Abstract
A field survey of mercury pollution in environmental media and human hair samples obtained from residents living in the area surrounding the Chatian mercury mine (CMM) of southwestern China was conducted to evaluate the health risks of mercury to local residents. The results showed that mine waste, and tailings in particular, contained high levels of mercury and that the maximum mercury concentration was 88.50 μg g−1. Elevated mercury levels were also found in local surface water, paddy soil, and paddy grain, which may cause severe health problems. The mercury concentration of hair samples from the inhabitants of the CMM exceeded 1.0 μg g−1, which is the limit recommended by the US EPA. Mercury concentrations in paddy soil were positively correlated with mercury concentrations in paddy roots, stalks, and paddy grains, which suggested that paddy soil was the major source of mercury in paddy plant tissue. The average daily dose (ADD) of mercury for local adults and preschool children via oral exposure reached 0.241 and 0.624 μg kg−1 body weight per day, respectively, which is approaching or exceeds the provisional tolerable daily intake. Among the three oral exposure routes, the greatest contributor to the ADD of mercury was the ingestion of rice grain. Open-stacked mine tailings have resulted in heavy mercury contamination in the surrounding soil, and the depth of appreciable soil mercury concentrations exceeded 100 cm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A significant body of research on mercury (Hg) has been performed because of its adverse impacts on public health and environmental quality (Horvat et al. 2003; Taylor et al. 2005; Appleton et al. 2006; Feng et al. 2006; Qiu et al. 2006; Zheng et al. 2007; Kim and Jung 2012). Currently, the global input of anthropogenic mercury to the environment is estimated to be 2,000–2,200 ton yr−1 (Seigneur et al. 2004), 12 % of which is derived from the mining–refining industry (Han et al. 2006). Once released into the environment, mercury is redistributed between and within the environmental compartments. Therefore, mercury is an acute environmental problem due to its bioaccumulative and persistent characteristics in the ecosystem, especially within agro-ecosystems.
Blood, urine, and hair have been successfully used as biomarkers to reflect the health risks of mercury exposure (Adimado and Baah 2002; Mortada et al. 2002). Hair in particular is a suitable indicator for estimating long-term mercury exposure (McDowell et al. 2004; Yasutake et al. 2004). Although preliminary investigations of the daily intake (DI) of mercury via several types of exposure routes have been performed (Taylor et al. 2005; Feng et al. 2006), most DIs used in mercury assessments related to populations affected by mercury contamination have been based on fish and other seafood, which accumulate mono- and dimethylmercury in fatty tissues. Studies in areas where seafood consumption does not represent the main exposure route, such as those surrounding inland mercury mines, have been rare. For people who live in inland areas, the consumption of fish or shellfish is not common; therefore, other pathways for exposure, such as freshwater consumption, grain and vegetable intake, and inhalation, may contribute to the mercury DI of inland residents. A quantitative understanding of the mechanism by which each pathway contributes to mercury exposure is essential for developing effective mitigation strategies, especially for residents living in contaminated sites and adjacent areas in southwestern China, where remediation efforts are constrained by costs or other factors and nutritional challenge may exacerbate exposure outcomes.
Within the context outlined above, the main objectives of this study were as follows: (1) to delineate mercury contamination in a historic mercury mining area located in southwestern China by analyzing soil, mine waste, water, paddy plant tissue, rice grain, and human hair and (2) to assess the health risk of mercury pollution via oral exposure to inhabitants in the vicinity of the area.
Materials and methods
Study area
The Chatian mercury mine (CMM) has been one of the largest producers of mercury in China, producing approximately 10 % of the total national production of mercury, which supplies both domestic demands and exports. The CMM is located in the town of Chatian, southwestern China, at 27°46′00″–27°47′30″ north latitude and 109°20′30″–109°21′10″ east longitude (Fig. 1). The area has a subtropical humid monsoon climate, and the annual average temperature and rainfall are 13–18 °C and 1,100–1,500 mm, respectively. Mining activities in the area date back to the Qin Dynasty (221 BC), but only in the early 1950s were its mines rediscovered, and an intensive mining activity was started. Between the late 1950s and the early 1990s, for instance, approximately 150 ton yr−1 of mercury had been produced from the CMM. Because of an increasing health concern about mercury and the importance of decline of its price, the mines in the CMM were abandoned gradually until the late 1990s. However, long-term mining operations, random tailing disposal, and poor governmental control have resulted in heavy mercury pollution. The emission of metallic mercury from the CMM and the adjacent area has become one of the largest and most serious environmental problems in southwestern China in recent decades (Horvat et al. 2003; Li et al. 2009).
Sampling and preparation
In the fall of 2005, a total of 158 samples (29 soil samples, 14 mine waste samples, 13 water samples, 72 paddy tissue samples, 15 rice grains, and 15 human hairs) were collected within the CMM. The soil samples included 18 paddy soils and 11 profile soils. To study mercury translocation and accumulation in paddy soil–paddy plant systems, 18 sets of paddy plant tissues (including paddy roots, paddy stalks, paddy leaves, and paddy grains) and corresponding paddy soils were sampled. A soil profile below a historic mine tailing pile was sampled to determine the impact of slag piles on the migration and distribution of mercury. At the tested soil profile site, intensive sampling was performed to a depth of 110 cm, and 11 samples were collected at 10-cm intervals using a cylindrical auger. The hand auger was drilled into the soil and removed, and the resulting sample was carefully cut into 10 cm sections. Mine wastes were also collected from discarded piles. In total, 13 water samples were tested, including 4 drinking water samples from mountain streams and 9 irrigation water samples, which were obtained from reservoirs, ponds, and ditches. Moreover, 15 sets of hair and rice grain samples were collected from local adult inhabitants to evaluate the health response of the residents to mercury exposure. All of the solid samples were sealed in polyethylene bags to avoid cross-contamination. The sampling sites of the CMM are shown in Fig. 1.
In the laboratory, the soil and mine waste samples were air-dried, ground, and separated using a 150-mesh sieve. Water samples were filtered in situ through a 0.45 μm precleaned cellulose membrane filter, added to 1 % (v/v) HCl, and stored at 4 °C prior to analysis. Paddy plants were rinsed several times using flowing demineralized water to remove external attached soil particles. Subsequently, the plants were separated into 4 parts (root, stalk, leaves, and paddy grain without the hull, i.e., brown rice) and were cut into small pieces. All of the plant samples were oven-dried at 40 °C for 48 h, ground, sieved to less than 50 μm, thoroughly mixed, and stored in sealed polyethylene bottles. Each hair sample was washed with nonionic detergent, tap-water, and demineralized water. The washed samples were air-dried at room temperature in a dust-free area and were cut into pieces smaller than 1 cm in length prior to storage or digestion.
Sample analysis
All of the reagents used in this study were of analytical-reagent grade. For the dilutions, high-purity demineralized water provided by a Milli-Q Plus filter apparatus (Millipore Inc., USA) was used.
For the total mercury analysis, the appropriate amounts of prepared soil and mine waste were collected for digestion, which was performed using a water bath containing a mixture of HCl-HNO3 (9:1 in v/v) (Horvat et al. 1991; Qiu et al. 2006). Dry hair samples, rice grains, and different tissues from paddy plants were digested in a water bath with a fresh mixture of HNO3/H2SO4 for the total mercury analysis (Horvat et al. 1991). The total mercury content of mine waste, soil, water, paddy tissue, rice grains, and human hair samples was determined using cold vapor atomic absorption spectrometry (CV-AAS). For quality assurance and quality control (QA/QC), method blanks, certified reference materials (CRMs), and blind duplicates were used during analyses. CRMs obtained from China National Standard Materials Research Center including soil (GBW07404), water (GBW08603), bush branches and leaves (GSV-1), and human hair (GSH-1). The analytical results of mercury in the CRMs were in good agreement with the certified values, with RSDs in range from 2.32 to 12.58 %. The recovery of mercury on spiked samples varied from 87 to 110 %, and the relative percent difference in the total mercury content of duplicate samples was less than 11 %.
The following soil properties were also tested: pH (the ratio of soil to Milli-Q water is 1:2.5, W/V) and soil organic matter (OM), according to the conventional methods issued by ISSCAS (1978), and the grain size of the soil was determined by laser-diffraction analysis (Mastersizer 2000, Malvern, UK).
Exposure assessment
In the exposure assessment stage, the average daily dose [ADD, μg kg−1 of body weight (BW) per day] was used to quantify mercury exposure from water, rice grain consumption, and other pathways. The following equation was used in the exposure assessment (US EPA 1992):
where C is the mercury concentration in the environmental sample (e.g., soil, rice grain, water in μg kg−1, μg kg−1, μg L−1, etc.), IR is the ingestion rate (kg day−1, L day−1), ED is the exposure duration (day), BW is body weight (kg), and AT is the time period over which the dose is averaged (day). The total ADD was equal to the sum of ADDs via various exposure routes.
Results
Mercury levels in mine wastes
In the CMM, mine wastes, such as tailings and waste rock, are usually stocked in piles on the ground without a proper disposal system. Similar to most mercury mines, the mine waste from the CMM exhibited a high mercury concentration (Table 1). The concentration of mercury in mine waste ranged from 0.47 to 88.50 μg g−1, which was 5.88–1 106 times higher than the crustal average mercury content of 0.08 mg kg−1 suggested by Habashi (1997). In general, mining and ore processing techniques have greatly increased the surface area of mercury-containing mine wastes, thus facilitating the interaction of mercury phases with surface and rain water (Kim et al. 2000). As surface and/or rain water passes through the highly permeable piles, it can leach and mobilize mercury in both the dissolved and particulate phase. This process is particularly enhanced at mines where acid mine drainage increases the degree of leaching (Kim et al. 2000). The elevated mercury levels for these mine wastes suggest that significant amounts of mercury can be released into surrounding compartments, including soil and surface water.
Mercury levels in surface water
Overall, the mercury concentrations of surface water samples collected from the CMM ranged from 0.09 to 9.92 μg L−1 (Table 1), which is lower than the reported concentration at a mercury mine located in Almadén, Spain (water mercury: 0.01–20.30 μg L−1) (Berzas Nevado et al. 2003) and the Palawan Quicksilver mine (Philippines, water mercury: 0.12–31.00 μg L−1) (Gray et al. 2003) but is comparable to those found at the Wanshan mercury mine in China (water mercury: 0.02–10.58 μg L−1) (Horvat et al. 2003). Nevertheless, the mercury concentrations determined in surface water in the CMM, specifically the maximum observed values, were higher than those found at mercury mines located in Idrija, Slovenia (water mercury: 0.00–0.32 μg L−1) (Horvat et al. 2002), Nevada, USA (water mercury: 0.001–2.11 μg L−1) (Bonzongo et al. 1996), and Alaska, USA (water mercury: 0.00–2.50 μg L−1) (Gray et al. 2003).
The mercury concentrations of irrigation water samples varied greatly and showed remarkable heterogeneity in the CMM. Almost all of the irrigation water samples exceeded the national guidelines for mercury in agricultural water in China (1 μg L−1). Compared to irrigation water, the concentrations of mercury in drinking water were much lower and ranged between 0.09 and 0.18 μg L−1. However, because this water was derived from the CMM, which contains a high geochemical background of mercury, these values were greater than the national guidelines for mercury in drinking water in China (0.05 μg L−1). As a result, the local population is exposed to mercury through the consumption of drinking water as well as crops and vegetables that are irrigated using contaminated surface water.
Vertical distribution of mercury in soil
The enrichment factor (EF), which is the ratio between the content of an element in any given horizon and that of the parent rock, was used to describe the mobility of mercury in the soil profile, as proposed by Rahn and McCafrfrey (1979). The formula is as follow:
where X s and I s represent the contents of element X and immobile element I in the soil sample, respectively, while X r and I r represent those in the parent rock. Martínez Cortizas et al. (2003) developed this approach to estimate the extent of heavy metal migration and enrichment in soil profiles. In the current study, because the soil was too deep and soil profile was too shallow to reach the parent rock, the deepest layer (100–110 cm) was used as the substitute. The enrichment factor of mercury in any given horizon is calculated by using Ti as the stable element (Table 2).
A maximum mercury concentration of 640 μg g−1 was observed in the topsoil (0–10 cm) below the stacked tailings. However, the mercury enrichment factor abruptly decreased from 23.7 in topsoil to 11.2 in the subsoil (10–20 cm), which indicated that open-stacked tailings without proper disposal led to heavy mercury pollution in the surrounding soil. With respect to the mobility of mercury, the enrichment factor decreased with an increase in the depth of the soil profile (Table 2). However, at a depth of 90–100 cm, the soil horizon still contains elevated levels of mercury (the mercury enrichment factor remained constant at 1.2), which suggested that mercury has migrated downward to at least 100 cm depth in the soil profile.
Mercury levels in paddy soil–paddy plant systems
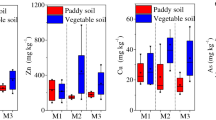
The mercury concentration in paddy soil and paddy plant tissue is shown in Fig. 2. The concentration of mercury in paddy soil ranged from 3.90 to 55.70 μg g−1, which exceeded the benchmark value of Grade II Environmental Quality for Soils in China (0.5 μg g−1). The amount of mercury in paddy soil varied considerably due to anthropogenic disturbances. The concentration of mercury abruptly decreased as the distance from the mine increased, especially in soil located upstream of the mine well. The mercury concentration in paddy grains varied between 10 and 150 ng g−1 based on dry weight (DW), and the average mercury content was 30.6 ng g−1 (DW). Among the paddy grain samples, the mercury content of 11 samples exceeded the maximum permissible level of mercury for grain in China [20 ng g−1 (DW)].
Table 3 showed a comparison of our results with several earlier reports on mercury concentrations in rice grains. As can be seen, mercury in rice commonly consumed in China averages 0.58 ng g−1 (range, 0.02–31 ng g−1; Qian et al. 2010), whereas a value of 5.21 ng g−1 was reported for commercial rice from Italy (Horvat et al. 2003). The mean value of mercury concentrations found in the CMM rice grains is 52.8 and 5.9 times above the Chinese and Italian background value, respectively, indicating that rice grains from the CMM are at higher risk of mercury exposure. Actually, mercury in rice grains from the CMM in general is much higher than reported concentrations in mercury polluted areas from Philippines, such as the Manila mercury mines (range, 3.08–3.61 ng g−1; Maramba et al. 2006) and Mt. Diwata gold mining areas (range, 9–58 ng g−1; Appleton et al. 2006), but is comparable to those found in Wanshan, China (range, 4.9–214.7 ng g−1; Feng et al. 2008) and Wuchuan, China (range, 6.0–113.0 ng g−1; Li et al. 2008), especially concerning the mean values. Nevertheless, the mean concentration found in the studied area is higher than concentrations found in Zhejiang e-waste recycling areas, China (range, 15.6–68.4 ng g−1; Fu et al. 2008) and Jiangsu rural-industrial zone, China (range, 1–13 ng g−1; Cao et al. 2010).
The average concentration of mercury in different paddy plant tissues displayed the following trend: paddy roots > paddy leaves > paddy stalks > paddy grains. Significant positive correlations (p < 0.05) were observed between mercury in paddy roots, paddy stalks, paddy grains, and paddy soil (Table 4), which implied that the paddy soil was the major source of mercury in paddy roots, stalks, and grains. However, statistically significant correlations between mercury in paddy leaves and paddy soil were not observed. This indicates that the uptake and retention of mercury in paddy leaves is influenced by a number of factors, of which the concentration of mercury in paddy soil may only be one of the variables. Our findings are similar to the results illustrated by Horvat et al. (2003) who found mercury concentrations in rice from two stations in Wanshan and Quingzhen with similar concentrations of mercury in paddy soil but distinct differences in mercury concentrations in paddy plant. Because the amount of mercury in paddy leaves was greater than that of paddy stalks, paddy leaves also absorbed particle-bound mercury from the atmosphere through foliage stomata exchange as well as via a soil source (Patra and Sharma 2000). A large amount of mercury is emitted into the atmosphere during mining. For example, Horvat et al. (2003) demonstrated that the mercury content of the atmosphere at the Guizhou mercury mine was 500–1,500 ng m−3.
Mercury in hair
Hair analysis is a valuable indicator for estimating long-term heavy metal exposure, especially for cadmium, lead, and mercury (Mortada et al. 2002). The average concentration of mercury in the hair of Chinese citizens is estimated to be 1.36 μg g−1 (Qin 2004). In the present study, the mercury content of residents from the CMM ranged between 1.04 and 10.32 μg g−1, and the average concentration was 3.73 μg g−1. In total, 87 % (13/15) of the residents possessed a hair mercury concentration greater than 1.36 μg g−1, thereby indicating that residents in the CMM may suffer from severe health problems caused by mercury exposure. Moreover, of the hair samples that were tested for mercury content, all were found to exceed 1.0 μg g−1, which is the limit recommended by the US EPA (Hightower and Moore 2003).
Discussion
ADD of mercury via oral routes
Generally, oral routes to ADD include drinking water intake, grain products intake, fish and meat intake, fruit and vegetable intake, soil ingestion and pica, etc. (US EPA 1997). Given that the CMM is a poor area of southwestern China and very far from any lake and ocean. Those people who live in the CMM eat rare amount of fish, meat, fruit, and vegetable (3, 22, 20, and 125 g day−1 based on fresh weight, respectively) (FBS 2008); however, rice grain is a staple food and provided more calories than any other single food (FBS 2008). Previous studies indicated that meat and vegetables contained very low methylmercury compared to rice grain in mercury mining area in southwestern China (Feng et al. 2008). In the present study, data on mercury in fish, meat, fruit, and vegetable in the CMM are not available; nevertheless, it is reasonable to assume that drinking water intake, rice grain intake, and soil ingestion are the major oral routes to the ADD of mercury in inhabitants in the CMM.
The joint FAO/WHO Expert Committee on Food Additives recommends that the provisional tolerable weekly intake (PTWI) of mercury should be 3.3 μg kg−1 BW per week (namely 0.47 μg kg−1 BW per day) (UNEP 2002). The average daily drinking water intakes for adults and children were considered to be 2 and 1 L person−1 day−1 (US EPA 1991), respectively, while the average adult and preschool child BW were considered to be 60 and 16 kg, respectively, as used in previous studies (Fu et al. 2008; Li et al. 2008). Therefore, the ADD of mercury via drinking water consumption by local adults and children is 0.005 and 0.009 μg kg−1 BW per day, respectively.
As rice grain is a staple food for daily consumption in China, especially in the region of our study site, mercury in rice grain may contribute a major part to the total ADD. Based on the national survey of nutrition and health of Chinese people (Wang 2005), average daily consumption of rice grain is 402 g for a general adult; according to field investigation on local resident’s diet, 200 g day−1 is a conservative estimate of the mean rice grain ingestion for local preschool children. Therefore, local adults and children would receive an ADD of mercury from rice grain that is 0.205 and 0.383 μg kg−1 BW per day, respectively.
The incidental oral ingestion of contaminated soil was considered to be another important pathway for heavy metal absorption, especially for preschool children with regular hand-to-mouth activity. According to the US EPA, a soil intake is 200 mg day−1 for children (US EPA 2002) and 100 mg day−1 for adults (US EPA 1997) through inadvertent ingestion; however, certain researchers use lower levels of soil ingestion for adults (Edward et al. 1997). In the present study, 100 mg day−1 was used as the direct soil ingestion rate for adults due to their participation in regular farming activities, such as the plowing, weeding, and fertilizing of paddy plots. Therefore, the ADD of mercury via soil ingestion by local preschool children reached 0.232 μg kg−1 BW per day, which is greater than that of adults (0.031 μg kg−1 BW per day).
The total ADD of mercury via oral routes, including drinking water, rice grain ingestion, and incidental soil ingestion, was calculated to be 0.241 μg kg−1 BW per day for adults and 0.624 μg kg−1 BW per day for preschool children (Table 5), which approaches or exceeds the PTDI recommended by the joint FAO/WHO Expert Committee on Food Additives (0.47 μg kg−1 BW per day). Among the aforementioned exposure routes, rice grain ingestion contributed 85 and 61 % of the total ADD of mercury for adults and preschool children, respectively, which indicated that rice grain ingestion is the primary pathway of mercury exposure to humans in the CMM. Although obvious health problems have not been observed in the CMM, long-term exposure to mercury through the regular consumption of rice grain and the incidental oral ingestion of soil may pose a potential health risk.
Mercury migration through the soil profile
In general, when mercury in forest soil originates from a deposition or polluted water, mercury does not move downward through soil profile but primarily remains in the topsoil (Sterckeman et al. 2000; Feng et al. 2006; Ettler et al. 2007). However, the mercury concentrations of the tested soil profile in the CMM were indicative of a high vertical mobility, thereby allowing us to draw a different conclusion. Soil horizons in the tested soil profile were enriched in mercury with respect to the deepest soil horizon concentration (23.7–1.2 times higher). The observed increase in the mercury concentration with depth could be attributed to inputs from tailings due to weathering, eluviation, and downward migration. Previous study (He et al. 1995) indicates that the hydrothermal fluids in the CMM are characterized by high Cl− and low SO4 2− concentrations (Cl−: 34.03–64.87 g L−1, 46.44 g L−1 in average; 2.88–42.24 g L−1, 20.16 g L−1 in average); thus, mercury may have immigrated as Hg2Cl2 and HgCl4 2− rather than HgS when the mercury ore was formed (Varekamp and Buseck 1984; He et al. 1995). Hg2Cl2 is less thermodynamically stable than HgS, and the solubility products of HgS and Hg2Cl2 are 1.0 × 10−53 and 4.1 × 10−23, respectively. Therefore, Hg2Cl2 becomes more active when the Cl− content is high (Hahne and Heiss 1974). Brandon et al. (2001) demonstrated that Cl− greatly extends the area of stability of Hg+ and Hg2 2+ ions and that Hg2Cl2 and HgCl4 2− ions form in Hg–Cl–H2O systems. Thus, during long-term weathering in hot, wet environments, the mercury present in Cl-rich tailings likely combines with Cl− to form HgCl2, HgCl4 2−, or HgClOH, which can migrate downward through the soil profile. The amount of mercury in different soil layers showed slight fluctuations due to variances in the physicochemical characteristics of the soil profile, such as high silt and sand contents (Table 2), which facilitate mercury movement (Sterckeman et al. 2000).
References
Adimado, A. A., & Baah, D. A. (2002). Hg in human blood, urine, hair, nail, and fish from the Ankobra and Tano river basins in Southwestern Ghana. Bulletin of Environmental Contamination and Toxicology, 68, 339–346.
Appleton, J. D., Weeks, J. M., Calvez, J. P. S., & Beinhoff, C. (2006). Impacts of mercury contaminated mining waste on soil quality, crops, bivalves, and fish in the Noboc River area, Mindana, Philippines. Science of the Total Environment, 354, 198–211.
Berzas Nevado, J. J., García Bermejo, L. F., & Rodríguez Martín-Doimeadios, R. C. (2003). Distribution of mercury in the aquatic environment at Almadén, Spain. Environmental Pollution, 122, 261–271.
Bonzongo, J. C., Heim, K. J., Warwick, J. J., & Lyons, W. B. (1996). Mercury levels in surface waters of the Carson River–Lahontan Reservoir system, Nevada: Influence of historic mining activities. Environmental Pollution, 92, 193–201.
Brandon, N. P., Francis, P. A., Jeffrey, J., Kelsall, G. H., & Yin, Q. (2001). Thermodynamics and electrochemical behaviour of Hg–S–Cl–H2O systems. Journal of Electroanalytical Chemistry, 497(1–2), 18–32.
Cao, H., Chen, J., Zhang, J., Zhang, H., Qiao, L., & Men, Y. (2010). Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu, China. Journal of Environmental Sciences, 22, 1792–1799.
Edward, J. C., Ramon, B., & Penelope, P. (1997). Soil ingestion in adults—Results of a second pilot study. Ecotoxicology and Environmental Safety, 36, 249–257.
Ettler, V., Rohovec, J., Navrátil, T., & Mihaljevič, M. (2007). Mercury distribution in soil profiles polluted by lead smelting. Bulletin of Environmental Contamination and Toxicology, 78, 12–16.
FBS (Fenghuang Bureau of Statistics). (2008). Fenghuang statistical yearbook 2007. Wuhan: Tianyi Statistics Press. (in Chinese).
Feng, X., Li, G., & Qiu, G. (2006). A preliminary study on mercury contamination to the environment from artisanal zinc smelting using indigenous methods in Hezhang County, Guizhou, China: Part 2. Mercury contaminations to soil and crop. Science of the Total Environment, 368, 47–55.
Feng, X., Li, P., Qiu, G., Wang, S., Li, G., Shang, L., et al. (2008). Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou Province, China. Environmental Science and Technology, 42, 326–332.
Fu, J., Zhou, Q., Liu, J., Liu, W., Wang, T., Zhang, Q., et al. (2008). High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere, 71, 1269–1275.
Gray, J. E., Greaves, I. A., Bustos, D. M., & Krabbenhoft, D. P. (2003). Mercury and methylmercury contents in mine–waste calcine, water, and sediment collected from the Palawan Quicksilver Mine, Philippines. Environmental Geology, 43, 298–307.
Habashi, F. (1997). Handbook of extractive metallurgy. Weinheim: Wiley–VCH. (901 pp).
Hahne, F. J. W., & Heiss, W. D. (1974). The effect of compound states on collective states singularities in the optical potential. Annals of Physics, 89, 68–95.
Han, F. X., Su, Y., Monts, D. L., Waggoner, C. A., & Plodinec, M. J. (2006). Binding, distribution, and plant uptake of Hg in a soil from Oak Ridge, Tennessee, USA. Science of the Total Environment, 368, 753–768.
He, J., Ma, D., & Liu, Y. (1995). Geochemistry of mercury mineralization, Chatian, West Hunan. Chinese Journal of Geochemistry, 14(4), 361–370. (in Chinese with English abstract).
Hightower, J., & Moore, D. (2003). Mercury levels in high-end consumers of fish. Environmental Health Perspectives, 111(4), 604–608.
Horvat, M., Jereb, V., Fajon, V., Logar, M., Kotnik, J., Faganeli, J., et al. (2002). Mercury distribution in water, sediment and soil in the Idrijca and Soča river systems. Geochemistry: Exploration, Environment, Analysis, 2(3), 287–296.
Horvat, M., Lupšina, V., & Pihlar, B. (1991). Determination of total mercury in coal fly ash by gold amalgamation cold vapor atomic absorption spectrometry. Analytica Chimica Acta, 243, 71–79.
Horvat, M., Nolde, N., Fajon, V., Jereb, V., Logar, M., Lojen, S., et al. (2003). Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Science of the Total Environment, 304, 231–256.
ISSCAS (Institute of Soil Science, Chinese Academy of Sciences). (1978). Physical and chemical analysis of soils. Shanghai: Shanghai Science Press. (in Chinese).
Kim, C. S., Brown, G. E., Jr, & Rytuba, J. J. (2000). Characterization and speciation of mercury-bearing mine wastes using X-ray absorption spectroscopy. Science of the Total Environment, 261, 157–168.
Kim, H. S., & Jung, M. C. (2012). Mercury contamination in agricultural soils from abandoned metal mines classified by geology and mineralization. Environment Geochemistry and Health, 34, 55–69.
Li, P., Feng, X., Qiu, G., Shang, L., & Wang, S. (2008). Mercury exposure in the population from Wuchuan mercury mining area, Guizhou, China. Science of the Total Environment, 395, 72–79.
Li, Y., Yang, L., Ji, Y., Sun, H., & Wang, W. (2009). Quantification and fractionation of mercury in soils from the Chatian mercury mining deposit, Southwestern China. Environment Geochemistry and Health, 31, 617–628.
Maramba, N. P. C., Reyes, J. P., Francisco-Rivera, A. T., Panganiban, L. C. R., Dioquino, C., Dando, N., et al. (2006). Environmental and human exposure assessment monitoring of communities near an abandoned mercury mine in the Philippines: A toxic legacy. Journal of Environmental Management, 81, 135–145.
Martínez Cortizas, A., García-Rodeja Gayoso, E., Nóvoa Muňoz, J. C., Pontevedra, X., Buurman, P., & Terribile, F. (2003). Distribution of some selected major and trace elements in four Italian soils developed from the deposits of the Gauro and Vico volcanoes. Geoderma, 117, 215–224.
McDowell, M. A., Dillon, C. F., Osterloh, J., Bolger, P. M., Pellizzari, E., Fernando, R., et al. (2004). Hair mercury levels in US children and women of childbearing age: Reference range data from NHANES 1999–2000. Environmental Health Perspectives, 112, 1165–1171.
Mortada, W. I., Sobh, M. A., El-Defrawy, M. M., & Farahat, S. E. (2002). Reference intervals of cadmium, lead, and mercury in blood, urine, hair, and nails among residents in Mansoura city, Nile delta. Egypt. Environmental Research, 90, 104–110.
Patra, M., & Sharma, A. (2000). Mercury toxicity in plants. The Botanical Review, 66(3), 379–422.
Qian, Y., Chen, C., Zhang, Q., Li, Y., Chen, Z., & Li, M. (2010). Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control, 21, 1757–1763.
Qin, J. F. (2004). The upper limit of normal value of hair Pb, Cd, As, Hg in Chinese resident. Guangdong Trace Elements Science, 1(4), 29–37. (in Chinese with English abstract).
Qiu, G., Feng, X., Wang, S., & Xiao, T. (2006). Mercury contaminations from historic mining to water, soil and vegetation in Lanmuchang, Guizhou, southwesten China. Science of the Total Environment, 368, 56–68.
Rahn, K. A., & McCaffrey, R. J. (1979). Compositional differences between Arctic aerosol and snow. Nature, 280, 479–480.
Seigneur, C., Vijayaraghavan, K., Lohman, K., Karamchandani, P., & Scott, C. (2004). Global source attribution for mercury deposition in the United States. Environmental Science and Technology, 38(2), 555–569.
Sterckeman, T., Douay, F., Proix, N., & Fourrier, H. (2000). Vertical distribution of Cd, Pb and Zn in soils near smelters in the North of France. Environmental Pollution, 107, 377–389.
Taylor, H., Appleton, J. D., Lister, R., Smith, B., Chitamweba, D., Mkumbo, O., et al. (2005). Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining centre, Geita District, Tanzania. Science of the Total Environment, 343, 111–133.
UNEP. (2002). Global mercury assessment. United Nations Environment Programme Chemicals, Geneva, Switzerland. http://www.unep.org/gc/gc22/Document/UNEP-GC22-INF3.pdf. Accessed 20 June 2011.
US EPA. (1991). National primary drinking water regulation. Final rule. Federal Register, 56(20), 3526–3597.
US EPA. (1992). Guidelines for exposure assessment. US Environmental Protection Agency, Washington, DC, EPA/600/Z–92/001.
US EPA. (1997). Exposure factors handbook. US Environmental Protection Agency, Washington, DC, EPA/600/P-95/002Fa.
US EPA. (2002). Child-specific exposure factors handbook. US Environmental Protection Agency, Washington, DC, EPA 600–P–00–002B.
Varekamp, J. C., & Buseck, P. R. (1984). The speciation of mercury in hydrothermal systems, with application to ore deposition. Geochimica et Cosmochimica Acta, 48, 177–185.
Wang, L. D. (2005). Report on the national survey of nutrition and health of Chinese people: Summary report 2002. Beijing: People’s Medical Publishing House. (in Chinese).
Yasutake, A., Matsumoto, M., Yamaguchi, M., & Hachiya, N. (2004). Current hair mercury levels in Japanese for estimation of methylmercury exposure. Journal of Health Science, 50, 120–125.
Zheng, N., Wang, Q., & Zheng, D. (2007). Mercury contamination and health risk to crops around the zinc smelting plant in Huludao City, northeastern China. Environment Geochemistry and Health, 29, 385–393.
Acknowledgments
This work was supported by the Key Project of the Knowledge Innovation Program of IGSNRR (Grant No. 2012ZD002) and the Natural Science Foundation of China (Grant No. 41040014; Grant No. 40571008). The author would like to thank two anonymous reviewers for their valuable comments. The author is also thankful to Dr. H.F. Sun for helping to prepare the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y. Environmental contamination and risk assessment of mercury from a historic mercury mine located in southwestern China. Environ Geochem Health 35, 27–36 (2013). https://doi.org/10.1007/s10653-012-9470-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-012-9470-2