm). All groundwater complies with the permitted temperature the limit of WHO and NT.09.14 (25 °C). The TDS content in all groundwater above was within 100 % of the limit of WHO standards and within 96 % of the Tunisian standards.
The results of physical and chemical analyses (Table 5) were compared with the WHO and the Tunisian standards (NT.09.14) for drinking water (SONEDE 2012). Table 5 shows that all groundwater samples of Sminja aquifer exceeded the chemical characteristic recommended guidelines of WHO and Tunisian standards (NT.09.14) for potability but with different percentages. The percentages of cations (Na+, Mg2+, Ca2+ and K+) values were above the permissible limits of WHO (83, 4, 43 and 4 %, respectively) and exceed Tunisian standards with 13 % of Ca2+ and 4 % of Mg2+ for drinking water. All major anions (Cl−, SO4 2− and HCO3 −) in groundwater samples of Sminja aquifer exceed the permissible level of WHO and NT.09.14. The presence of major ions in excess in drinking water can cause many health effects (Table 5). Human activities generate significant pollution by heavy metals, mainly from domestic sewage, industrial waste, household waste and especially agricultural activities (Thévenot et al. 2009). The water samples of Sminja aquifer have been the subject of some heavy metal analyses: lead, cadmium, aluminium, chrome, manganese and arsenic (Table 5). The decision to analyse these heavy metals is based on their significant threats to human health proved by the World Health Organisation through many studies about drinking water (Rajeswari and Sailaja 2014). Drinking water with excessive quantities of metals, over a long period of exposure, can cause serious health problems like Alzheimer’s disease, blood poisoning and skin cancer. Most heavy metals are naturally present in the rocks. The metals presence in aquatic system is a result of natural weathering of rocks and soils as well as of anthropogenic activities (smelting and mining). Consequently, this can cause the release of heavy metals in lakes and rivers. The components of the environment, including groundwater, are contaminated by heavy metals due to the weathering processes and to the mobilisation of toxic elements by acid rock from primary minerals in order to form secondary minerals essentially arsenates, hydroxides and oxides and carbonates (Andráš et al. 2013). The majority of mineral ores present variable quantities of many heavy metals which can be free in the environment through the ore-processing activities, geological and geochemical processes from a low-grade crust (Table 6; Mitchell 1955).
The waters of Sminja aquifer are in accordance with the drinking water standards prescribed by WHO for some heavy metals such as manganese and nickel, but exceed the limit values for some metals like aluminium, arsenic, barium, cadmium, chromium, lead and antimony (Fig. 11). All groundwater samples exceed the desirable limit (<0.2 mg/l) and the maximum permissible limit (=0.2 mg/l) of aluminium and 100 % lie in the class of not permissible limit (Table 7; WHO 2011). The majority of boreholes of Sminja aquifer with about 61 % are situated in the category of maximum permissible limit of arsenic and about 39 % are located in the not permissible limit class (>0.01 mg/l) (Fig. 12). A pre-treatment of waters is necessary before consumption as drinking water. Many medical researchers have shown a close relationship between the contamination of drinking water and many serious diseases, including Alzheimer’s, liver cirrhosis, renal failure and chronic anaemia (Salem et al. 2000).
Irrigation water quality of Sminja aquifer
The water for irrigation should not have problems like high quantities of chemical substances in solution and dissolved salts that can deteriorate soil fertility and reduce crop yield to maintain sustainable agriculture (Phocaides 2000). The major important parameters that measure salinity hazards in Sminja aquifer are EC, TDS, sodium adsorption ratio (SAR), sodium per cent (%Na) and residual sodium carbonate (RSC). In the study area, fertilisation is a principal cause of aquifer salinisation.
Sodium adsorption ratio (SAR)
SAR is one of the main parameters that verify the irrigation water quality because a great level of sodium in the water generates an alkalisation process and reduces the soil permeability and water infiltration (Gupta 2012). SAR values were determined by the proportions concentrations of sodium (Na+), calcium (Ca2+) and magnesium (Mg2+). The SAR was assessed using the following equation given by Richards (1954), where the concentrations are presented in milliequivalents per litre:
The SAR value for irrigation water is ranked into three groups (Table 8). Based only on the SAR values, the majority of water samples of Sminja aquifer are suitable for irrigation with an excellent water quality and can be utilised for most types of soil, except the borehole FS1, from which the water is poor and unsuitable for irrigation (SAR > 26).
Electrical conductivity (EC)
The electrical conductivity (EC) reflects the total dissolved solid (TDS) in water (Ayers et al. 1985). The maximum permissible limit of EC in groundwater is 1500 μS/cm because a high value of electrical conductivity in water can cause a physiological dryness and reduce the crop yield. According to Kelly (1940), Paliwal and Singh (1967) and Wilcox (1955), the graphical plot (Fig. 13) expresses the combination effect between salinity (EC) and sodium (SAR) hazards. All water samples of Sminja aquifer have high salinity and low, medium or high sodium hazards. The results illustrated in Fig. 13 show six categories of water, with 2, 1, 5, 8, and 2 of 23 groundwater samples are located in C3S1 class with high salinity and low alkalinity and in C3S2 class with high salinity and medium sodium hazards, C4S1 class with high salinity and low sodium, C4S2 class with high salinity and medium sodium hazards and the C4S3 field with high salinity and high sodium, respectively. Five groundwater samples exceed all classes of USSL diagram with very high salinity and high alkalinity hazards. The majority of samples of Sminja aquifer are unsuitable for irrigation and cannot be used for all types of soil. The TDS values combined with the interval of EC (Table 9) show that thirteen samples of water of Sminja aquifer are slightly saline, six samples have a medium salinity, three of the groundwaters have a high salinity and one sample is very saline.
USSL diagram of Sminja aquifer (Richards 1954)
Sodium percentage (%Na)
Sodium percentage is among the most important parameters characterising the suitability of water quality for agriculture (Wilcox 1948). A high concentration of %Na in water can cause an impairment, reduce soil permeability and touch plant growth (Karanth 1987). %Na can be calculated by using the following formula of Wilcox (1955).
The %Na in Sminja aquifer varies from 20 to 86 % (Table 10). Five and fourteen samples of water were good to permissible for irrigation purposes, respectively. Three groundwater samples ranging from 60 to 80 % are doubtful and one sample (FS1) is unsuitable for irrigation uses. Figure 14, where % Na is plotted against EC (µS/cm), shows that the samples of study area are good to unsuitable for irrigation.
Residual sodium carbonate
The quantity of sodium bicarbonate and bicarbonate has been calculated with the RSC formula that reveals the hazardous effect of water irrigation (Eaton 1950) if the sum of carbonate and bicarbonate is greater than the sum of calcium and magnesium concentration, making it very probable that Ca and Mg complete precipitation (Ragunath 1987).
The RSD is estimated by the following equation, where all ionic concentrations are represented in meq/l.
The value of RSC of Sminja aquifer ranges from −28.7 to 1 with an average value −10.35. All groundwater samples of the study area have values less than 1.25 of residual sodium carbonate; therefore, this water is safe for irrigation purposes.
Kelly’s ratio (KR)
The KR (Kelly 1951) is defined by concentration of sodium (meq/l) measured against magnesium and calcium concentration. KR is obtained by the following equation:
Also, 60 % of groundwater samples of Sminja aquifer are less than 1 KR, indicating a good water quality for irrigation uses, and 40 % were more than 1, showing unsuitable water quality for irrigation purposes (Table 11).
Table 11 shows the classification of groundwater on the basis of Kelly’s ratio.
Doneen’s permeability index (PI)
According to Doneen (1964), PI value for irrigation water is determined by the following formula:
PI of the study area varies from 43 to 88. The majority of water samples are categorised in class I and class II as good for irrigation (75 % maximum of permeability), except for the FS1 borehole that is unsuitable for irrigation (class III) purposes with 25 % maximum permeability (Fig. 15).
Magnesium hazard (MH)
According to Szabolcs and Darab (1964), magnesium ratio (MR) is calculated as follows:
Magnesium plays a very important role in plant growth and soil productivity if the magnesium ratio is <50 meq/l, but MR >50 is considered to be unsuitable and harmful for irrigation purposes. The MR in the Sminja aquifer varies from 34 to 65 meq/l. Overall, 78 % of the water samples of the study area are suitable for irrigation, whereas 22 % of groundwater samples are considered unsuitable and should be used following treatment. The spatial distribution maps of the SAR, NA % and PI of the study area (Fig. 16) show that the deterioration of the quality of the groundwater samples of Sminja aquifer, for irrigation purposes, is very visible from upstream to downstream of the aquifer and therefore from the recharge zone (Bir Hlima and Mograne) to the discharge zone (Sminja).
The calculation of SAR, MH, % Na, KR, PI and EC shows that one, five, one, nine, one and four groundwater samples, respectively, in Sminja aquifer are unsuitable for irrigation, whereas all twenty-three samples fall into the suitable category for RSC (Table 12).
Conclusion
In recent decades, groundwater has become a very important resource for many countries in the world (UNESCO 2004). In the study area, the interpretation of geochemical analyses, using multivariate statistical and geochemical methods, aims to assess the water quality of the Sminja aquifer and its suitability for irrigation and domestic uses. The order of the abundance of major cations and anions is Na > Ca > Mg > K = Cl > HCO3 > SO4. The dominant hydrochemical facies of Sminja aquifer is Cl–Na and Na–Ca–Cl–SO4. Piper diagram shows that all sample waters fall within the chloride–sodium–bicarbonate category. The chemical evolution of Sminja water samples is controlled by aquifer minerals and groundwater interaction. The multivariate statistical analysis has confirmed this classification of Sminja aquifer water samples mineralisation through PCA and HCA. The PCA shows that Sminja aquifer waters are identified with three major factors presenting 80 % of total variance and HCA identified with four major groups controlling the water chemistry.
All groundwater samples of Sminja aquifer exceed chemical characteristic guideline limits recommended by WHO and Tunisian standards (NT.09.14) for potability but with different rates. The TDS content in all groundwater falls beyond the permissible limit of WHO standards to a level of 100 and 96 % was within the Tunisian standards. By assembling various geochemistry methods and clues (SAR, % Na, PI, MH, KR, EC) to assess the Sminja aquifer groundwater for irrigation, the majority of groundwater samples are not suitable for irrigation purposes and should be used with treatment.
References
Acero, P., Gutierrez, F., Galve, J. P., Auque, L. F., & Carbonel, D. (2013). Hydrogeochemical characterization of an evaporite karst area affected by sinkholes (Ebro Valley, NE Spain). Geologica Acta., 11(4), 389–407.
Ahmadi, S. H., & Sedghamiz, A. (2007). Geostatistical analysis of spatial and temporal variations of groundwater level. Environmental Monitoring and Assessment, 129, 277–294.
Alaya, M. B., Saidi, S., Zemni, T., & Zargouni, F. (2014). Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environmental Earth Sciences, 71, 3387–3421.
Andráš, P., Turisová, I., Lacková, E., Kharbish, S., Krnáč, J., & Čmielová, L. (2013). Environmental risk due to heavy metal contamination caused by old copper mining activity at Ľubietová Deposit, Slovakia. Energy and Environment Research, 3(2), 16p.
APHA. (1995). American public health association. Standard Methods (19th ed.). Washington, DC: American Public Health Association.
Appelo, C. A. J., & Postma, D. (2005). Geochemistry, groundwater and pollution (2nd ed.). Netherlands: Balkerma.
Arnauld, M. (1946). Etude géologique et hydrogéologique du bassin de l’Oued Zaghouan-Oued Rmel, plaine à l’Est de Zaghouan. Ste Marie du Zit, Zriba: DGRE. Tunis, Rapp. Int.
Attia, R., Agrébaoui, S., & Hamrouni, H. (2005). Application des Directives CAR/PAP pour la formulation d’un programme de gestion de contrôle de l’érosion et de la désertification. Cas du bassin versant de l’Oued Rmel. Rapport final. Programme d’Actions Prioritaires (PNUE), p 78.
Ayedi, M. (1995). Ressources en eaux du gouvernorat de Zaghouan. DGRE: Tunis, Rapp. Int.
Ayers, R. S., & Westcot, D. W. (1985). Water quality for agriculture, irrigation and drainage. No. 29, food and agriculture organization of the United Nations. FAO, Rome.
Belkhiri, L., Mouni, L., & Tiri, A. (2012). Water–rock interaction and geochemistry of groundwater from the Ain Azel aquifer, Algeria. Environ Geochem Health, 34, 1–13.
Berthon, L. (1911). Note hydrogéologique sur le massif du Djebel Zaghouan. DGRE: Tunis, Rapp. Int.
Bratchell, N. (1989). Cluster analysis. Chemometrics and Intelligent Laboratory Systems, 6, 105–125.
Brereton, R. G. (2007). Applied chemometrics for scientists. Chichester: Wiley.
BRGM. (2012). Eaux souterraines, les mystères d’une ressource invisible. France: Synthèse de la conférence.
Bussard, T. (2005). Méthodologie de dimensionnement des zones de protection des captages d’eaux souterraines contre les polluants chimiques persistants. Thèse Doc. L’Université de Lausanne, p 172.
Deshpande, S. M., & Aher, K. R. (2012). Evaluation of groundwater quality and its suitability for drinking and agriculture use in parts of Vaijapur, District Aurangabad, MS, India. Research Journal of Chemical Sciences. 2(1), 25–31.7.
Deutsch, W. J. (1997). Groundwater geochemistry: fundamentals and applications to contamination. Boca Raton, New York: Fla, Lewis Publishers.
DGRE. (2008). Annuaires annuels d’exploitation des nappes profondes. Tunis: Direction Générale des Ressources en Eaux.
Doneen, l. D. (1964). Notes on water quality in agriculture, water science and engineering. Department of Water Science and Engineering, University of California.
Eaton, F. M. (1950). Significance of carbonates in irrigated waters. Soil Science, 69, 123–134.
Elango, L., & Kannan, R. (2007). In D. Sarkar, R. Datta, & R. Hannigan (Eds.), Rock-water interaction and its control on chemical composition of groundwater, in: Concepts and applications in environmental geochemistry (pp. 229–246). Amsterdam: Elsevier Science.
Freeze, R. A., & Cherry, J. A. (1979). Groundwater: Prentice-Hall Inc. New Jersey: Englewood Cliffs. 604 p.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science (New York, NY), 170, 1088–1090.
Güler, C., Thyne, G. D., Mccray, J. E., & Turner, K. A. (2002). Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeology Journal, 10, 455–474.
Gupta, S. (2012). Geochemistry of the River Damodar-the influence of the geology and weathering environment of the dissolved load. International Journal of Geomatics and Geosciences, 3, 853–867.
Hamzaoui, F., Bouhlila, R., & Gueddari, M. (2009). Geochemistry of Fluoride and major ion in the Groundwater samples of Triassic aquifer (South Eastern Tunisia), through multivariate and hydrochemical techniques. Journal of Applied Sciences Research, 5(11), 1941–1951.
Hamzaoui-Azaza, F. (2011). Géochimie et Modélisation des nappes de Zeuss- Koutine, des grès du Trias et du Miocène de Jorf- Jerba- Zarzis. Tunis: Thèse Doc. Fac. Sci.
Hamzaoui-Azaza, F., Ameur, M., Bouhlila, R., & Gueddari, M. (2012). Geochemical Characterization of groundwater in a Miocene Aquifer, Southeastern Tunisia. Environmental and Engineering Geoscience, 18, 159–174.
Hamzaoui-Azaza, F., Tlili, B., Bouhlila, R., & Gueddari, M. (2013). An integrated statistical methods and modelling mineral-water interaction to identifying hydrogeochemical processes in groundwater in Southern Tunisia. Chemical Speciation and Bioavailability, 25, 165–178.
Hassan, W. F. (2007). The physio-chemical characteristic of Sawa lake water in Samawa city-Iraq. Marina Mesopotamica., 22(2), 167–179.
Hassan, A. R., & Holly, D. A. (2008). Les Nations Unies et le problème de l’eau dans le monde essai sur ONU-EAU. Montréal, Université du Québec à Montréal. 123p. (http://www.archipel.uqam.ca/1414/).
Hill, R. A. (1940). Geochemical patterns in Coachella Valley. Eos. Transactions American Geophysical Union., 21, 46–53.
Hill, R. A. (1942). Salts in irrigation waters. Transaction American Society of Civil Engineering, 107, 1478–1493.
Hounslow, A. W. (1995). Water quality data: Analysis and interpretation. Boca Raton, FL: Lewis.
IAO. (2004). Land Evaluation in the Oued Rmel Catchment in Tunisia. Italy: Istituto Agronomico per l’Oltremare. Florence.
Kaiser, H. F. (1960). The application of electronic computers to factor analysis. Educational and Psychological Measurement, 20, 141–151. doi:10.1177/001316446002000116.
Karanth, K. R. (1987). Groundwater assessment, development and management. New Delhi: Tata McGraw Hill.
Kelly, W. P. (1940). Permissible composition and concentration of irrigation water, In Proc of American Society for Civil Engineering. pp 607–609.
Kelley, W. P. (1951). Alkali, soils, their formation. New York: Properties and Reclamation. Reinhold Pub.
Kellner, R., Mermet, J. M., Otto, M., Valcarcel, M., & Widmer, H. M. (2004). Analytical chemistry: A modern approach to analytical science (2nd ed., pp. 176–189). New york: Wiley.
Khanfir, R. (1988). Etude des eaux souterraines de la nappe de Sminja. DGRE. Tunis, Rapp. Int, p 22.
Kumar, M., Sharma, B., Ramanathan, A., Someshwar Rao, M., & Kumar, B. (2009). Nutrient chemistry and salinity mapping of the Delhi aquifer, India: Source identification perspective. Environmental Geology, 56, 1171–1181.
Mitchell, R. L. (1955). Trace elements. Chemistry of the soil. New York: Reinhold Publishing Corporation.
Narany, S. T., Ramli, M. F., Aris, A. Z., Sulaiman, W. N. A., & Fakharian, K. (2014). Spatiotemporal variation of groundwater quality using integrated multivariate statistical and geostatistical approaches in Amol-Babol Plain, Iran. Environmental Monitoring and Assessment., 186, 5797–5815.
Paliwal, K. V., & Singh, S. (1967). Effect of gypsum application on the quality of irrigation water. The Madras Agricultural Journal, 59, 646–647.
Parkhurst, D. L., & Appelo, C. A. J. (1999). User’s guide to PHREEQC. A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Survey Water- ResourcesInv.Rep. 99-4259, 312p.
Phocaides, A. (2000). Technical handbook on pressurized irrigation techniques. Rome: Food and Agriculture. Organization of the United Nations. FAO.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water-analyses, Eos. Transactions American Geophysical Union., 25, 914–928.
PNUD (2006). Programme d’action régional de lutte contre la désertification du Gouvernorat de Zaghouan. Rapport final. Ministère de l’environnement et du développement durable. p 99.
Ragunath, H. M. (1987). Groundwater. New Delhi: Wiley Eastern.
Rajeswari, R., & Sailaja, N. (2014). Impact of heavy metals on environmental pollution. Journal of Chemical and Pharmaceutical Sciences. p 7.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils. U.S. Salinity laboratory staff, USDA Handbook. pp 60–160.
Salem, H. M., Eweida, E. A., & Farag, A. (2000). Heavy metals in drinking water and their environmental impact on human health (pp. 542–556). Egypt: ICEHM; Cairo University.
SONEDE. (2012). Rapport mensuel du laboratoire .Norme NT.09.14.1p.
Szabolcs, I., & Darab, C. (1964). The influence of irrigation water of high sodium carbonate content of soils. Proceedings of the International Congress, 2, 803–812.
Thévenot, D. R., Lestel, L., Tusseau-Vuillemin, M-H., Gonzalez, J-L., & Meybeck, M. (2009). Les métaux dans le bassin de la Seine. Programme PIREN Seine, fascicule no 7, 60 p. Editions IFREMER.
UNESCO. (2004). Groundwater resources of the world and their use. IHP-vi, series on groundwater.No.6. p 342.
Vesper, D. J. (2009). Aquifer in a bottle: Detecting geologic origin from bottled water chemistry. In P. Basu & M. E. Johnson (Eds.), The integrated approach to chemistry laboratory: Selected experiments (pp. 19–24). Lancaster: DEStech Publications Inc.
Ward, J. H. (1963). Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association, 58, 236–244.
WHO. (2011). Guidelines for drinking–water quality-4th ed. Geneva. World Health Organization. p 564.
Wilcox, L. V. (1948). The quality of water for irrigation use, U.S.D.A. Tech. Bull. 962, Washington D.C., p. 40.
Wilcox, L. V. (1955). Classification and use of irrigation Waters, U.S. Geological Department Agric. Circ. 969, Washington, DC.
Yidana, S. M., Banoeng- yakubo, B., & Sakyi, P. A. (2012). Identifying key processes in the hydrochemistry of a basin through the combined use of factor and regression models. Journal of Earth System Science, 121(2), 491–507.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ameur, M., Hamzaoui-Azaza, F. & Gueddari, M. Suitability for human consumption and agriculture purposes of Sminja aquifer groundwater in Zaghouan (north-east of Tunisia) using GIS and geochemistry techniques. Environ Geochem Health 38, 1147–1167 (2016). https://doi.org/10.1007/s10653-015-9780-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9780-2