Abstract
E-waste is a global environmental problem that must not be underestimated. To ascertain the environmental pollution status of electronic waste dumpsites, an assessment of seasonal variation in concentrations of heavy metals in the soils and plants in electronic waste dumpsites in Lagos metropolis was undertaken during the two major seasons (wet and dry seasons) in Nigeria. The soils and plants samples collected in August during the rainy season and December during the dry season were digested using aqua regia, a wet digestion method. The heavy metal concentrations were quantified with flame atomic absorption spectrophotometer (FAAS) fitted with a hollow cathode lamp (Perkin-Elmer AA Analyst 200) using an air-acetylene flame. The soil samples and the plants analyzed were found to have high concentrations of heavy metals. All the plants analyzed were identified and classified as excluders and accumulators of heavy metals based on their shoot/root quotients. The results obtained experimentally were analyzed using statistical and multivariate techniques. The soil quality of the study area was evaluated using correlation factor, pollution load index, and geoaccumulation index. The heavy metal assessment in comparison with the EU and Canadian standards for both agricultural and residential soils showed that the e-waste dumping sites are highly polluted. The results obtained for both the soil and plants analyzed confirmed the great impact of the hazardous components of e-waste on the ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electronic waste (e-waste) pollution is a major global problem because e-waste consists of toxic substances which can contaminate the environment and possibly affect the human health (Hilty, 2005).
The world at large is affected by e-waste pollution since electronic appliances are used by a larger percentage of the global population (Kiddee et al., 2013). China which is the world’s leading manufacturing country is the largest dumping ground for e-waste (Song & Li, 2014). The countries that are mostly affected by e-waste pollution are countries that lack comprehensive e-waste management plan, especially countries that permit the dumping and burning of e-waste (Huang et al., 2014). The improper e-waste management (such as dumping and burning of these obsolete electronics and their various parts) impacts on the soil ecosystem and the air quality (Ogunseitan et al., 2009). Nigeria like most other developing countries source their potable water from underground; therefore, leaching of the toxic substances in e-waste into underground and surface water can occur (Olafisoye et al., 2013; Sun et al., 2001). Therefore, improper e-waste management has the potential to affect all the components of the environment (Sullivan, 2014; Onwughara et al., 2010; Manhart et al., 2011).
Multiple studies have shown that e-waste consists of different hazardous substances especially halogenated compounds (PCB, PBB, PBDE) and heavy metals (Pb, Cd, Cu, Ni, and Cr) (Cui & Zhang, 2008; Kang & Schoenung, 2005; Babu et al., 2007). Heavy metals have the tendency to accumulate in soils and plants, thereby having negative influence on the physiological activities of plants such as photosynthesis, gaseous exchange, and nutrient absorption (Baker, 1981; Devkota & Schmidt, 2000).
According to the Basel Action Network (BAN), a Seattle-based environmental group, an estimated 500 shipping containers with a load equal in volume to about 400,000 computer monitors or 175,000 large TV sets enter Lagos, Nigeria, each month and about 75% of some of these shipments are classified as e-waste (Sullivan, 2014). The reasons for this huge influx of e-waste into Lagos are not farfetched; Lagos has a large seaport where these items easily slip through. There is a huge desire for very cheap second-hand imported electronic items in the city, but the lifespan of these items is usually short, thereby resulting in most of these imported items being discarded almost as soon as they are shipped into Lagos. The environment in Lagos is negatively impacted by the influx of these e-waste because of the poor e-waste management (Nnorom et al., 2007). Therefore, the aim of this study was to investigate the impact of electronic waste on the environment by measuring the concentration of the heavy metals in soils and plants from electronic waste dumpsites in Lagos metropolis during the wet and dry seasons, thereby providing a comprehensive understanding of the seasonal variation effect of e-waste on the environment.
Materials and methods
Study location
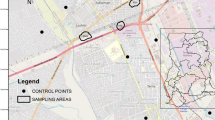
This study was carried out at two different electronic waste dumpsites in the metropolis of Lagos, Southwest Nigeria (Fig. 1). The two electronic waste dumpsites used for this study are Alaba electronic waste dumpsite, which is situated within Alaba international market in Ojo local government area in Lagos, Nigeria, and Westminster electronic waste dumpsite, which is situated within Westminster electronics market along Apapa road in Apapa local government area in Lagos (Fig. 2). Some of the electronic wastes found on these sites include mother board, monitors, television, radio, keyboard, phone batteries, finger-like batteries, printers, video players, fluorescent lamps, and broken parts of other electrical devices.
Sampling
Soil samples were collected at different points at Alaba and Westminster dumpsites. The samples were collected at the surface level (topsoil: 0–10 cm) and sub-surface (sub-soil: 20–30 cm). The soil was collected with the aid of a soil auger from five points to make a sample and transferred into a black polyethylene bag and labeled appropriately. Plant samples found around the dumpsites were collected by uprooting them carefully from the soil, and the plant samples were kept in a brown envelope and identified in the Herbarium, Botany Department of University of Lagos. The plant species found at the study area are Eleusine indica, Amaranthus viridis, Aspilia thouarsii, Phyllantus muellarianus, and Cyperus echinatus. The sampling was done on a seasonal basis (wet and dry season); for the wet season, the samples were collected in August, while for the dry season, the samples were collected in December.
Sample treatment and chemical analysis
The adhered soils on the plant samples collected from the dumpsites were thoroughly removed with the use of hands. The plant samples were then separated into parts (i.e., roots and shoots) and air dried in the environmental laboratory for 4 and 3 weeks for wet and dry seasons, respectively. The properly dried samples were ground using agate mortar and pestle and transferred into polyethylene bags for storage until further analysis. The soil samples were also air dried in the laboratory for 4 and 3 weeks for wet and dry seasons, respectively. The stones and glasses in the soils were removed by hand picking, after which they were ground using mortar and pestle. Thereafter, the samples were sieved to 2-mm diameter size and transferred into clean polyethylene bags for storage until later analysis.
The pH of the soil samples was measured in 0.111 M CaCl2 solution at a ratio of 1:2 with the aid of a pH meter. The moisture content of the soil was determined by constant weight method via oven-drying. The total organic matter (TOM) of the soil samples was determined by Walkey-Black chromic acid wet oxidation method (Rhodes et al., 1981; Sleutel et al., 2007). The cation exchange capacity (CEC) of the soil samples was determined by the standard magnesium acetate method. The bioavailable metals in the soil were determined by using 1 g of soil sample with 20 ml of 0.5 M HCl solution and shaking for 1 h at a speed of 120 excursions per minute (e.p.m) as described by Sutherland method (Sutherland, 2002), after which the solution was filtered with Whatman filter papers into properly acid washed bottles for analysis. The plant and soil samples were digested by wet digestion method via the use of aqua regia (HCl: HNO3 in 3:1).
The concentration of the potential toxic metals in both the plant and soil samples was quantified with flame atomic absorption spectrophotometer (FAAS) fitted with a hollow cathode lamp (Perkin-Elmer AA Analyst 200) using air-acetylene flame.
Statistical analysis and heavy metal assessment
The experimental data obtained were statistically analyzed using Pearson correlation and the following multivariate techniques: principal component analysis (PCA) and cluster analysis. The heavy metals were assessed using contamination factor (CF), pollution load index (PLI), and the geoaccumulation index (Igeo) to evaluate the soil quality of the study area.
Statistical and multivariate analysis
Pearson correlation and PCA were used to determine the relationship between the soil parameters at each sampling point. The PCA and the cluster analysis were carried out using Microsoft Excel Addin XLSTAT 2021.1.1107 software. The cluster analysis was used to investigate the similarity among all the e-waste sampling points based on the heavy metal concentration values using agglomerative hierarchical clustering (single linkage) analysis method.
Heavy metal assessment
Contamination factor
The contamination factor for each of the metal was calculated using: \(CF= \frac{{C}_{M}}{{C}_{B}}\), where CM is the measured value of the concentration of each of the metal in the soil, and CB is the background value for the metal. The results obtained from the calculations were classified using the classes of contamination factor and contamination degree reported by Hakanson (1980) (Table 1).
Pollution load index
This index is usually determined by calculating the nth root of the product of the CF, where n is the total number of metals analyzed. When PLI < 1, there is no metal pollution, but PLI > 1 indicates metal pollution.
Geoaccumulation index
This index is usually used to assess soil quality by comparing the measured concentration obtained for the heavy metals to the background concentration:
where Cn is the measured concentration for the metal n and Bn is the background concentration for the metal n, while 1.5 is used as a factor to minimize the effect of possible variations in the background data. The results obtained from the calculations are analyzed and classified using Müller’s classifications (Müller, 1981) as presented in Table 2.
Results and discussion
Wet season
The physicochemical properties of the soils analyzed in wet season are presented in Table 3. The pH of the soil samples was observed to be slightly alkaline with values ranging from 7.2 to 7.6, which is an indication of the availability of the nutrients in the soil (though the ideal pH for soil is mainly less than 7), therefore indicating a level of contamination. ALAT has the highest TOC, TOM, and CEC compared to other soil samples investigated. To adequately compare the results obtained for the investigated soil samples, reference soil samples are analyzed to compare their measured values with certified values as presented in Table 4. The measured values obtained for SO-2 was within the certified values for the heavy metals that were determined, and this indicates the accuracy in values of the concentration of heavy metals obtained for the samples. The recovery of spiked samples ranged from 86.25 to 108.75%. ALAT had the highest pH of 7.6, CEC of 87.5 meq/100 g, organic matter of 6.58, and the highest concentration of all heavy metals investigated. There were also noticeable differences in the concentration of metals in each of the samples depending on pH values and organic matter content. The soil samples with alkaline pH and high organic matter (OM) content accumulated significantly higher concentrations of heavy metals compared with other soil samples. Organic matter content is one of the most important factors that control the accumulation, mobility, and bioavailability of heavy metals in soils. Increase in soil organic matter (SOM) content can lead to elevation of the soil adsorption capacity by which accumulation of heavy metals will be enhanced (Lo et al., 2008). Multiple studies have shown that organic matter form complexes with heavy metals and other contaminants; therefore, increase in SOM may serve as a natural barrier against heavy metal in plants (Kwiatkowska-Malina, 2018; Chibuike & Obiora, 2014).
Distribution of heavy metals in soils and plant species
Amaranthus spinosus and Cyperus echinatus were predominant plant species at the Alaba site followed by Aspilia thouarsii, while in Westminster site, very few plants were seen on the site; Phyllantus muellarianus and Eleusine indica were the only plants seen at the site during the wet season. The presence of other plant species in Alaba site was sporadic. The concentration of heavy metals in plants and soils is as given in Tables 5, 6, and 7. Soil contamination with Pb, Ni, and Cu was very obvious in the topsoil of Alaba dumpsite. Pb, Ni, and Cu levels in soils from Alaba and Westminster sites ranged from 224.4 to 1890 mg/kg, 6.2 to 125.9 mg/kg, and 31 to 180.9 mg/kg for total metals present, while for bioavailable metals, Pb, Ni, and Cu ranged from 80.4 to 975.4 mg/kg, ND to 17.6 mg/kg, and 9.4 to 142.2 mg/kg, respectively. The concentrations of Cd and Cr observed in soils ranged from 1.4 to 3.6 mg/kg (total) and 3.1 to 7.2 mg/kg (total), respectively, while the concentrations of bioavailable metals in Cd and Cr ranged from 1.1 to 2.7 mg/kg and 0.1 to 2.0 mg/kg.
Among all the sampling points, ALAT had the highest concentrations of Pb and Cu as shown in Table 5. At the Alaba dumpsite, these e-wastes were burnt which led to the increase in the surface area of these components, thereby impacting the air quality and soil quality of the environment. Pb, Cd, Ni, Cu, and Cr concentrations in ALAW were obtained as 254.3 mg/kg, 3.2 mg/kg, 10.8 mg/kg, 102 mg/kg, and 4.2 mg/kg, respectively, while the heavy metal concentrations obtained for ALAS were 224.4 mg/kg, 1.7 mg/kg, 9.3 mg/kg, 102 mg/kg, and 3.9 mg/kg, respectively, though sub-soil was not expected to have high levels of heavy metals. This result showed that the leaching of these metals from the topsoil to the sub-soil did occur which might have been due to rainfall during the wet season. The concentrations of Pb, Cd, Ni, Cu, and Cr obtained from ALAW were higher than that of ALAS but lower than that of ALAT; this could be due to the landscape of the area which is sloppy; therefore, rainfall could have played a major role in the washing off of the ALAT soil towards ALAW.
The results obtained for the mean concentrations of the heavy metals in soils and plants (Tables 6 and 7) at the e-waste dumpsites showed that the root to shoot transfer of Pb, Cd, Cr, and Cu in A. spinosus and Eleusine indica is greater than the other plants. This showed that A. spinosus and E. indica readily allow the translocation of the heavy metals within them compared to the other plants. This also mean that A. spinosus and E. indica maintained high concentrations of these metals in their shoot over a wide range of concentrations; therefore, their heavy metal uptake is very high.
Extraction coefficient and shoot/root quotient
Excluders are plants that limit the levels of heavy metal translocation within them and maintain relatively low concentrations in their shoot over a wide range of soil concentrations. They are mostly employed in re-vegetation and stabilization of heavy metal-contaminated soils (Baker, 1981). Plants that accumulate heavy metals are commonly reported with shoot/root quotients greater than 1, while shoot/root quotients less than 1 characterize heavy metal excluders. Three of the plants analyzed had extraction coefficient greater than 1; Aspilia thouarsii had an extraction coefficient of 2.07 for Cd; Amaranthus spinosus had an extraction coefficient of 1.33 for Cd; and Eleusine indica had an extraction coefficient of 1.19, 12.58, and 1.16 and 8.08 for Cd, Ni, Cu, and Cr, respectively. Most of the plants have their shoot/root quotient greater than 1, especially Eleusine indica which had its shoot/root quotient greater than 1 for all the metals analyzed. The concentrations of heavy metal concentrations in the plants varied with plant species and levels of heavy metals in the soils. Amaranthus spinosus and Eleusine indica can be referred to as accumulators of heavy metals based on the result in Table 8 because the shoot/root quotient of all the heavy metals analyzed in Amaranthus spinosus and Eleusine indica exceeded 1.0. All the plants had their shoot/root quotient for Cd being greater than 1.0; therefore, they are all accumulators of Cd, while other plant species with shoot/root quotient less than 1 can be regarded as excluders.
Comparison of soils and plants between the two locations
The levels of heavy metals found in soils and plants on Alaba site and that of Westminster site were compared; it was observed that the levels of heavy metals (Pb, Ni, Cu, Cd, and Cr) in soils from Alaba site were extremely greater than that of soils from Westminster site, especially when values of ALAT and WSTT were compared and were probably because the electronic wastes in Alaba site were burnt which does not occur in Westminster site as the surface area of the components of electronic waste would have increased after being burnt. Also, the lower levels of heavy metals in Westminster may be due to the short time that these e-wastes used on the site, as investigation from the market sellers in the warehouse near the site revealed that the e-wastes on the site were usually removed by LAWMA (Lagos State Waste Management Agency) every two Saturdays. Therefore, the e-waste removal after a short period of being dumped can be said to be the reason for the low level of heavy metals in the soil samples obtained from Westminster site since these wastes stay on the site for a shorter period and burning of these e-wastes does not occur at this dumpsite compared to Alaba dumpsite.
Comparison of soil samples, reference soil sample (SO-2), and quality standards
According to the results of the different soil samples recorded in Table 5 for wet season analysis, ALAT had the highest Pb content of 1890 mg/kg which when compared with the EU and Canadian standards; it showed a high level of contamination because it did exceed the quality standards, except for the level of Cr which was within the certified standard’s value; all other heavy metals such as Cd, Ni, and Cu levels in ALAT were very high and exceeded the EU (EEA, 2000) and Canadian (CCME, 1991) standards for both agricultural and residential soils (Table 9).
The level of pollution of the soil samples was in the order: ALAT > ALAW > ALAS > WSTT > WSTA. ALAW showed a higher level of metal concentration than ALAS despite the fact that ALAW soil sample was taken few meters away from the major dumpsite and this may be due to movement of people across the path, wind, and washing of the metals via rainfall from the dumpsite to the soil area few meters away from the site. The pollution trend for the soil samples was expected since burning of e-waste occurred at Alaba site compared to Westminster site. The concentrations of heavy metals such as Ni, Cu, and Cr in WSTT were lower than that of the quality standards except for Pb and Cd. Concentrations of heavy metals in WSTA all satisfied the quality standards except for Pb that was 3 times greater than the quality standard for soils. All the soil samples from the two sites except for Cr have the concentrations of their heavy metals higher than that of the reference soil sample (SO-2). The level of pollution of the soil samples was in the order of ALAT > ALAW > WSTT > ALAS > WSTA. When the level of the individual heavy metals in the soil samples from both sites was compared with the quality standards, it became clear that for Pb, only ALAT and ALAW exceeded the 70 mg/kg recommended by the EU and Canada standard for agricultural soil, while ALAS, WSTT, and WSTA had Pb concentrations that were lower than the concentration recommended. ALAT and WSTT exceeded 1.0 mg/kg and 1.4 mg/kg of Cd (EU and CanadaAGR) which was recommended according to the quality standards. ALAW only exceeded the 1.0 mg/kg (EU) by 0.1 but satisfied the CanadaAGR standard of 1.4 mg/kg for Cd. The concentration of Cu in the samples was observed in the order ALAT > WSTT > ALAS. The three (3) soil samples have their concentrations of Cu being higher than that of the quality standard with ALAT having the highest concentration. In the case of Ni, only ALAT and ALAW exceeded the required limit, but for Cr, all the soil samples satisfied the required limit.
Based on these results, ALAT is a highly contaminated soil; therefore, the high concentrations of these metals present in Alaba site present a potentially hazardous and highly inimical to food chain and biological life in the environment. This makes remediation of the site a matter of urgency for safe biological life and for a clean environment.
Dry season
The pH values obtained for the soil samples (Table 10) during the dry season did not differ from that obtained for wet season except in the case of ALAW and ALAS where a slight change was observed. Also, a little increase in the TOM for dry season soil samples was observed especially in the case of ALAT, ALAW, and ALAS compared to the values obtained for wet season, and this could be attributed to the bush burning practiced during the dry season.
Concentration of heavy metals in plant species and soils
Among all the plants analyzed from Alaba site (Table 11), Cyperus echinatus accumulated the highest concentration of Pb, Ni, and Cu in its root and shoot with concentrations level of 83.2 mg/kg and 10.5 mg/kg; 68.5 mg/kg and 14.9 mg/kg; and 1722.5 mg/kg and 643.9 mg/kg, respectively, followed by Amaranthus spinosus with high levels of Pb, Ni, and Cu in its root and shoot as 13.2 mg/kg and 4.9 mg/kg; 15.6 mg/kg and 15.0 mg/kg; and 527.6 mg/kg and 288.8 mg/kg. Eleusine indica accumulated a higher level of metals than Amaranthus viridis though they were both sampled from Westminster site (Table 12). Amaranthus spinosus accumulated higher concentrations of Pb, Ni, and Cu than Amaranthus viridis. The level of heavy metals accumulated by the plant is dependent on the plant species as well as the concentration of these metals in the soils (Yadav, 2009). Table 13 shows the mean concentrations of heavy metals in the soil samples investigated during the dry season. All the soil samples were contaminated with heavy metals with ALAT being the most contaminated and WSTA as the least contaminated.
Seasonal variations in the concentration of heavy metals in soils and plants obtained from the two locations
During the dry season, dumping and burning of wastes are a common practice at the dump sites. These and more intense evaporation in the dry season make soil solution more concentrated. Generally, higher mean heavy metal concentrations are usually recorded in soil during the dry season than in the wet season (Yahaya et al., 2009).
The mean concentration of heavy metals in all the three plants in Table 14 for dry season was greater than those obtained for wet season except in the case of Pb and Cd. The standard deviation values for Pb, Cd, Ni, Cu, and Cr in Cyperus echinatus were calculated at 95% confidence level and obtained as 1.63, 0.05, 0.09, 0.09, and 0.05, respectively. In the case of A. spinosus, the standard deviation values for Pb, Cd, Ni, Cu, and Cr at 95% confidence level were obtained as 0.46, 0.27, 0.37, 0.09, and 0.19, respectively. The standard deviation values of Pb, Cd, Ni, Cu, and Cr in E. indica at 95% confidence level are 0.79, 0.19, 0.37, 0.37, and 4.62, respectively. The high values of Pb and Cd obtained for wet season could be due to the wet deposition of Pb and Cd from the air to the soil via rainfall. All the heavy metals analyzed in the various plants exceeded the toxic concentration expected for plants according to Tisdale et al. (1985).
Table 15 shows that the concentrations of heavy metals of soils obtained during the wet season were lower than the concentrations of heavy metals in the dry season except in the case of Pb. This is probably because of lower e-waste with Pb content being dumped and burnt during the dry season. ArcGIS 10.2 version is employed to show the distribution of the heavy metals in the study area as presented in Figs. 3, 4, 5, 6 and 7. The distribution of these heavy metals gave better understanding on the seasonal variation effect on the concentration of these heavy metals.
Statistical analysis and heavy metal assessment
Pearson correlation was applied to the results obtained for wet season for all the heavy metals analyzed, and a positive association was obtained for each of the metals (Table 16). The correlation coefficient of ≈ ± 1 shows that the variables are perfectly correlated. When the correlation coefficient magnitude is > 0, it indicates a positive association, and when the magnitude is < 0, it denotes a negative association. The correlation coefficient values of 0.1 to 0.3 indicate a small correlation and 0.3 to 0.5 denote a medium correlation, while 0.5 to 10 indicate large correlation (Laerd Statistics, 2020). According to the correlation coefficients obtained and presented in Table 16, there is no low or medium correlation between the heavy metals rather a large correlation was observed. The correlation results obtained showed that the source of these heavy metals is mainly electronic waste. The soil quality at the dumpsites was evaluated by assessing the level of heavy metal contamination at the sites using the multivariate techniques.
Principal component analysis
The principal component analysis of the studied sites shows a total variability of 91.43% with the PC1 having a variability of 49.09% (eigenvalue 2.46) and 42.35% (eigenvalue 2.46) for PC2 as shown in Fig. 10. The bi-plot factors analysis shown in Fig. 8 indicates that ALAT and WSTT have negative correlations at PC1 and positive correlations at PC2. ALAW showed positive correlation at PC1 and PC2, and WSTA showed negative correlation at PC1 and PC2, while ALAS showed a positive correlation at PC1 but a negative correlation at PC2.
Cluster analysis
The spatial similarity matrix among sampling points based on the heavy metals was determined using XLSTAT software. The single linkage agglomeration method was carried out to obtain and analyze the clusters. The dendrogram (Fig. 9) obtained from the analysis summarizes and classifies the clustering. ALAS showed a similarity of about 94% with WSTA, ALAT, and ALAS as clearly shown in the dendrogram. WSTA formed clusters with ALAT and ALAW, thereby showing a similarity of approximately 97%.
Contamination factors
The contamination factors and the degree of contamination for four heavy metals (Pb, Ni, Cu, and Cr) were calculated for all the sampling points at the two dumpsites (Table 17); all the sites were highly contaminated with Pb and Cu (CF ≥ 6). The contamination factors obtained for Ni at all the sampling points indicated low to high contamination; ALAT showed a very high level of Ni contamination, while WSTA showed a low level of Ni contamination. All the sampling points showed low Cr contamination except for ALAS that indicated a moderate contamination. The degree of heavy metal contamination for all the sampling points was evaluated and considered to be very high (32 ≤ Cdeg) at ALAT and ALAS, while other sampling points indicated considerable contamination (16 ≤ Cdeg < 32). The degrees of contamination of heavy metals within the study area are presented in Figs. 10 and 11.
Pollution load index
The pollution load indices (PLI) were calculated for all the sampling points using the following elements: Pb, Ni, Cu, and Cr. All the sampling points indicated that the dumpsites are highly polluted with heavy metals (PLI > 1); ALAT had the highest PLI value of 12.0 as shown in Table 17. Therefore, these e-waste dumpsites need urgent clean up.
Geoaccumulation index
The Igeo values (Table 18) for all the sampling points were analyzed according to Muller’s classifications, and all the sampling points showed that all the sampling sites investigated were heavily contaminated with Pb except for ALAT which was extremely contaminated (5 < Igeo). The Igeo values for Ni varied from − 0.7 to 3.6 which indicates that the soil quality at the sampling sites ranged from uncontaminated to heavily contaminated. The Igeo value for the sampling point, ALAT, showed that the site is heavily to extremely contaminated with Cu, while other sampling points showed that they are moderately to heavily contaminated. Therefore, all the sites are contaminated with Cu. The Igeo values for all the sampling sites showed that the soil is practically uncontaminated with Cr.
Comparison of present study with other studies
The heavy metal concentrations obtained in this present study are compared with other relevant studies as presented in Table 19. Adesokan et al. (2016) reported high heavy metal concentrations in the soils of Ogunpa e-waste dumpsites in Ibadan, Nigeria. Tokumaru (2015) also reported extremely high heavy metal contamination in soil within Agbogbloshie e-waste dismantling site in Accra, Ghana. The extremely high values in the concentration of heavy metals from the Agbogbloshie site were reported to be due to dismantling and burning of e-waste dumpsites. In addition, Kumar and Fulekar (2019) have reported high concentrations of heavy metals in soil from three e-waste dumpsites at Ahmedabad in north central Gujarat, India.
Conclusions
E-waste pollution is a global problem since electronic appliances are used by a larger percentage of the global population. The results obtained from the investigation of the e-waste dumpsites in Lagos metropolis confirmed that e-waste pollution is a significant problem in today’s society. The analytical results obtained for this research showed that most of the soils and plants studied were highly contaminated with Pb, Ni, and Cu for both wet and dry seasons suggesting that the site poses potential hazards to grazing animals and the food chain especially soils obtained from Alaba site. The results obtained from the CFs, PLIs, and Igeo showed that these sites are highly contaminated with heavy metals especially Pb. The result obtained from the comparison of the present study with other studies showed that the site is highly contaminated with heavy metals and the range of heavy metal concentrations obtained are close to those obtained at e-waste dismantling sites reported by Tokumaru (2015) and other e-waste dumpsites reported by Adesokan et al. (2016). Most of the plants analyzed accumulated high concentration of heavy metals most especially Amaranthus spinosus. Among all the plants analyzed, Eleusine indica (a weed and herb) was observed to be an accumulator of all the heavy metals. Therefore, it can be used to decontaminate the studied sites and other polluted soils in Nigeria, thereby reducing potential dangers of heavy metal toxicity to ecology. These findings suggest that there is a need for urgent attention to proffer far-reaching solutions to the problems of the contaminated electronic waste dumpsites.
Recommendation
It is highly recommended that recycling of electronic waste should be encouraged and employed immediately in Nigeria, thereby putting an end to the burning of electronic wastes. The members of public should be enlightened on the dangers involved in the harvesting of edible vegetables and herbs from dumpsites irrespective of the type of dumpsite, most especially from e-waste dumpsites as these vegetables and herbs could be highly contaminated.
Data availability
Additional data will be provided on request by the corresponding author.
References
Adesokan, M. D., Adie, G. U., & Osibanjo, O. (2016). Soil pollution by toxic metals near E-waste recycling operations in Ibadan. Nigeria. Journal of Health Pollution, 6(11), 26–33.
Babu, B. R., Parande, A. K., & Basha, C. A. (2007). Electrical and electronic waste: A global environmental problem. Waste Management & Research, 25, 307–318.
Baker, A. J. (1981). Accumulators and excluders-strategies in the response of plants to heavy metals. Journal of Plant Nutrition, 3, 643–654.
CCME (CANADIAN COUNCIL OF MINISTERS OF THE ENVIRONMENT). (1991). Canadian environmental quality criteria for contaminated sites (Canadian Soil Quality Guidelines for the protection of Environmental and Human Health), 1–6.
Chibuike, G. U., & Obiora S. C. (2014). Heavy metal polluted soils: Effect on plants and bioremediation methods. Applied and Environmental Science, 2014, 1–12. Article ID 752708.
Cui, J., & Zhang, L. (2008). Metallurgical recovery of metals from electronic waste: A review. Journal of Hazardous Materials, 158, 228–256.
Devkota, B., & Schmidt, G. (2000). Accumulation of heavy metals in food plants and grasshoppers from the Taigetos Mountains, Greece. Agriculture, Ecosystems & Environment, 78, 85–91.
EEA (EUROPEAN ENVIRONMENT AGENCY). (2000). European soil quality standards.
ESG International Inc. (2002). Environmental quality standards for soil and land ESG 2002. Quantification of the exposure concentrations and toxicity of BTEX compounds in soil. Report prepared for the Soil Quality Guidelines Task Group, Canadian Council of Ministers of the Environment. June 2002.
Food and Agriculture Organization of the United Nations Council Directive EEC. (1991). Council Directive 91/676/EEC concerning the protection of waters against pollution caused by nitrates from agricultural sources.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A Sedimentological Approach. Water Res, 14(8), 975–1001.
Hilty, L. M. (2005). Electronic waste—An emerging risk? Environmental Impact Assessment Review, 5, 431–435.
Huang, J., Nkrumah, P. N., Anim, D. O., & Mensah, E. (2014). E-waste disposal effects on the aquatic environment: Accra. Springer.
Kang, H. Y., & Schoenung, J. M. (2005). Electronic waste recycling: A review of US infrastructure and technology options. Resources, Conservation and Recycling, 45, 368–400.
Kiddee, P., Naidu, R., & Wong, M. H. (2013). Electronic waste management approaches: An overview. Waste Management, 33, 1237–1250.
Kumar, P., & Fulekar, M. H. (2019). Multivariate and statistical approaches for the evaluation of heavy metals pollution at e-waste dumping sites. SN Applied Sciences, 1, 1506.
Kwiatkowska-Malina, J. (2018). Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Applied Soil Ecology, 123, 542–545.
LAERD STATISTICS. (2020). Pearson's product moment correlation. Statistical tutorials and software guides. Retrieved July 3, 2021 from https://statistics.laerd.com/statistical-guides/pearson-correlation-coefficient-statistical.gov.php
Lo, K. S. L., Yang W. F., Lin Y. C. (2008). Effects of organic matter on the specific adsorption of heavy metals by soil. Toxicological & Environmental Chemistry, 34, 2–4.
Manhart, A., Osibanjo, O., Aderinto, A., & Prakash, S. (2011). Informal e-waste management in Lagos, Nigeria–Socio-economic impacts and feasibility of inter-national recycling co-operations. Final Report of Component, 3, 1–129.
Muller, G. (1981). The heavy metal pollution of the sediments of neckars and its tributary: A stocktaking. Chemiker Zeitung, 105, 157–164.
Nnorom, I. C., Osibanjo, O., & Nnorom, S. O. (2007). Achieving resource conservation in electronic waste management: A review of options available to developing countries. Journal of Applied Sciences, 7, 2918–2933.
Ogunseitan, O. A., Schoenung, J. M., Saphores, J.-D.M., & Shapiro, A. A. (2009). The electronics revolution: From e-wonderland to e-wasteland. Science, 326, 670–671.
Olafisoye, O. B., Adefioye, T., & Osibote, O. A. (2013). Heavy metals contamination of water, soil, and plants around an electronic waste dumpsite. Polish journal of environmental studies, 22.
Onwughara, N., Nnorom, I., Kanno, O., & Chukwuma, R. (2010). Disposal methods and heavy metals released from certain electrical and electronic equipment wastes in Nigeria: Adoption of environmental sound recycling system. International Journal of Environmental Science and Development, 1, 290.
Rhodes, E., Kamara, P., & Sutton, P. (1981). Walkley-Black digestion efficiency and relationship to loss on ignition for selected Sierra Leone soils. Soil Science Society of America Journal, 45, 1132–1135.
Sleutel, S., de Neve, S., Singier, B., & Hofman, G. (2007). Quantification of organic carbon in soils: A comparison of methodologies and assessment of the carbon content of organic matter. Communications in Soil Science and Plant Analysis, 38, 2647–2657.
Song, Q., & Li, J. (2014). Environmental effects of heavy metals derived from the e-waste recycling activities in China: A systematic review. Waste Management, 34, 2587–2594.
Sullivan, J. (2014). Trash or treasure: Global trade and the accumulation of e-waste in Lagos, Nigeria. Africa Today, 61, 89–112.
Sun, B., Zhao, F., Lombi, E., & McGrath, S. (2001). Leaching of heavy metals from contaminated soils using EDTA. Environmental Pollution, 113, 111–120.
Sutherland, R. A., (2002). Comparison between non-residual Al Co, Cu, Fe, Mn, Ni, Pb and Zn released by a three-step sequential extraction procedure and a dilute hydrochloric acid leach for soil and road deposited sediment. Applied Geochemistry, 17, 353–365.
Tisdale, S. L., Nelson, W. L., & Beaton, J. D. (1985). Soil fertility and fertilizers, Collier Macmillan Publishers.
Tokumaru, T. (2015). Determination of the extent of pollution in soils, water bodies and human hair at the e-waste recycling sites in Ghana. Activity report of field research. Global leadership training programme in Africa, 1–13.
Yadav, S. K. (2009). Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. SA. J Botany, 76(2), 167–179.
Yahaya, M., Mohammad, S., & Abdullahi, B. (2009). Seasonal variations of heavy metals concentration in abattoir dumping site soil in Nigeria. Journal of Applied Sciences and Environmental Management, 13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olusegun, O.A., Osuntogun, B. & Eluwole, T.A. Assessment of heavy metals concentration in soils and plants from electronic waste dumpsites in Lagos metropolis. Environ Monit Assess 193, 582 (2021). https://doi.org/10.1007/s10661-021-09307-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09307-4