Abstract
Non-ferrous metal mining and smelting has emitted large amounts of associated toxic elements into the environment and poses potential health risks to human health. In this study, local residents’ and miner’s hair and urine samples were collected as bioindicators to assess the health risks and soil, vegetable, rice, and surface water samples were also collected to calculate the probably daily intake of the lead (Pb), zinc (Zn), copper (Cu), and arsenic (As). The results showed that all the collected paddy and vegetable soils were contaminated according to the assessment of impact index of comprehensive quality. About 32% of the rice samples exceeded the As concentration limit of the national food safety standards, but the other toxic element concentrations in rice and all the toxic elements in the vegetables were lower than the threshold limits. Multi-pathway intake of toxic elements and target hazard quotient showed rice was the most dominate pathway of the toxic element exposure, accounting for 68–82% of the total exposures. The excessive daily exposure to the toxic elements posed a high non-carcinogenic risk for residents. Hair Cu and As concentrations exceeded the Chinese resident normal hair concentrations by factors of 1.7–6.0, especially for the miners. The implication of this study is that proper mitigation strategy on toxic element pollution and human toxic element exposure is to reduce the intake of local agricultural products, especially rice and soil remediation are encouraged to improve the health of local residents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Toxic element pollution caused by industrial activities, such as the non-ferrous metal mining, fossil fuel combustion, and metal processing, is a global concern. These activities released a large number of toxic elements into air, soil, and water (Du et al. 2020; Kavehei et al. 2021; Zhu et al. 2020). According to a global inventory of trace element emissions, about 2246–46,328 tons of lead (Pb), zinc (Zn), copper (Cu), arsenic (As), and cadmium (Cd) were emitted to the environment in 2012 and the emissions of these elements are still increasing (Zhu et al. 2020). Non-ferrous metals mining and smelting is considered as one of the most important anthropogenic emission sources of these toxic elements, accounting for 25–73% of the global emissions (Zhu et al. 2020). Among the non-ferrous metal mining and smelting, Pb–Zn mining and smelting is the second-largest toxic element emission source, which poses potential health risks due to the toxicity not only from Pb and Zn, but also from the associated toxic elements (e.g., As, Cu, and Cd) (Kavehei et al. 2021; Qin et al. 2021). Asia, one of the most economically dynamic regions, has emitted about 38–67% of the global Pb, Zn, As, Cu, and Cd to the environmental, especially China, accounting for 60% of the Asian emissions (Zhu et al. 2020). Large amounts of toxic elements emitted to the environment have resulted in serious contaminations around the mining and smelting areas. For instance, Li et al. (2014) reviewed heavy-metal contamination in mine areas of China and found that soils around the examined mines are severely contaminated, posing high health risks to human health.
The toxic elements in the environment can potentially enter the food chains and accumulate in human organs and living organisms (Briffa et al. 2020; Engwa et al. 2019; Järup 2003). Millions of people are exposed to toxic elements in mining areas worldwide (Plumlee et al. 2013; Wcisło et al. 2002). Chronic exposure to Pb even at low dosages can damage the nervous system, kidney, and liver, and cause Alzheimer’s disease (Bihaqi 2019; Chibowska et al. 2016; Moody et al. 2018). Similarly, chronic exposure to As is associated with the occurrence of various kinds of human cancers (e.g., lung, skin, liver, bladder, and kidney), and other nonmalignant disorders, such as respiratory illnesses, cardiovascular diseases, diabetes, neurotoxicity, and renal diseases (Cancer 2006; Lamm et al. 2021). Excessive Zn intake can cause cholesterol balance, diminish immune system function, and even cause infertility (Agnew and Slesinger 2022). Excess Cu intake can induce DNA damage and reduce cell proliferation (Royer and Sharman 2020; Xu et al. 2018).
For residents living in the mining area, there are multiple exposure pathways including food ingestion (wheat, corn, rice, vegetables, meat, etc.), inhalation, drinking, and contacting with soil fine particles (accidental oral intake, skin contact, and inhalation) (Bai et al. 2019; Cao et al. 2020; Zhang et al. 2019; Zhou et al. 2019c). Among all of the exposure pathways, dietary intake is considered as the main route for most populations, although inhalation can also play an important role in contaminated sites with exhaust emission or occupational exposure (Chen et al. 2022; Sah et al. 2019; Zhuang et al. 2014). Risk assessment calculated by simple media or pathway-specific method may underestimate the risk and fail to ensure public safety, so it is necessary to apply multi-pathway risk analysis and identify the potential dominant pathway from the major relevant environmental media (Dong and Hu 2012; Zhang et al. 2019).
Human hair, urine, and blood have been widely used as heavy-metal bio-indicators to estimate human exposure levels (Appenzeller and Tsatsakis 2012; Yang et al. 2019). Hair sample is a useful assessment tool to characterize long-term exposure of contaminant, whereas urine and blood always reflect most recent exposures (Du et al. 2021; Zhou et al. 2019a). Hair has been widely used in biomonitoring environmental and occupational exposures of various pollutants as well as large cohort survey due to its less invasive, more convenient to store and transport, and less toxic to handle (Appenzeller and Tsatsakis 2012). However, urine and blood were widely used in occupational exposure, as they are sensitive and timely (Nasirzadeh et al. 2022).
In this study, we collected soil, vegetable, rice, surface water, and residents’ hair and urine samples to examine environmental pollution and human exposure around an active Pb–Zn mine. The primary aims of this research are as follows: (1) to build a connection between those risks with environmental and human health; and (2) to evaluate the potential health risks of the residents associated with environmental exposure by hair and urine analysis. Our study would provide scientific data for risk assessment as well as risk control of human toxic element exposure in non-ferrous mining areas.
Materials and Methods
Study Site
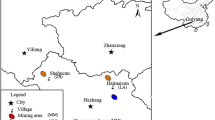
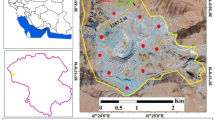
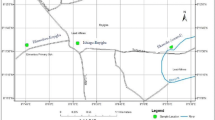
This study was performed in the area around the Lengshui Pb–Zn mine in Guixi City, Jiangxi Province [Fig. S1 in the Supporting Information (SI)]. The explored reserves are considered as the largest porphyry Pb–Zn–Ag deposit in China (Huang et al. 2020; Zhu et al. 2019). During our sampling period, only serval small mines were mining and most of the ores had not been mined, but a large amount of mine slag was discharged and piled up in the open field. The mine slag was eroded and leached to the adjacent stream and agricultural lands by rain, resulting in agricultural soils suffering from toxic element pollution (Du et al. 2020). In this study, the study area is dived into three sub-areas according to the watersheds: M1, M2 and M3 (Fig. S1).
Sample Collection
The exact sampling point are shown in Fig. S1. Totally, 36 agricultural soils were collected in the three watersheds, 24 and 12 of which were collected from the vegetable and paddy fields. For each soil sample, three representative soils were collected from the surface layer (0–15 cm) in a plot (3 m × 3 m) to form a composite sample. For the crops, six kinds of the corresponding vegetables were sampled and the staple food, rice samples were not able to collect in the field due the seasonal nature. Instead, the rice samples were provided by the local residents. Totally, 26 rice and 36 vegetable samples were collected.
Hair and urine samples were collected from both the miners and local residents in the three watersheds, where 11 hair samples and 12 urine samples were obtained from the miners (occupationally exposed populations), and 39 urine samples and 39 hair samples were obtained from the residents. Hair near the scalp was scissored and morning urine was collected to a 50-mL bottles. Questionnaires were filled by all the volunteers, and their basic information were showing in Table S1. All the residents participated of their own accord and the local Government also permitted our sampling scheme. The study was based on the Declaration of Helsinki.
All the samples were stored in the cooler with ice bags during the transport to our lab. HNO3 was added to preserve the urine samples (v:v = 5%) and then reserved < − 20 °C. Hair samples were washed based on the standardized washing procedure according to the IAEA (Pozebon et al. 2017). Soil samples were air-dried to a constant weight, while plant samples were washed with tap water and then distilled water before dried at 75 °C to a constant weight. All the solid samples were ground into powder and passed through a 1 mm sieve. Detailed methods can be found in the previous study (Du et al. 2020).
Sample Analysis
Surface water samples were filtered through a 0.45 μm filter membrane. Soil were digested by HNO3, HF, and HClO4 (v:v:v = 5:10:5) at the temperature of 120–240 °C on an hot plate, which were detailed in our previous study (Zhou et al. 2019b). Hair and urine samples were digested with 5 mL HNO3, while plant samples were digested by mixed HNO3 and HClO4 (v:v = 5:1) at the temperature of 80–120 °C on an hot plate. The analysis precision and accuracy were conducted the Pb, Zn, Cu, and As in the digested solutions and soluble deposition were measured by inductively coupled plasma mass spectrometry. Hair samples (0.05 g), urine sample (2 mL), and food sample (rice and vegetables, 0.5 g) were digested with mixed HNO3 and HClO4 (v:v = 5:1) of 3 mL, 6 mL and 6 mL on an electric hot plate with the temperature 80–120 °C, respectively. Then the Pb, Zn, Cu, and As in the digested solutions were determined by inductively coupled plasma mass spectrometry (ICP-MS, Perkin Elmer®). Blank duplicates, parallels and certified reference materials for Pb, Zn, Cu, and As was measured during the experiments. The limits of detection for Pb, Zn, Cu, and As, based on three times the standard deviation of replicate measurements of a blank samples, was above 0.002 μg L−1, and the corresponding limits of detection obtained for Pb, Zn, Cu, and As in samples were 0.01 μg kg−1. The instrumental blanks were under the detection limit. Certified reference materials (CRMs) comprising human hair (GBW07601a), spinach [GBW10015 (GSB-6)], and single element solution of Pb, Zn, Cu, and As (GSB04-1721-2004) were used for quality control. The recovery rates of elements in the certified reference materials ranged between 96 and 105%. The corresponding analytical results of CRMs are listed in Table S2. The toxic element concentrations in the vegetable samples were reported in fresh weight and in the soil, rice, and hair samples are in dry weight. Cd in all the samples were reported and obtained from the previous study (Du et al. 2020).
Impact Index of Comprehensive Quality (IICQ)
IICQ index synthesized the soil pollution and food contamination is applied to assess the soil environmental qualities (Huang et al. 2020), calculating based on the following equation:
where the S and C are farmland soil and crops, respectively. IICQ contains five classes (I‒V), flowing unpolluted, slightly polluted, moderately polluted, heavily polluted, and extremely polluted. The detailed approach of this assessment and classification are reported by Wu et al. (2019), as well as shown in the SI (Text S1, Tables S3 and S4).
Bio-accumulation Factor (BAF)
Toxic element concentrations in soils and crops were in dry weight. The BAF which is an index of the ability of the vegetable (Cplant) to accumulate a particular metal with respect to its concentration in the soil substrate (Csoil), was calculated as follows:
Health Risk Assessment
The probably daily intake (PDI), target hazard quotient (THQ), and hazard index (HI) of the residents in studying area were calculated and analyzed.
The Probably Daily Intake (PDI) and Hazard Quotient (HQ)
The PDI (μg kg−1 day−1) of toxic elements (Pb, Zn, Cu, As, and Cd) from foodstuffs ingestion, drinking water and dermal absorption was calculated by the following formulae:
where the parameters in Eqs. (4) and (5) used for PDI estimation were well discussed in previous study (Du et al. 2020) and presented in Table S5, which is according to the methods from US EPA and literatures. C represents the chemical concentrations in rice and vegetables (mg kg−1), respectively; IR is the daily consumed amount of rice and vegetable (g day−1), respectively (USEPA 2011); BW is the average body weight of the corresponding population (kg body weight, kg BW, USEPA 2011). The C of rice was converted with a factor of 0.86 due to commonly 14% water content in home-stored rice (Zhuang et al. 2009).
The non-carcinogenic risk for Pb, Zn, Cu, As, and Cd is calculated by the HQ, which is calculated by dividing the PDI for the three exposure pathways by the reference dose (RfD) (μg BW kg−1 day−1). The exposed people are unlikely to undergo adverse effects if the ratio is lower than one. On the contrary, there is potential health risk if the ratio is equal to or greater than one, and relevant protection measure should be taken. The formula of HQs supplied by the United States Environmental Protection Agency (USEPA 2011) was as follows:
where RfDingest and RfDdermal represents the oral reference dose and dermal reference dose (μg BW kg−1 day−1), respectively. In this assessment, the Cd concentrations in all our collected samples are used from our previous study (Du et al. 2020) to comprehensive assessment the toxic element exposure in this area. The RfDingest for Pb, Zn, Cu, As, and Cd are 1.4, 300, 40, 0.3, and 1 μg BW kg−1 day−1, while RfDdermal for the them are 0.525, 60, 1.9, 0.123, and 0.05 μg BW kg−1 day−1, respectively (USEPA 2011).
Target Hazard Quotient (THQ) and Hazard Index (HI)
The THQ represents a comprehensive risk of different sources of a certain kind of metal on human health:
The HI assesses the overall potential risk of non-carcinogenic effects composed of more than one element (Saha and Zaman 2013). The HI was calculated by the following formula:
There is an un-acceptable risk of non-carcinogenic effects on health assessment if HI > 1, whereas there is an acceptable risk if HI < 1.
Statistical Analysis
The SPSS22.0 and OriginPro 9.1 were used for the statistical analysis and figure plot. Comparisons of datasets were subjected to one-way ANOVAs analysis of variance test. The correlation was analyzed by Pearson’s Correlation Tests. All differences in means and correlation coefficient with p values are significantly at the level of 0.05.
Results and Discussion
Toxic elements in Agricultural Soils
Soil contaminants have been widely used to characterize the environmental toxic element pollution (Li et al. 2014; Włostowski et al. 2016). The mean concentrations of paddy soil Pb, Zn, Cu, and As were 120 ± 22, 186 ± 80.2, 21.1 ± 10.0, and 6.6 ± 5.3 mg kg–1 in the studying area (Fig. 1 and Table S6). For the paddy soils, the Pb, Zn, and Cu concentrations showed the highest in the watershed M1 (138 ± 22, 227 ± 134, and 25 ± 8.6 mg kg–1) and followed by M2 and M3, while the As concentrations showed the highest in the watershed M2 and followed by M1 and M3. The mean Pb, Zn, Cu, and As concentrations in the vegetable soil was 178 ± 110, 353 ± 241, 37.0 ± 16.7, and 14.8 ± 10.6 mg kg–1 and the toxic element concentrations showed the highest in the watershed M2 and followed by M3 and M1.
All (100%) of the paddy soils and 98% of the vegetable soils exceeded the soil Pb threshold values of agricultural soil standards in China (80 and 70 mg kg–1 for paddy and vegetable soils), respectively (GB15618-2018, Table S4). A total of 33% paddy soils and 69% vegetable soils exceeded the soil Zn threshold values of the standard (200 mg kg–1 for both paddy and vegetable soils). About 19% of vegetable soils exceeded the soil Cu threshold values of the standard (50 mg kg–1 for both paddy and vegetable soils). Except one vegetable soil sample in M2, soil As concentrations were below the soil threshold values (30 and 40 mg kg–1 for paddy and vegetable soils, respectively). Paddy soils in M2 had the highest percent of soil exceeding the thresholds than those in M1 and M3.
In our studying area, some mines were operating and ores were elementarily processing, which resulted in plenty of mineral waste residue storage in the open field. The Pb and Zn concentrations in the waste residue can be up to 2995 and 5986 mg kg−1, and the Pb and Zn concentrations gradually decreased from mean concentrations of 2586 and 5686 mg kg−1 in the new mineral waste to 1966 and 3742 mg kg−1 in the 12-month old waste (Zhu et al. 2019). No coverage over the mineral waste would promote large amounts of erosion and leaching of the mineral waste to nearby agricultural land during rain events (Du et al. 2020; Huang et al. 2020; Zhu et al. 2019), which results in large areas of agricultural soil contamination around these mines. We also found higher toxic element concentrations in the surface water and the concentrations decreased gradually away from the mining areas (in section “Toxic Elements in Surface Water and Agricultural Products”), which can support the toxic elements is leaching from the waste residue.
Toxic Elements in Surface Water and Agricultural Products
To a certain extent, toxic element concentrations in surface water is also an important indicator of environmental toxic element contamination. The Pb, Zn, Cu, As, and Cd concentrations in surface water were 9.2 ± 8.0, 308 ± 261, 2.0 ± 0.85, and 15.9 ± 6.9 ng L−1, respectively (Fig. 2 and Table S7). About 41% of the water samples exceeded the Pb concentration limit of the Chinese Environmental quality standards for class II surface water (GB3838-2002) (10 ng L−1 for Pb), while all the Zn, Cu, and As concentrations were below their limits (1,000, 1,000, and 50 ng L−1). In M2 area, Pb concentrations in surface water at sites 9 and 10 were 4704 and 945 ng L−1, and relatively higher than other sites, which proved that mining wastewater was an important Pb source of the surrounding environment, because the outlet of the ore processing factory was located at about several meters in the upstream.
The Pb, Zn, Cu, and As concentrations in the rice samples were 0.043 ± 0.014, 15 ± 2.7, 2.8 ± 0.57, and 0.44 ± 0.20 mg kg−1 (Fig. 2 and Table S8). About 32% of the rice samples in our research exceeded the As concentration limit of the national food safety standards in China (GB2762-2017), while all of the rice Pb concentrations were below this standard (0.5 and 0.1 mg kg−1 for As and Pb, respectively). The Pb, Zn, Cu, and As concentrations in the vegetables (fresh weight) were 0.032 ± 0.027, 12 ± 12, 0.45 ± 0.22, and 0.12 ± 0.063 mg kg−1, respectively (Fig. 2 and Table S8). The Pb and As concentrations in all the vegetables were under the threshold limit concentrations (GB2762-2017). Zn and Cu are essential elements for human body, so no threshold limits in food are given by the government. Toxic element concentrations were different among the vegetables, where leeks had the highest Pb and As concentrations while radish leaves had the highest Zn concentration (Table S9).
The BAF of vegetables to Pb, Zn, Cu, and As were 0.00024, 0.035, 0.014, and 0.012, respectively, which were much lower than other research in some other contaminated area. For instance, a study on vegetables around battery plant showed that the BAFs of vegetables to Pb, Zn, Cu, and As were 0.026, 0.088, 0.042, and 0.0036, respectively (Bu et al. 2012). The speciation of the toxic elements was the crucial factor affecting the plant uptake and BAF. Usually, exchangeable fractions are regarded as the bioavailable toxic elements in soils and the iron and manganese oxidized, organic matter bounded, and residual fractions are regarded as un-bioavailable species (Ahmed et al. 2018). In this mining area, we speculate that toxic elements in surrounding soils were mainly iron and manganese oxide species and residue species by the influence of ore waste and tailings, and finally caused low BAFs of vegetables.
Impact Index of Comprehensive Quality (IICQ)
The IICQ index and soil environmental quality proportions are shown in Fig. 3 and Table S10. The IICQ of M1, M2, and M3 were 6.4, 6.0, and 7.1, respectively. For soils, the IICQS of the three sites were 5.5, 5.5, and 6.7, and the IICQC of the three sites were 0.97, 0.50, and 0.43 for crops, respectively, indicating soil environmental quality mainly from the weighted soil qualities (IICQS). Totally, 58% of the samples in our studying area were extremely contaminated (V), 38% of the samples were from slightly to heavily contaminated (II-IV), while only 2% of the samples were uncontaminated (I). For the three areas, the highest IICQ was in M3, followed by M1and M2, suggesting highest contamination occurred in the M3. Although M3 was the farthest to the mines and slag stacks, it located in the downstream where rivers from M1 and M2 meets (Fig. S1). Highest Zn and As has been observed in the surface water in M3 confirmed that toxic elements were transported from upstream (M1 and M2).
Although in this study, the toxic elements in slugs and waste water were not studied, previous study showed that the Pb, Zn, and Cd concentrations in the new mineral waste can be up to 2799, 5198, and 23.9 mg kg−1, while these in the mineral waste stacked for about 12 months were decreased to 1305, 2899, and 9.4 mg kg−1, suggesting that the these metals were leached during the rain event (Zhu et al. 2019). Our results also showed that in some sites near the mineral waste, the Pb concentrations of water exceeded the limit of the Chinese Environmental quality standards for class II surface water (GB3838-2002, as shown in 3.2). Therefore, stabilization the toxic elements in the mineral waste or the leaching water should be treated before discharge to ensure that toxic elements in the wastewater were under the limits of emission standards for heavy non-ferrous metal industrial pollutants (GB4913-1985). Meanwhile, some moderate detoxification activities, such as soil remediation such as chemical immobilization, foliar sprayings of benefit elements to crops, and phytoremediation, should also be taken to reduce the toxic elements accumulation in the crops (Li et al. 2021). Chemical immobilization can effectively reduce the transportation of toxic elements between soil and crop, while phytoremediation can accumulate toxic elements into the hyper-accumulators and move out toxic elements from soils (Li et al. 2019; Paz-Ferreiro et al. 2014; Su 2014).
Health Risk Assessment
The Probably Daily Intakes (PDIs)
The exposure pathways considered in this assessment are from three medium: food (rice and vegetable ingestion), soil (ingestion and dermal absorption), and drinking water. The total PDIs of Pb, Zn, Cu, and As were 0.48, 118, 17, and 2.9 μg kg−1 day−1, respectively (Fig. 4 and Table S11). The contributions to the total PDIs of Zn, Cu, As, and Cd decreased in the same order: rice ingestion (64–94%) > vegetable ingestion (11–36%) > soil ingestion (0.04–0.62%) > drinking water (2.8E−3–3.3E−2%) > dermal absorption of soil (7.2E−5–7.0E−4%) (Fig. 4). For Pb intake, the major pathway is also rice ingestion, averaging for 51%, but followed by soil ingestion (28%), vegetable ingestion (21%), and drinking water (< 0.07%), and dermal absorption of soil (< 0.02%), respectively. By reviewing literatures, rice consumption is the main contributor to dietary toxic element intake, especially in East and Southeast Asia where rice is the staple food (Davis et al. 2017; Himeno and Aoshima 2019; Lee et al. 2008; Li et al. 2021; Pateriya et al. 2020; Praveena and Omar 2017; Songprasert et al. 2015; Yadav et al. 2017; Zakaria et al. 2021). Combined with vegetable toxic element intake, the dietary intake accounts for 49–96% of the total exposure (Du et al. 2020; Hu et al. 2019; Ikeda et al. 2000; Mondal et al. 2021). Therefore, reduction of toxic element concentrations in the foodstuff, especially toxic element in rice can effectively reduce the exposure in the mine areas. Although PDIs of all the toxic elements were much higher than national residents by the 5th China total diet study (Wei et al. 2019), our estimates would still underestimate the PDIs of toxic elements in this area. The PDIs of toxic element near a Cu smelting factory 30 km away from our sampling area and showed meat and egg were contributed approximately 10% of Zn exposure (Hu et al. 2019). The inhalation and food ingestion would be also contributed half and half of the exposure to As near the largest coking plant (Cao et al. 2014). However, in this study, the lack of enough food source and inhalation and ingestion of atmospheric particles may underestimate PDIs of the toxic elements.
The calculated hazard quotient (HQ) were shown in Fig. 5 and Table S12. HQs of each median among all of the toxic elements were less than one except for As in five rice samples. Among all the toxic elements, HQs caused by rice ingestion (0.16–0.85) were the highest, followed by vegetable ingestion (0.023–0.11). While HQs of dermal absorption and water drinking were almost negligible (44E−6–27E−4 and 1.4E−6–3.5E−4, respectively). It is worth to mention that HQs of Pb from soil ingestion (0.08–0.12) were more than 10 times higher than other toxic elements (0.0005–0.005), which suggested that soil ingestion is also a considerable source for Pb exposure.
Target Hazard Quotient (THQ) and Hazard Index (HI)
By referring the HQ of Cd in the previous study (Du et al. 2020), we calculated the THQ and HI of the five toxic elements (Pb, Zn, Cu, As, Cd) in studying area (Fig. 6 and Table S13). THQs followed the order: rice ingestion (2.5–3.0) > vegetable ingestion (0.56 − 1.2) > Soil ingestion (0.09–0.13) > dermal absorption (0.02–0.05) > drinking water (0.0007–0.0008). The THQs through rice, vegetable, and water ingestion were higher in M1 than the other two sites, while THQ though soil ingestion was slightly higher in M2. Totally, rice and vegetable ingestion contributed 78–97% to the THQ, while the THQ though soil and water ingestion were about negligible.
The HI of the residents in our studying area was 3.9, among which the HIs of the residents from M1, M2, and M3 were 4.4, 3.6, and 3.6. The HIs of all the residents in the three watersheds were higher than one, indicating a high non-carcinogenic risk for residents exposed to toxic elements in this Pb–Zn mining areas.
As for the residents and miners who have already exposed toxic element, the possible detoxification was recommended that: (1) The miners should car about the inhalation exposure to toxic elements during working as inhalation may be one of the most important toxic element exposure pathways for occupational exposure (Mahurpawar 2015; Omrane et al. 2018); (2) the local government should prohibit local residents’ agricultural activities in the polluted areas, or cultivate low-heavy-metal-accumulating vegetables, such as radish or cabbage to reduce the exposure risk (as shown in 3.2); (3) previous studies found that selenium supplementation may have positive affect on human health after toxic element exposure (Aaseth et al. 2021; Zhou et al. 2019a, b, c) due to the antagonism between selenium and toxic elements (Fu et al. 2019; Jamwal et al. 2016; Skalny et al. 2016).
Toxic Elements in Hair
Hair Pb, Zn, Cu, and As concentrations of the miners were 2.6, 157, 16, and 1.8 mg kg−1, respectively, while Pb, Zn, Cu, and As concentrations of the residents from the three sampling sites were, 0.74, 157, 13, and 1.2 mg kg−1, respectively (Fig. 7). The Pb, Cu, and As concentrations in miners’ hair were significantly higher than those in the residents’ hair (p < 0.01), while no significant differences of Zn were observed between miners and residents (p > 0.05). Researchers did plenty of work on toxic elements in residents’ hair in China, showing in Table S14. Hair Cu and As in this study were 1–3 times higher than those compared to other studies and hair Zn and Cd were comparable with the results of most the other studies. Hair Pb in this study were relatively lower than most of the human hair in mining areas, accounting for only 1/2–1/30. However, hair Cu and As in this study exceeded the Chinese normal resident hair concentrations (11.3 and 0.68 mg kg−1 for Cu and As) by factors of 1.7–6.0 (H/ZWY03-2005 and H/ZWY01-2007).
The Pb and Cu concentrations in hair were well correlated with corresponding PDIs in the study areas (p < 0.01, Fig. S2), indicating hair Pb and Cu concentrations can well indicate the exposure. However, no correlations were observed between hair Zn and As with the corresponding PDIs. As we discussed above, meat would be contributing relatively higher Zn that is not calculated in this study. As and its compound (As2O3) trioxide would be volatilized during the ore preprocessing before smelting and accumulate in atmospheric dust, so inhalation exposure would be also an important exposure pathway for As (Yüksel et al. 2013; Zhang et al. 2021), which can be also evidenced by significantly higher As concentration in the miner’s hair in this study (Fig. 7).
Toxic Elements in Urine
Urinary heavy-metal levels are an important sensitive index of heavy-metal exposure and can represent acute intoxication (Ahmed et al. 2021). Urine Pb, Zn, Cu, and As concentrations of miners from the three sampling sites were 1.9, 128, 48, and 21 µg L−1, while these concentrations of residents were 1.9, 122, 41, and 25 µg L−1, respectively (Fig. 4). Urine Cu were slightly higher in miners than in residents, though no significant differences of all the toxic elements were observed (p > 0.05). As shown in Table S15, urine Pb Zn and As of both miners and residents in this study were lower than workers from nonferrous plants, miners and residents from mining area, and general populations in Guangzhou and Wuchuan. However, urine Cu and Cd concentrations of miners and residents in this study were relatively high, which were 2–10 times higher than workers, miners, and residents in previous studies.
Conclusion
All the paddy and vegetable soils were contaminated and exceeded the soil threshold values of agricultural soil standards in China. About 32% of the rice samples exceeded the As concentration limit of the national food safety standards, but the toxic element concentrations in the vegetables were under the threshold limit concentrations. The IICQ indicated that 98% of the monitoring sites was contaminated with 58% of extreme contamination. The results from multi-pathway intake of toxic elements and TTHQ showed rice was the most dominate pathway of toxic element exposure. The HIs showed a high non-carcinogenic risk for residents exposed to toxic elements in this Pb–Zn mining area. Hair Cu and As concentrations exceeded the Chinese resident normal hair concentrations by factors of 1.7–6.0. The implications are that proper mitigation strategies on toxic element pollution and human exposure are to reduce the intake of local agricultural products, especially rice. Additionally, soil remediation is encouraged to improve the crop toxic element accumulation and local residents’ health.
Data Availability
The data will be available by the corresponding author (Jun Zhou).
References
Aaseth J, Alexander J, Alehagen U, Tinkov A, Skalny A, Larsson A et al (2021) The aging kidney—as influenced by heavy metal exposure and selenium supplementation. Biomolecules 11:1078
Agnew UM, Slesinger TL (2022) Zinc toxicity. StatPearls Publishing, Treasure Island. https://www.ncbi.nlm.nih.gov/books/NBK554548/?report=reader#_NBK554548_pubdet_
Ahmed AY, Osman MR, Abdulah MP, Wood AK, Hamza MS, Siddeeg SM (2018) Speciation of trace metals in sediment: modified Tessier sequential extraction scheme versus BCR scheme. Int Res J Nat Appl Sci 5:115
Ahmed HAM, Goher AS, Alzubaidi AM (2021) Risk assessment of heavy metals content in different hair dyes and their users’ urine samples. Int J Environ Anal Chem 101:1116–1125
Appenzeller BR, Tsatsakis AM (2012) Hair analysis for biomonitoring of environmental and occupational exposure to organic pollutants: state of the art, critical review and future needs. Toxicol Lett 210:119–140
Bai L, He Z, Ni S, Chen W, Li N, Sun S (2019) Investigation of PM2.5 absorbed with heavy metal elements, source apportionment and their health impacts in residential houses in the North-east region of China. Sustain Cities Soc 51:101690
Bihaqi SW (2019) Early life exposure to lead (Pb) and changes in DNA methylation: relevance to Alzheimer’s disease. Rev Environ Health 34:187–195
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Bu YJ, Zhang HX, Yang ZZ, Wang SY, Li Hb, Zhang RF (2012) Bioenrichment of heavy metals in different crops. J Xin Med Coll 29:569–571
Cancer IAfRo (2006) Monographs on the evaluation of carcinogenic risks to humans. http://monographs.iarc.fr/ENG/Classification/index.php
Cao S, Duan X, Zhao X, Ma J, Dong T, Huang N (2014) Health risks from the exposure of children to As, Se, Pb and other heavy metals near the largest coking plant in China. Sci Total Environ 472:1001–1009
Cao S, Chen X, Zhang L, Xing X, Wen D, Wang B (2020) Quantificational exposure, sources, and health risks posed by heavy metals in indoor and outdoor household dust in a typical smelting area in China. Indoor Air 30:872–884
Chen X, Ward TJ, Sarkar C, Ho KF, Webster C (2022) Health risks of adults in Hong Kong related to inhalation of particle-bound heavy metal(loid)s. Air Qual Atmos Health 15:691–706
Chibowska K, Baranowska-Bosiacka I, Falkowska A, Gutowska I, Goschorska M, Chlubek D (2016) Effect of lead (Pb) on inflammatory processes in the brain. Int J Mol Sci 17:2140
CNEMC (1990) Elements background value of China soil. China Environmental Press, Beijing
Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T (2017) Assessment of human dietary exposure to arsenic through rice. Sci Total Environ 586:1237–1244
Dong Z, Hu J (2012) Development of lead source-specific exposure standards based on aggregate exposure assessment: Bayesian inversion from biomonitoring information to multipathway exposure. Environ Sci Technol 46:1144–1152
Du B, Zhou J, Lu B, Zhang C, Li D, Zhou J (2020) Environmental and human health risks from cadmium exposure near an active lead-zinc mine and a copper smelter. China Sci Total Environ 720:137585
Du B, Li P, Feng X, Yin R, Zhou J, Maurice L (2021) Monthly variations in mercury exposure of school children and adults in an industrial area of southwestern China. Environ Res 196:110362
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poisoning in the modern world-new tricks for an old dog 10:70–90
Fu J, Yang T, Wang W, Xu S (2019) Effect of selenium antagonist lead-induced damage on Th1/Th2 imbalance in the peripheral blood lymphocytes of chickens. Ecotoxicol Environ Saf 175:74–82
Himeno S, Aoshima K (2019) Cadmium toxicity: new aspects in human disease, rice contamination, and cytotoxicity. Springer, Singapore
Hu Y, Zhou J, Du B, Liu H, Zhang W, Liang J (2019) Health risks to local residents from the exposure of heavy metals around the largest copper smelter in China. Ecotoxicol Environ Saf 171:329–336
Huang YN, Dang F, Li M, Zhou DM, Song Y, Wang JB (2020) Environmental and human health risks from metal exposures nearby a Pb-Zn-Ag mine. China Sci Total Environ 698:134326
Ikeda M, Zhang ZW, Shimbo S, Watanabe T, Nakatsuka H, Moon CS (2000) Exposure of women in general populations to lead via food and air in east and southeast Asia. Am J Human Biol 38:271–280
Jamwal A, Naderi M, Niyogi S (2016) An in vitro examination of selenium–cadmium antagonism using primary cultures of rainbow trout (Oncorhynchus mykiss) hepatocytes. Metallomics 8:218–227
Järup L (2003) Hazards of heavy metal contamination. Brit Med Bull 68:167–182
Kavehei A, Hose GC, Gore DB (2021) History of environmental contamination at Sunny Corner Ag-Pb-Zn mine, eastern Australia: a meta-analysis approach. Environ Pollut 273:115742
Lamm SH, Boroje IJ, Ferdosi H, Ahn J (2021) A review of low-dose arsenic risks and human cancers. Toxicol 456:152768
Lee JS, Lee SW, Chon HT, Kim KW (2008) Evaluation of human exposure to arsenic due to rice ingestion in the vicinity of abandoned Myungbong Au–Ag mine site, Korea. J Geochem Explor 96:231–235
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Li C, Zhou K, Qin W, Tian C, Qi M, Yan X et al (2019) A review on heavy metals contamination in soil: effects, sources, and remediation techniques. Soil and Sediment Contam 28:380–394
Li Z, Liang Y, Hu H, Shaheen SM, Zhong H, Tack FG (2021) Speciation, transportation, and pathways of cadmium in soil-rice systems: a review on the environmental implications and remediation approaches for food safety. Environ Int 156:106749
Mahurpawar M (2015) Effects of heavy metals on human health. Int J Res Granthaalayah 530:1–7
Mondal D, Rahman MM, Suman S, Sharma P, Siddique AB, Rahman MA (2021) Arsenic exposure from food exceeds that from drinking water in endemic area of Bihar. India Sci Total Environ 754:142082
Moody EC, Coca SG, Sanders AP (2018) Toxic metals and chronic kidney disease: a systematic review of recent literature. Curr Pollut Rep 5:453–463
Nasirzadeh N, Mohammadian Y, Dehgan G (2022) Health risk assessment of occupational exposure to hexavalent chromium in Iranian workplaces: a meta-analysis study. Biol Trace Elem Res 200:1551–1560
Omrane F, Gargouri I, Khadhraoui M, Elleuch B, Zmirou-Navier D (2018) Risk assessment of occupational exposure to heavy metal mixtures: a study protocol. BMC Public Health 18:1–11
Pateriya A, Verma RK, Sankhla MS, Kumar R (2020) Heavy metal toxicity in rice and its effects on human health. Lett Appl NanoBioScience 10:1833–1845
Paz-Ferreiro J, Lu H, Fu S, Mendez A, Gasco G (2014) Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth 5:65–75
Plumlee GS, Durant JT, Morman SA, Neri A, Wolf RE, Dooyema CA (2013) Linking geological and health sciences to assess childhood lead poisoning from artisanal gold mining in Nigeria. Environ Health Perspect 121:744–750
Pozebon D, Scheffler GL, Dressler VL (2017) Elemental hair analysis: a review of procedures and applications. Anal Chim Acta 992:1–23
Praveena S, Omar N (2017) Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis. Food Chem 235:203–211
Qin W, Han D, Song X, Liu S (2021) Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb-Zn mine, Southwest China. Environ Pollut 277:116774
Royer A, Sharman T (2020) Copper toxicity. StatPearls Publishing, Treasure Island
Sah D, Verma PK, Kumari KM, Lakhani A (2019) Chemical fractionation of heavy metals in fine particulate matter and their health risk assessment through inhalation exposure pathway. Environ Geochem Health 41:1445–1458
Saha N, Zaman MR (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ Monit Assess 185:3867–3878
Skalny AV, Skalnaya MG, Nikonorov AA, Tinkov AA (2016) Selenium antagonism with mercury and arsenic: from chemistry to population health and demography selenium. Springer, Cham, pp 401–412
Songprasert N, Sukaew T, Kusreesakul K, Swaddiwudhipong W, Padungtod C, Bundhamcharoen K (2015) Additional burden of diseases associated with cadmium exposure: a case study of cadmium contaminated rice fields in Mae Sot District, Tak Province, Thailand. Int J Env Res Public Health 12:9199–9217
Su C (2014) A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environ Skept Crit 3:24
USEPA (2011) Exposure factors handbook, 2011th edn. National Center for Environmental Assessment, Washington, DC
Wcisło E, Ioven D, Kucharski R, Szdzuj J (2002) Human health risk assessment case study: an abandoned metal smelter site in Poland. Chemosphere 47:507–515
Wei J, Gao J, Cen K (2019) Levels of eight heavy metals and health risk assessment considering food consumption by China’s residents based on the 5th China total diet study. Sci Total Environ 689:1141–1148
Włostowski T, Kozłowski P, Łaszkiewicz-Tiszczenko B, Oleńska E (2016) Cadmium accumulation and pathological alterations in the midgut gland of terrestrial snail Helix pomatia L. from a zinc smelter area: role of soil pH. Bull Environ Contam Toxicol 96:484–489
Wu TL, Cui XD, Cui PX, Ata-Ul-Karim ST, Sun Q, Liu C (2019) Speciation and location of arsenic and antimony in rice samples around antimony mining area. Environ Pollut 252:1439–1447
Xu L, Zhou M, Yuan Y, Wang YM, Tang DD, Zhang XH (2018) Study on cadmium and lead concentrations in soils and atmospheric particles and their contributions to rice in the typical agricultural area of southern Jiangsu Province. J Ecol Rural Environ 34:201–206
Yadav P, Singh B, Garg V, Mor S, Pulhani V (2017) Bioaccumulation and health risks of heavy metals associated with consumption of rice grains from croplands in Northern India. Hum Ecol Risk Assess 23:14–27
Yang S, Huang Q, Cheng X, Qi W, Zhang X, Xiang Y (2019) A review of human hair heavy metal concentration characteristics from mines in China. IOP Conf Ser 362:012162
Yüksel B, Kayaalti Z, Türksoy VA, Tutkun E, Söylemezoglu T (2013) Evaluation of arsenic levels in biological samples of smelter workers in Turkey. Toxicol Lett 221:251
Zakaria Z, Zulkafflee NS, Mohd NA, Selamat J, Ismail MR, Praveena SM (2021) Understanding potential heavy metal contamination, absorption, translocation and accumulation in rice and human health risks. Plants 10:1070
Zhang H, Mao Z, Huang K, Wang X, Cheng L, Zeng L (2019) Multiple exposure pathways and health risk assessment of heavy metal(loid)s for children living in fourth-tier cities in Hubei Province. Environ Int 129:517–524
Zhang L, Zhou H, Chen X, Liu G, Jiang C, Zheng L (2021) Study of the micromorphology and health risks of arsenic in copper smelting slag tailings for safe resource utilization. Ecotoxicol Environ Saf 219:112321
Zhou J, Du B, Hu Y, Liang J, Liu H, Fan X (2019a) A new criterion for the health risk assessment of Se and Pb exposure to residents near a smelter. Environ Pollut 244:218–227
Zhou J, Du B, Liu H, Cui H, Zhang W, Fan X (2019b) The bioavailability and contribution of the newly deposited heavy metals (copper and lead) from atmosphere to rice (Oryza sativa L.). J Hazard Mater 384:121285
Zhou L, Liu G, Shen M, Hu R, Sun M, Liu Y (2019c) Characteristics and health risk assessment of heavy metals in indoor dust from different functional areas in Hefei. China Environ Pollut 251:839–849
Zhu X, Cao L, Liang Y (2019) Spatial distribution and risk assessment of heavy metals inside and outside a typical lead-zinc mine in southeastern China. Environ Sci Pollut Res 26:26265–26275
Zhu C, Tian H, Hao J (2020a) Global anthropogenic atmospheric emission inventory of twelve typical hazardous trace elements, 1995–2012. Atmos Environ 220:117061
Zhuang P, McBride MB, Xia HP, Li NY, Lia ZA (2009) Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 407(5):1551–1561
Zhuang P, Lu H, Li Z, Zou B, McBride MB (2014) Multiple exposure and effects assessment of heavy metals in the population near mining area in South China. PLoS ONE 9:e94484
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (42207277), General project of Natural Science Research in Colleges and Universities of Jiangsu Province (21KJB610019), the “Green blue project” of Jiangsu University, Project of Industry and School and Research Institution of Jiangsu Province (BY 2021529), and the Qinlan project of Jiangsu Province (2021SZJS-003).
Funding
This work was financially supported by the National Natural Science Foundation of China (42207277), General project of Natural Science Research in Colleges and Universities of Jiangsu Province (21KJB610019), the “Green blue project” of Jiangsu University, Project of Industry and School and Research Institution of Jiangsu Province (BY 2021529), and the Qinlan project of Jiangsu Province (2021SZJS-003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, B., Zhang, H., Ji, D. et al. Environmental Contamination and Health Risk Assessment to Toxic Elements in an Active Lead–Zinc Mining Area. Expo Health 15, 687–698 (2023). https://doi.org/10.1007/s12403-022-00515-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-022-00515-y