Abstract
Toxicity imposed by organophosphate pesticides to the freshwater cultivable fish species mrigal (Cirrhinus mrigala) was assessed under laboratory conditions. Healthy juveniles were exposed to chlorpyrifos, dichlorvos, and their equitoxic mixture in geometric series. Median lethal concentrations of chlorpyrifos were found to be 0.906 (0.689–1.179), 0.527 (0.433–0.633), 0.435 (0.366–0.517) and 0.380 (0.319–0.450) mg/L and dichlorvos were found to be 38.432 (33.625–47.866), 22.477 (19.047–26.646), 12.442 (9.619–14.196) and 11.367 (9.496–13.536) mg/L after 24 h, 48 h, 72 h and 96 h of exposure respectively. Surprisingly, the joint toxicity of these organophosphates in the binary mixture was less than additive during most of the exposure periods. Behavioral changes exhibited by individual as well as mixture pesticide treatments were loss of schooling behavior, aggregating at corners of the test chamber, elevated opercular beatings, surplus mucus secretion, slight color changes and sudden and rapid body movements before death. Loss of fish equilibrium was noticed only in chlorpyrifos treated fish, whereas sluggish behavior was noticed only in mixture pesticide treatment. Such behavioral studies can be applied as a non-invasive bio-monitoring tool for water quality assessment for fish growth and development. Despite the same mode of action of both pesticides, the antagonistic action in the binary mixture is an interesting outcome of this research that requires further investigation for a lucid understanding of the joint toxicity mechanism of such pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are toxic compounds and their capacity to harm fish and aquatic animals depends on exposure time, concentration, and persistence in the environment (Sabra and Mehana 2015). Pesticides can be classified as minimal, slight, moderate, high, extreme and super-toxic compounds based on their LC50 values (Sabra and Mehana 2015). There are a number of studies that estimated median lethal concentration (LC50) of chlorpyrifos (Bhatnagar et al. 2017), dichlorvos (Velmurugan et al. 2009, Srivastava et al. 2012), carbaryl, carbofuran, profenfos and triazophos (Mahboob et al. 2015), endosulfan (Ilyas and Javed 2013) and malathion (Rauf 2015) in mrigal (Cirrhinus mrigala).
Pesticides are one of the most potentially harmful chemicals and even small amounts of pesticides can be fatal (Jokanović, 2009), therefore increasing pesticide application is a serious threat to human health and biodiversity. Chlorpyrifos is a broad-spectrum organophosphate pesticide. It is extensively used for pest control throughout the world for agriculture and domestic purposes (Ali et al. 2009, Sun et al. 2015). Dichlorvos is another commonly used organophosphate pesticide (Ural and Çalta 2005, Sun et al. 2015). It is one of the main chemical agents used in bath treatment against fish ectoparasites (Varó et al. 2007). A small fraction of the applied pesticide reaches the target pests and the majority of it is released into the environment (Tišler et al. 2009). During the rainy season, the pesticides from agricultural fields are flushed away and drained into the aquatic system (Adhikari et al. 2004, Ramesh and Saravanan 2008) that alter physico-chemical parameters of water and ultimately affect the performance of aquatic organisms inhabiting there (Muthukumaravel et al. 2013). Since the final destination of all applied pesticides is the water bodies, organisms thriving there are always threatened by the mixture of various pesticides which can be more hazardous than single pesticide exposure. Laetz et al. (2009) also reported that pesticide mixtures can be much more toxic compared to single pesticide exposure.
Pesticides become fatal to fish at higher concentrations but even at lower concentrations they are able to generate biochemical modifications without any fatality (Kunwar et al. 2022). Such sub-lethal effects are generally ignored and receive less attention since no direct mortality is observed, but these effects ultimately determine the overall success of any species and their population. Behavioral change is one of the sub-lethal effects which is an important indicator of water pollution and stress (Chebbi and David 2010, Kesharwani et al. 2018). In recent studies, behavioral observations have gained popularity because they are noticed at low chemical concentrations and are non-invasive.
The global fisheries sector is threatened by aquatic pollution and pesticides are one of the serious sources of pollution. In this context, we selected mrigal as a model organism to evaluate the toxicity of pesticides. This is an important aquaculture candidate species in Nepal which is successfully cultivated under single stocking and multiple harvesting techniques. This farming technique, locally called Chhadi farming, is gaining popularity in Nepal (Mishra and Kunwar 2014, Kunwar and Adhikari 2016 and 2017, Adhikari et al. 2018). Chlorpyrifos and dichlorvos were selected for a toxicity assessment because they are commonly used in many countries (Sun et al. 2015) and their traces were detected in nature, fish and fisheries products (Kafle et al. 2015, Singh et al. 2015, Akoto et al. 2016, Zahran et al. 2018, Nag et al. 2020). Chlorpyrifos residue was reported to be 0.0091 ± 0.0020 mg/L in the river Deomoni, West Bengal, (Singh et al. 2015). Similarly, water sampled from the Chilika lake, India contained chlorpyrifos with concentration ranging 0.019–2.73 µg/L (Nag et al. 2020).
Individual effects of chlorpyrifos and dichlorvos on mrigal had already been documented but their joint toxic effect on this species is still lacking. Pesticides in a mixture can interact with each other (additive or competitive) to modulate the overall resultant toxicity effect, therefore water quality assessment based on single pesticide toxicity can be misleading. Wang et al. (2015) had also highlighted that single pesticide risk assessments are more likely to underestimate the impacts of these pesticides to aquatic organisms. Therefore, the present study was designed with the aim to elucidate lethal toxicity as well as behavioral manifestation of mrigal in response to chlorpyrifos and dichlorvos not only individually but also in combination. The results obtained from this study are expected to enrich the existing knowledge on joint pesticide toxicity.
Materials and methods
Fish acclimatization
Mrigal hatchlings (one week after hatching) were purchased from Fish Pure-line Breed Conservation and Promotion Centre, Bhairahawa, Rupandehi, Nepal and transported in oxygen-packed polythene bags to Central Fisheries Promotion and Conservation Centre (CFPCC) Balaju, Kathmandu, Nepal. Hatchlings were grown in an earthen pond for two months until they reached finger size. Healthy fingerlings with uniform size (exact weight provided below) were transferred to a 350-L indoor glass aquarium of CFPCC for acclimatization. The aquarium was fitted with a water filter and aeration system. Fish were regularly fed ad libitum with commercial pellet feed having 32% protein (Sreema feed Pvt. Ltd., India). Uneaten food and fecal matter were removed with the help of a scoop net and siphon. Everyday approximately half of the aquarium water was exchanged with freshwater to maintain optimum water quality. Water pumps, air stones, pipes and filters were cleaned twice a week. Water temperature, pH, dissolved oxygen and total ammonia ranged between 23.97–24.62 °C, 7.68–7.85, 5.80–6.74 mg/L and 0.20–0.23 mg/L, respectively. Fish were acclimatized for 15 days before using them for the lethal toxicity experiment.
Pesticides selection
Two pesticides-chlorpyrifos and dichlorvos were selected for the present toxicity experiment. These are commonly applied organophosphate insecticides in crops for pest control. Dichlorvos (G-VAN-80%) Greenriver Industry Co., Ltd., ShenZhen, China and chlorpyrifos (Dursban-20%) Dow AgroSciences Pvt. Ltd., India were the commercial- grade pesticides used in this study.
Lethal toxicity test
Lethal toxicity tests were conducted according to the standard guidelines (OECD 1992) in semi-static conditions. Well cleaned glass aquaria (35-L capacity) with 25-L water volume were used for the test. The average weight of the fish used in this experiment was 5.52 ± 0.91 g (mean ± SD). Feeding was ceased 24 hours prior to the exposure experiments to avoid any interference of waste with pesticides. Stock solutions were freshly prepared in distilled water and calculated amounts of the solution were added in the aquaria to obtain five different pesticide concentrations in geometric series (chlorpyrifos: 0.25, 0.5, 1.0, 2.0 and 4.0 mg/L; dichlorvos: 4.0, 8.0, 16.0, 32.0 and 64.0 mg/L). The exposure range was determined by performing a range finding test for the pesticides chlorpyrifos and dichlorvos. The actual concentration of the pesticide in the water was not measured but the water was renewed on a daily basis to maintain the desired pesticide concentration at the same level (Nwani et al. 2013). There were four replicates for each concentration with 5 five fish in each group. In total 200 fish were used for the lethal toxicity tests. Exposed fish were regularly monitored and dead fish were immediately removed from the aquaria; no fish died in the control. Mortality was recorded after 24 h, 48 h, 72 h and 96 h of exposure. The mortality data obtained from chlorpyrifos and dichlorvos treatments were analyzed to estimate lethal concentrations (24 h to 96 h-LC10–90) of both pesticides by using a log probit analysis program.
After estimation of individual pesticide toxicity, their joint toxicity was assessed. For this, five geometric series of pesticides mixtures were prepared by adding both pesticides in equitoxic concentration i.e. 12.5%, 25%, 50%, 100% and 200% 96 h-LC50 of chlorpyrifos and dichlorvos. Fish mortality was recorded as described above. As before, no fish mortality was observed in the control. Similar to individual pesticides, 24 h to 96 h-LC10 to LC90 of the mixture pesticide toxicity was also calculated; where LC10 and LC90 indicate pesticide concentrations required to kill 10% and 90% of the fish population, respectively.
Joint toxicity assessment
The joint toxicity of pesticide was assessed by the additive index (AI). This was calculated according to Marking (1985).
where, AI represents additive index and S represents the sum of biological activity
where, A and B represent two different pesticides, ‘m’ represents LC50 of pesticides in mixture, ‘i’ represents LC50 of individual pesticides.
AI values equal, greater or less than zero indicates additive, synergistic or antagonistic action of the pesticide mixture respectively.
Fish behavior
Fish exposed to chlorpyrifos, dichlorvos and mixture pesticides in geometric series (doses mentioned above) for lethal toxicity assessment were carefully observed during the whole experimental period and their behavior like body movements, operculum movements, color change, swimming pattern, schooling behavior, mucus secretion was recorded. Although the behavior study was started with five concentrations of each pesticide, data from all treatments could not be presented due to fish mortality in higher pesticide concentrations.
Data presentation and analysis
The average weight, standard deviation and mortality percentage of fish were calculated in Microsoft Excel. The diagrams were also prepared in Microsoft Excel. The lethal concentrations (24 h, 48 h, 72 h and 96 h LC10 to LC90) of individual and mixture pesticides were calculated by log probit analysis using statistical program SPSS version 20. The lethal concentration data are presented with their upper and lower limit at a 95% confidence interval. The joint toxicity of the pesticides was analyzed in Microsoft Excel based on formulae described by Marking (1985).
Results
Lethal toxicity of chlorpyrifos
Lethal toxicity testing of chlorpyrifos was conducted in five different concentrations ranging from 0.25 mg/L to 4 mg/L. Chlorpyrifos at a low dose (0.25 mg/L) was slightly toxic to fish where mortality started at a later phase (after 72 h) where only 10% of the fish population died by the end of the experiment (96 h). In contrast, chlorpyrifos concentrations 1 mg/L, 2 mg/L and 4 mg/L caused 100% fish mortality after 72 h, 48 h and 24 h of exposure periods, respectively (Fig. 1).
The median lethal concentrations (LC50) of chlorpyrifos with their 95% confidence limit were found to be 0.906 (0.689-1.179), 0.527 (0.433–0.633), 0.435 (0.366–0.517) and 0.380 (0.319–0.450) mg/L at 24 h, 48 h, 72 h and 96 h, respectively (Table 1). The range of LC10 to LC90 at 24 h, 48 h, 72 h and 96 h were 0.312 (0.167–0.445) to 2.630 (1.873–4.737), 0.311 (0.203–0.388) to 0.894 (0.727–1.316), 0.287 (0.195–0.345) to 0.659 (0.547–0.973) and 0.251 (0.172–0.302) to 0.575 (0.479–0.829) mg/L, respectively, showing a decreasing trend of lethal pesticide concentration with increasing time of exposure (Table 1).
Lethal toxicity of Dichlorvos
Lethal toxicity of dichlorvos to mrigal was assessed by exposing fish to five different concentrations ranging from 4 mg/L to 64 mg/L. The lower dichlorvos concentrations (4 mg/L and 8 mg/L) exhibited no fish mortality during 96 h exposure. Other pesticide concentrations viz. 16 mg/L, 32 mg/L and 64 mg/L killed 100% of stocked fish after 96 h, 48 h and 24 h of exposure, respectively (Fig. 2).
The median lethal concentrations (LC50) at 24 h, 48 h, 72 h and 96 h with 95% confidence limits were 38.432 (33.625–47.866), 22.477 (19.047–26.646), 12.442 (9.619–14.196) and 11.367 (9.496–13.536) mg/L, respectively (Table 2). The range of LC10 to LC90 at 24 h, 48 h, 72 h and 96 h were calculated to be 28.710 (20.917–32.898) to 51.447 (43.129–87.287), 17.453 (13.322–20.394) to 28.948 (24.723–38.344), 9.837 (6.003–11.713) to 15.736 (13.759–19.275) and 9.068 (6.821–10.655) to 14.250 (12.146–18.718) mg/L, respectively, showing a decreasing trend of lethal pesticide concentration with increasing time of exposure (Table 2). The acute toxicity results of both pesticides clearly indicate that chlorpyrifos is more toxic than dichlorvos to freshwater fish such as mrigal (Tables 1 and 2).
Lethal toxicity of mixture pesticides
To assess the lethal toxicity of chlorpyrifos and dichlorvos mixture, fish were exposed to 5 different pesticide mixtures prepared in an equitoxic concentration ranging from 12.5% to 200% 96 h-LC50 values of the respective pesticide. 12.5% pesticide mixture caused no fish mortality whereas 25%, 50% and 100% of pesticide mixture killed 20%, 40% and 70% of the fish population respectively during 96 h of exposure. 200% pesticide mixture was highly toxic and killed all fish within 48 h of exposure (Fig. 3).
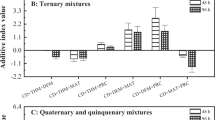
In the pesticides mixture, the LC50 values of chlorpyrifos with 95% confidence limit were 0.761 (0.535–1.665), 0.337 (0.279–0.413), 0.306 (0.252–0.374) and 0.217 (0.170–0.279) mg/L and dichlorvos were 22.774 (16.010–49.793), 10.088 (8.332–12.358), 9.155 (7.533–11.200) and 6.484 (5.089–8.333) mg/L at 24 h, 48 h, 72 h and 96 h, respectively (Table 3). In joint toxicity assessment, the dominating action of chlorpyrifos and dichlorvos mixture was found to be antagonistic (Table 4).
Fish behavior
In terms of body movement, fish treated with the pesticide mixture became hypo-active compared to chlorpyrifos and dichlorvos exposed fish. Such fish behavior was more intense with increasing concentrations of the pesticide mixture. Fish were unable to balance their body in chlorpyrifos treatments but such loss of equilibrium was not noticed in other pesticide treatments. Slight color changes were observed in fish; caudal and pectoral fins were reddish and the body became pale in higher dose of all treatment groups. Fish schooling behavior was influenced by chlorpyrifos, dichlorvos and the pesticide mixtures and changes became more distinct with increasing pesticide concentrations where swimming coordination among fish was lost and fish were scattered everywhere in the test chamber occupying a greater space than control fish. Frequently, fish were also aggregated at the corners of the test chambers in all pesticide treatments. In each treatment group, fish were overexcited and suddenly showed vigorous movements in different directions before death. The dead fish were found to be loaded with mucus around their respiratory organs in higher pesticide concentrations. Opercular movements of fish were also elevated after pesticide exposure in all treatment groups (Table 5).
Discussion
The first step in determining a chemical’s safety threshold in the aquatic environment is to ascertain its lethal toxicity. In our study 96 h-LC50 value of chlorpyrifos to mrigal was found to be 0.380 (0.319-0.450) mg/L. Our recent work on common carp (Cyprinus carpio) and golden mahseer (Tor putitora) reported 96 h median lethal concentration of chlorpyrifos to be 0.44 and 0.753 mg/L respectively (Kunwar et al. 2021a, b). Similarly, 96 h-LC50 values of chlorpyrifos were reported to be 0.44 mg/L in mrigal (Bhatnagar et al. 2017) and 0.58 mg/L in common carp (Xing et al. 2015). In this experiment, we found 96 h-LC50 of dichlorvos was 11.367 (9.496-13.536) mg/L. The present finding corroborates to other experiments that documented 96 h median lethal concentration of dichlorvos to be 9.1 mg/L in mrigal (Velmurugan et al. 2009), 9.41 mg/L (Ural and Çalta 2005) or 15.705 mg/L in common carp (Kunwar et al. 2021a) and 12.964 mg/L in golden mahseer (Kunwar et al. 2021b). Our results distinctly reveal chlorpyrifos is relatively more toxic than dichlorvos for mrigal.
In general, toxicity evaluations are based on single pesticide assessments but these compounds are oftentimes found as complex mixtures in nature; therefore such assessment studies on individual pesticides cannot represent the actual threats posed to aquatic organisms. The combined pesticide toxicity can be additive (an effect produced by mixture pesticides is exactly equal to the sum of individual pesticide’s effects), synergistic (an effect caused by mixture pesticides is higher than the sum of its individual pesticide’s effect) or antagonistic (an effect caused by mixture pesticides is less than the sum of its individual pesticide’s effect). In our study, the joint action of chlorpyrifos and dichlorvos to mrigal was observed to be antagonistic. An antagonistic effect of these two pesticides was also recorded in our recent experiment with golden mahseer (Kunwar et al. 2021b). Wang et al. (2017) documented antagonistic effect of chlorpyrifos and other pesticides mixture on zebrafish (Danio rerio). Antagonistic effects of chlorpyrifos and carbosulfan (Chen et al. 2014) and fenobucarb with triazophos or malathion (Wang et al. 2015) were also observed in common carp.
Chlorpyrifos and dichlorvos belong to the organophosphate group that have the same mode of action (MOA). Pesticides having the same MOA are not necessarily synergistic (LeBlanc et al. 2012, Wang et al. 2017). To the best of our knowledge, this is the first study to show that chlorpyrifos and dichlorvos have antagonistic effects in the freshwater fish mrigal. There are various explanations put forward by investigators for the antagonistic effect. Hernández et al. (2013) described that the pesticide mixture changes the toxicokinetics of the individual compounds, thus modifying their toxicity. Therefore, chemical interaction between two pesticides might be the reason for the antagonistic result (Imam et al. 2018). Likewise, authors (Stepić et al. 2013, Wang et al. 2017) stated enhanced metabolization processes leading to faster excretion of metabolites, eventually resulting in decreased pesticide toxicity. A potential basis for such an antagonistic outcome could be the elevation of carboxylesterases (CaEs) activity. CaEs can detoxify pesticides by hydrolysis (Jokanović 2001, Wheelock et al. 2005). Moreover, this enzyme is also believed to protect AChE from pesticide toxicity by direct binding and sequestration (Jokanović 2001, Maxwell 1992). Therefore, the observed outcome in this experiment might be due to the protective role of CaEs induced by the chlorpyrifos and dichlorvos mixture in mrigal. Another possible interpretation for antagonistic interaction could be Glutathione-S-transferase (GST) mediated pesticide detoxification. Available reports suggest that GST promotes cellular detoxification of xenobiotics including pesticides (Booth et al. 1998, Jin-Clark et al. 2002). In response to pesticide co-exposure, GST activity was reported to be significantly higher compared to individual pesticide exposure (Stepić et al. 2013). Based on this, we hypothesized that combined treatment of chlorpyrifos and dichlorvos might have elevated GST activity in our experimental animal which played a role in efficient detoxification of metabolites leading to reduced toxicity.
Fish behavior studies are an important non-invasive tool for toxicity assessment. Pesticide exposed fish exhibited loss of coordination with each other, residing at corners of the test chamber, excess mucus secretion, becoming pale, rapid opercular movements and abrupt swimming before death. Alike behavioral expressions in response to pesticide were exhibited by common carp and golden mahseer (Kunwar et al. 2021a, b) under similar exposure environments. Many other authors (Kavitha and Rao 2008, Halappa and David 2009, Nwani et al. 2013, Ullah et al. 2014, Padmanabha et al. 2015, Saha et al. 2016, Soni and Verma, 2018) have also documented comparable behavioral changes in fish after pesticides exposure. Inhibition of acetylcholinesterase (AChE) leads to accumulation of acetylcholine (ACh) in cholinergic synapses and overstimulation resulting behavioral changes in fish (Halappa and David 2009). The excess mucus secretion by pesticide exposed fish would protect them by avoiding contact with toxicants or by getting rid of it through the shedding of the mucus layer (Patil and David 2008, Halappa and David 2009). Fish are stressed when exposed to pesticides and their oxygen demand becomes high during such circumstances (Schmidt et al. 2005). Therefore rapid opercular movements after pesticide exposure in mrigal would have facilitated in supplying high oxygen to detoxify pesticides and protect them from deleterious effects. Most of the behavioral symptoms were similar in all pesticide treatments but only chlorpyrifos treated fish exhibited equilibrium loss. This behavioral difference might be due to the severe inhibition of chlorpyrifos on AChE leading to high accumulation of ACh in these fish groups compared to other pesticide treated groups.
Conclusion
Compared to dichlorvos, chlorpyrifos is highly toxic to fish. Despite the same mode of action, the majority of the binary mixture effects were antagonistic in the present study. This requires in-depth investigation such as measurement of ACh, AChE, CaE and GST to explore the toxicity mechanism of these pesticides in co-exposure. Behavioral manifestations detected even at low pesticide concentration suggest that such observations should be incorporated in toxicity studies because it is a highly sensitive and non-invasive bio-monitoring tool. Such assessments can be correlated to the health status and growth performance of fish under the available rearing condition. To sum up, the application of toxic pesticides should be regulated and the use of bio-pesticides and integrated pest management programs should be promoted to save precocious aquatic flora and fauna from pesticide threats.
References
Adhikari B, Kunwar PS, Jha SK (2018) Small-scale fisheries in Nepal. In: Giri SS (ed.) Small-scale fisheries in South Asia. SAARC Agriculture Centre, Dhaka, pp 42–66
Adhikari S, Sarkar B, Chatterjee A, Mahapatra CT, Ayyappan S (2004) Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicology and Environmental Safety 58:220–226. https://doi.org/10.1016/j.ecoenv.2003.12.003
Akoto O, Azuure AA, Adotey KD (2016) Pesticide residues in water, sediment and fish from Tono reservoir and their health risk implications. SpringerPlus 5(1849):1–11. https://doi.org/10.1186/s40064-016-3544-z
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B, Lakra WS (2009) Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food and Chemical Toxicology 47(3):650–656. https://doi.org/10.1016/j.fct.2008.12.021
Bhatnagar A, Cheema N, Yadav AS (2017) Alterations in haematological and biochemical profile of freshwater fish, cirrhinus mrigala (Hamilton) exposed to sub-lethal concentrations of chlorpyrifos. Nature Environment and Pollution. Technology 16(4):1189–1194
Booth LH, Heppelthwaite V, Eason CT (1998) Cholinesterase and glutathione S-transferase in the earthworm apporectodea caliginosa as biomarkers of organophosphate exposure. Proceedings of the New Zealand Plant Protection Conference 51:138–142
Chebbi SG, David M (2010) Respiratory responses and behavioural anomalies of the carp Cyprinus carpio under quinalphos intoxication in sublethal doses. Science Asia 36:12–17. https://doi.org/10.2306/scienceasia1513-1874.2010.36.012
Chen C, Wang Y, Zhao X, Wang Q, Qian Y (2014) The combined toxicity assessment of carp (Cyprinus carpio) acetylcholinesterase activity by binary mixtures of chlorpyrifos and four other insecticides. Ecotoxicology 23:221–228. https://doi.org/10.1007/s10646-013-1165-7
Halappa R, David M (2009) Behavioural responses of the freshwater fish, Cyprinus carpio (Linnaeus) following sublethal exposure to chlorpyrifos. Turkish Journal of Fisheries and Aquatic Sciences 9:233–238. https://doi.org/10.4194/trjfas.2009.0218
Hernández AF, Parrón T, Tsatsakis AM, Requena M, Alarcón R, López-Guarnido O (2013) Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 307:136–145. https://doi.org/10.1016/j.tox.2012.06.009
Ilyas R, Javed M (2013) Acute toxicity of endosulfan to the fish species Catla catla, Cirrhina mrigala and Labeo rohita. International Journal of Agriculture and Biology 15:149–152
Imam A, Sulaiman NA, Oyewole AL, Chengetanai S, Williams V, Ajibola MI, Folarin RO, Muhammad AS, Shittu ST, Ajao MS (2018) Chlorpyrifos- and dichlorvos- induced oxidative and neurogenic damage elicits neuro-cognitive deficits and increases anxiety-like behavior in wild-type rats. Toxics 6(71):1–17. https://doi.org/10.3390/toxics6040071
Jin-Clark Y, Lydy MJ, Zhu KY (2002) Effects of atrazine and cyanazine on chlorpyrifos toxicity in Chironomus tentans (Diptera: Chironomidae). Environmental Toxicology and Chemistry 21(3):598–603
Jokanović M (2001) Biotransformation of organophosphorus compounds. Toxicology 166:139–160
Jokanović M (2009) Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicology Letters 190(2):107–115. https://doi.org/10.1016/j.toxlet.2009.07.025
Kafle BK, Pokhrel B, Shrestha S, Raut R, Dahal BM (2015) Determination of pesticide residues in water and soil samples from Ansikhola watershed, Kavre, Nepal. International Journal of Geology, Earth and Environmental Sciences 5(2):119–127
Kavitha P, Rao JV (2008) Toxic effects of chlorpyrifos on antioxidant enzymes and target enzyme acetylcholinesterase interaction in mosquito fish, Gambusia affinis. Environmental Toxicology and Pharmacology 26(2):192–198. https://doi.org/10.1016/j.etap.2008.03.010
Kesharwani S, Dube KK, Khan R (2018) Effect of dichlorvos (Nvan) on behaviour, haematology and histology of freshwater teleost Labeo rohita. International Journal of Science and Reserach Methodology 8(3):132–146
Kunwar PS, Adhikari B (2016 and 2017). Status and development trend of aquaculture and fisheries in Nepal. Nepalese Journal of Aquaculture and Fisheries, 3 and 4, 1–11
Kunwar PS, Basaula R, Sinha AK, De Boeck G, Sapkota K (2021a) Joint toxicity assessment reveals synergistic effect of chlorpyrifos and dichlorvos to common carp (Cyprinus carpio). Comparative Biochemistry and Physiology Part C: Toxicology and Pharmacology 246:108975. https://doi.org/10.1016/j.cbpc.2021.108975
Kunwar PS, Parajuli K, Badu S, Sapkota B, Sinha AK, De Boeck G, Sapkota K (2021b) Mixed toxicity of chlorpyrifos and dichlorvos show antagonistic effects in the endangered fish species golden mahseer (Tor putitora). Comparative Biochemistry and Physiology Part - C: Toxicology and Pharmacology 240:108923. https://doi.org/10.1016/j.cbpc.2020.108923
Kunwar PS, Sinha AK, De Boeck G, Sapkota K (2022) Modulations of blood biochemical parameters of golden mahseer, Tor putitora following exposures to single and mixed organophosphate. Comparative Biochemistry and Physiology Part - C: Toxicology and Pharmacology 251:109207. https://doi.org/10.1016/j.cbpc.2021.109207
Laetz CA, Baldwin DH, Collier TK, Hebert V, Stark JD, Scholz NL (2009) The synergistic toxicity of pesticide mixtures: Implications for risk assessment and the conservation of endangered Pacific salmon. Environmental Health Perspectives 117(3):348–353. https://doi.org/10.1289/ehp.0800096
LeBlanc HMK, Culp JM, Baird DJ, Alexander AC, Cessna AJ (2012) Single versus combined lethal effects of three agricultural insecticides on larvae of the freshwater insect Chironomus dilutus. Archives of Environmental Contamination and Toxicology 63(3):378–390. https://doi.org/10.1007/s00244-012-9777-0
Mahboob S, Ghazala, Al-Ghanim KA, Sultana S, Alkahem Al-Balawi HF, Sultana T, Al-Misned F, Ahmed Z (2015) A study on acute toxicity of triazophos, profenofos, carbofuran and carbaryl pesticides on Cirrhinus mrigala. Pakistan Journal of Zoology 47(2):461–466
Marking LL (1985) Toxicity of chemical mixtures. In: Rand G and Petroceli S (eds.) Fundamentals of Aquatic Toxicology. Hemisphere Publishing Corporation, Washington DC, pp 164–176
Maxwell DM (1992) Detoxication of organophosphorus compounds by carboxylesterase. In: Chambers JE and Levi PE (eds.) Organophosphates: Chemistry, Fate, and Effects, Academic Press Inc, San Diego, pp 183–199
Mishra RN, Kunwar PS (2014) Status of aquaculture in Nepal. Nepalese Journal of Aquaculture and Fisheries 1:1–17. http://nefis.org.np/wp-content/uploads/2018/10/NJAF-Vol-1-2014.pdf
Muthukumaravel K, Sukumaran M, Sathick O (2013) Studies on the acute toxicity of pesticides on the freshwater fish Labeo rohita. International Journal of Pure and Applied Zoology 1(2):185–192. https://www.alliedacademies.org/articles/studies-on-the-acute-toxicity-of-pesticides-on-thefreshwater-fish-labeo-rohita.pdf
Nag SK, Saha K, Bandopadhyay S, Ghosh A, Mukherjee M, Raut A, Raman RK, Suresh VR, Mohanty SK (2020) Status of pesticide residues in water, sediment, and fishes of Chilika lake, India. Environmental Monitoring and Assessment 192(2):1–10. https://doi.org/10.1007/s10661-020-8082-z
Nwani CD, Ugwu DO, Okeke OC, Onyishi GC, Ekeh FN, Atama C, Eneje LO (2013) Toxicity of the chlorpyrifos-based pesticide Termifos®: effects on behaviour and biochemical and haematological parameters of African catfish Clarias gariepinus. African Journal of Aquatic Science 38(3):255–262. https://doi.org/10.2989/16085914.2013.780153
OECD (1992) Guideline for testing of chemicals, fish acute toxicity test no. 203. 1–9. https://doi.org/10.1787/9789264070684-en
Padmanabha A, Reddy H, Khavi M, Prabhudeva K, Rajanna K, Chethan N (2015) Acute effects of chlorpyrifos on oxygen consumption and food consumption of freshwater fish, Oreochromis mossambicus (Peters). International Journal of Recent Scientific Research 6(4):3380–3384
Patil VK, David M (2008) Behaviour and respiratory dysfunction as an index of malathion toxicity in the freshwater fish, Labeo rohita (Hamilton). Turkish Journal of Fisheries and Aquatic Sciences 8(2):233–237
Ramesh M, Saravanan M (2008) Haematological and biochemical responses in a freshwater fish Cyprinus carpio exposed to chlorpyrifos. International Journal of Integrative Biology 3(1):80–83
Rauf A (2015) Acute toxicity and effects of malathion exposure on behavior and hematological indices in Indian carp, Cirrhinus mrigala (Hamilton). International Journal of Aquatic Biology 3(4):199–207
Sabra FS, Mehana E-SE-D (2015) Pesticides toxicity in fish with particular reference to insecticides. Asian Journal of Agriculture and Food Sciences 3(1):40–60
Saha NC, Giri SK, Chatterjee N, Biswas SJ, Bej S (2016) Acute toxic effects of mancozeb to fish Oreochromis mossambicus (W. K. H. Peters, 1852) and their behaviour. International Journal of Advanced Research in Biological Sciences 3(6):40–44
Schmidt K, Staaks GBO, Pflugmacher S, Steinberg CEW (2005) Impact of PCB mixture (Aroclor 1254) and TBT and a mixture of both on swimming behavior, body growth and enzymatic biotransformation activities (GST) of young carp (Cyprinus carpio). Aquatic Toxicology 71(1):49–59. https://doi.org/10.1016/j.aquatox.2004.10.012
Singh S, Bhutia D, Sarkar S, Rai BK, Pal J, Bhattacharjee S, Bahadur M (2015) Analyses of pesticide residues in water, sediment and fish tissue from river Deomoni flowing through the tea gardens of Terai Region of West Bengal, India. International Journal of Fisheries and Aquatic Studies 3(2):17–23
Soni R, Verma SK (2018) Acute toxicity and behavioural responses in Clarias batrachus (Linnaeus) exposed to herbicide pretilachlor. Heliyon, 4(12). https://doi.org/10.1016/j.heliyon.2018.e01090
Srivastava N, Rai AK, Kumari U, Mittal S, Mittal AK (2012) Behavioural dysfunctions in relation to the toxicity of ‘NUVAN®’, an organophosphorus insecticide in an Indian major carp, Cirrhinus mrigala. Research in Environment and Life Sciences 5(4):245–250
Stepić S, Hackenberger BK, Velki M, Lončarić Ž, Hackenberger DK (2013) Effects of individual and binary-combined commercial insecticides endosulfan, temephos, malathion and pirimiphos-methyl on biomarker responses in earthworm Eisenia andrei. Environmental Toxicology and Pharmacology 36(2):715–723. https://doi.org/10.1016/j.etap.2013.06.011
Sun K-F, Xu X-R, Duan S-S, Wang Y-S, Cheng H, Zhang Z-W, Zhou G-J, Hong, Y-G (2015) Ecotoxicity of two organophosphate pesticides chlorpyrifos and dichlorvos on non-targeting cyanobacteria Microcystis wesenbergii. Ecotoxicology. https://doi.org/10.1007/s10646-015-1458-0
Tišler T, Jemec A, Mozetič B, Trebše P (2009) Hazard identification of imidacloprid to aquatic environment. Chemosphere 76(7):907–914. https://doi.org/10.1016/j.chemosphere.2009.05.002
Ullah R, Zuberi A, Ullah S, Ullah I, Dawar FU (2014) Cypermethrin induced behavioral and biochemical changes in mahseer, Tor putitora. The Journal of Toxicological Sciences 39(6):829–836. https://doi.org/10.2131/jts.39.829
Ural M-Ş, Çalta M (2005) Acute toxicity of dichlorvos (DDVP) to fingerling mirror carp, Cyprinus carpio L. Bulletin of Environmental Contamination and Toxicology 75(2):368–373. https://doi.org/10.1007/s00128-005-0763-3
Varó I, Navarro JC, Nunes B, Guilhermino L (2007) Effects of dichlorvos aquaculture treatments on selected biomarkers of gilthead sea bream (Sparus aurata L.) fingerlings. Aquaculture 266(1–4):87–96. https://doi.org/10.1016/j.aquaculture.2007.02.045
Velmurugan B, Selvanayagam M, Cengiz EI, Unlu E (2009) Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Brazilian Archives of Biology and Technology 52(5):1291–1296. https://doi.org/10.1590/S1516-89132009000500029
Wang Y, Chen C, Zhao X, Wang Q, Qian Y (2015) Assessing joint toxicity of four organophosphate and carbamate insecticides in common carp (Cyprinus carpio) using acetylcholinesterase activity as an endpoint. Pesticide Biochemistry and Physiology 122:81–85. https://doi.org/10.1016/j.pestbp.2014.12.017
Wang Y, Lv L, Yu Y, Yang G, Xu Z, Wang Q, Cai L (2017) Single and joint toxic effects of five selected pesticides on the early life stages of zebrafish (Denio rerio). Chemosphere 170:61–67. https://doi.org/10.1016/j.chemosphere.2016.12.025
Wheelock CE, Shan G, Ottea J (2005) Overview of carboxylesterases and their role in the metabolism of insecticides. Journal of Pesticide Science 30(2):75–83. https://doi.org/10.1584/jpestics.30.75
Xing H, Liu T, Zhang Z, Wang X, Xu S (2015) Acute and subchronic toxic effects of atrazine and chlorpyrifos on common carp (Cyprinus carpio L.): Immunotoxicity assessments. Fish and Shellfish Immunology 45(2):327–333. https://doi.org/10.1016/j.fsi.2015.04.016
Zahran E, Risha E, Awadin W, Palić D (2018) Acute exposure to chlorpyrifos induces reversible changes in health parameters of Nile tilapia (Oreochromis niloticus). Aquatic Toxicology 197:47–59. https://doi.org/10.1016/j.aquatox.2018.02.001
Acknowledgements
We are thankful to Central Fisheries Promotion and Conservation Centre, Balaju, Kathmandu, Nepal for providing research facilities. We extend our gratitude to Mr. Baikuntha Adhikari, Mr. Subhash Kumar Jha and Mr. Balaram Acharya for their help in different capacities.
Funding
No funding was received to assist with the preparation of this manuscript. However, laboratory facilities and chemicals required for the study were provided by Central Fisheries Promotion and Conservation Centre, Balaju, Kathmandu, Nepal.
Author information
Authors and Affiliations
Contributions
PSK designed this research, monitored the lab works and prepared the draft version of the manuscript. BS and SB conducted the exposure experiments and collected fish mortality and behavior data. KP compiled and analyzed the data. AKS and GDeB reviewed, corrected and improved the manuscript. KS approved the research design, validated the data and reviewed the manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Ethical approval for the study (Ref no. 1215) was received on 18 September 2019 from the Ethical Review Board of Nepal Health Research Council, Government of Nepal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kunwar, P.S., Sapkota, B., Badu, S. et al. Chlorpyrifos and dichlorvos in combined exposure reveals antagonistic interaction to the freshwater fish Mrigal, Cirrhinus mrigala. Ecotoxicology 31, 657–666 (2022). https://doi.org/10.1007/s10646-022-02534-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02534-6