Abstract
Polycyclic aromatic hydrocarbons are organic chemicals consisting of a small number of benzene rings. PAHs are exposed to the environment by events such as Crude oil spills, even though they are substances present in the environment. Exposure of PAHs to the environment will affect not only the environment, but also the living organisms and the ecosystem as a whole. The effects of PAHs vary widely depending on the type of PAHs and have been studied for a long time. However, there are only 16 kinds of PAHs defined by US EPA, and there are more kinds of PAHs present in the environment. Therefore, it is time- and space-limited to judge the toxicity of all kinds of PAHs by evaluating them. In all cases, the tendency of research is shifting toward predicting toxicity evaluation through modeling rather than the direction of toxicity evaluation. In this study, we constructed a quantitative structure-activity relationship (QSAR) model, one of the molecular structure activation models, and predicted the correlation between the toxicity value and the logKow value of PAHs. Basically, as the logKow value increases, the median effective concentration (EC50) tends to decrease. Compared with the previous studies, Hyalella azteca showed this tendency, but Daphnia magna showed different results when exposed to Naphthalene. The RMSE(Root Mean Square Error) values of Daphnia magna and Hyalella azteca were 6.0049 and 5.9980, respectively, when the QSAR model was constructed using the toxicity data for PAHs. We confirmed the validity of the QSAR model in this study by comparing the results of exposing Daphnia magna to PAHs and the ECOSAR data, one of the existing models. The R2 value was found to be 0.9356. This study suggests that it may be helpful to predict the toxicity evaluation and to prepare countermeasures for accidents such as Crude oil spill. It is thought that if more data base is created by using additional types of PAHs and species in the same way as this study in the future, it will help to construct the modeling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds in which two to six benzene aromatic rings are bonded and are composed primarily of carbon and hydrogen atoms. PAHs are semi-volatile organic pollutants that may or may not be persistent depending on the type of material (Chen 2007). PAHs predominantly originate from anthropogenic processes, especially from incomplete combustions of organic fuels. Certain naturally occurring processes, such as volcanic eruptions and forest fires, can also contribute to the increasing the amount of PAHs in the environment.
PAHs can be divided into two groups depending on the molecular weight. PAHs with two or three benzene aromatic rings are classified as light molecular weight (LMW) PAHs, while four to six benzene rings are considered high molecular weight (HMW) PAHs. LMW and HMW PAHs have different environmental impacts when exposed to the environment. Naphthalene, fluorine, and benzene belong to LMW PAHs while phenanthrene, benzo[a]pyrene, and pyrene belong to HMW PAHs. Although LMW PAHs are volatilized or naturally easily decompose when exposed to the environment, HMW PAHs can remain in water, sediments, and soils.
PAHs can spontaneously decompose and react with substances such as nitrogen oxide, ozone and sulfur dioxide present in the environment to produce byproducts (Lee 2010). PAHs can also be broken down by organisms, which is called biodegradation. Biodegradation can be caused by a wide variety of microorganisms or enzymes, which break down the chemical bonds in PAHs (Seo et al. 2009). The impact that PAHs have on the environment largely depends on the structure and weight of the molecule.
The presence of PAHs in aquatic environments is influenced by many complex organic substances present in the aquatic system (Amirhossein et al. 2016). PAHs present in the environment are oxidized or decomposed by light to form intermediates. These substances are more easily decomposed or naturally degraded by organisms than the original PAHs (Lehto et al. 2000).
Short-lived metabolic products produced by enzymatic degradation of PAHs have a more toxic effect on organisms than normal PAHs. Unless PAHs somehow avoid natural degradation, these PAHs exposed to the environment have been known to transform into carcinogens through degradation (Producers 2005). An example of this is fluoranthene, a class of PAHs known to cause acute toxicity; it lacks in carcinogenicity by itself, but is known to be toxic if it is present in a fluoranthene-induced form, such as with benzoic acid (Acros Organic, Safety Data Sheet; Science Lab). There are thousands of PAHs in the environment in many forms. Since PAHs exist in the environment and are decomposed or transformed through various pathways, it is difficult to conclude that there are only original PAHs.
There are several time and space constraints of toxicity and risk assessments for all types of PAHs, and making comprehensive evaluations often difficult. Therefore, models are being devised to predictively assess the toxicity and risk of PAHs.
A Quantitative Structure-Activity Relationship (QSAR) model is one such model. QSAR models can predict the degree of harmful effects that exposure to PAHs imposes on organisms, including the magnitude of toxicity, mutagenicity, and carcinogenicity, all based on the structural characteristics of the molecule. Based on the rapid development of computer science and theoretical quantum chemistry, it is now possible to calculate chemical quantum parameters accurately and quickly. Theories on quantum chemistry have successfully been applied to QSAR models (Seo et al. 2009; Amirhossein et al. 2016; Lehto et al. 2000). In this study, the QSAR model created was established using the Octanol-water coefficient (Kow), which every chemical substance has a different value of.
In this study, Daphnia magna and Hyalella azteca were exposed to 8 kinds of PAHs. After that, molecular structure activity model was created using toxicity evaluation data and logKow, one of the chemical properties of PAHs. The purpose of this study was to evaluate the acute toxicity of 8 selected PAHs and establish a QSAR model for them. The PAHs selected for this study are: benzene, toluene, dibenzothiophene, naphthalene, 2-methylnaphthalene, fluorene, phenanthrene, and pyrene. These PAHs have a logKow value between 2 and 5. The basic properties of the PAHs used in this study are shown in Table 1. PAHs were separately dissolved in water using dimethyl sulfoxide (DMSO). Toxicity tests were performed by exposing Daphnia magna and Hyalella azteca to PAHs dissolved in water, with the endpoint of the experiment being their mortality. When the QSAR model was established using the toxicity values and the logKow values of the PAHs, the y-intercept value was confirmed by applying it to the previously studied universal slope. The universal slope (−0.936) was applied to predict the y-intercept for each test organism in the QSAR model using the concentration value (EC50) and the logKow value. The QSAR model created in this study was verified by comparing it with results obtained through experiments using ECOSAR, another prediction model that estimates aquatic toxicity.
Materials and methods
Chemical substances
The following PAHs were used: naphthalene (99%, Sigma-Aldrich, Germany), fluorene (99%, Sigma-Aldrich, Germany), phenanthrene (99.5%, Sigma-Aldrich), pyrene (>99%, Sigma-Aldrich, Germany), dibenzothiophene (98%, Aldrich), 2-methylnaphthalene (*97%, Aldrich), toluene(99.8%, Sigma-Aldrich), benzene(>99.9%, Sigma-Aldrich), methanol (HPLC grade), and methyl chloride (>99.5%, Aldrich) were used for PAH-loading, -extraction, and -analysis (Merck, Darmstadt, Germany). Milli-Q water (Super Q-treated, Millipore, MA, USA) was used as a solvent, culture medium, and test solution in all experimental procedures.
Daphnia magna
The Daphnia magna was one of the organisms used in the test. The D.magna were obtained from the National Institute of Environmental Research (NIER). The culture conditions of the Daphnia magna were maintained at a pH of 7.6 ~ 8.0, a temperature of 20 ± 2 °C, a water hardness of 160 ~ 180 mg/L as CaCO3, an alkaline degree of 110 ~ 120 mg/L as CaCO3, and a dissolved oxygen density of (DO) 3.0 mg/L. The light condition was maintained at a lux of 1000 ~ 2000, with a light-dark cycle of 16-h light/8h-dark. The culture medium prepared for the D.magna was prepared using the reagents shown in the Table 2. The culture medium was changed daily, and the D.magna were fed with chlorella and YCT (yeast, chlorophyll, trout chow). The neonates of D.magna used in the experiment were less than 24 h old. Prior to the toxicity test, quality assurance and quality control (QA/QC) tests were conducted using potassium dichromate as a validation method for the D.magna.
Hyalella azteca
The Hyalella azteca was another organism used in the test. The H.azteca were obtained from the National Institute of Environmental Research (NIER). They were cultured according to the standard method of the US EPA (EPA 1996). The water conditions for culturing the H.azteca were as follows: water hardness of 90 to 100 mg/L as CaCO3, alkaline level of 50 to 70 mg/L as CaCO3, conductivity of 330 to 360 mS/cm, and pH of 7.8 to 8.2. The chemicals used to make the culture of the H.azteca are shown in the Table 3 below. The organisms were fed ca. 200 mg of finely ground Tetramin® fish flakes (Tetra US, Blackburg, USA) twice per week following each water change. Cotton gauze strips were added to the culture tank to serve as a substrate. The culture was illuminated by two 48“ fluorescent light bulbs emitting ca. 55 μEm-1s-1 with a light-dark cycle of 16h-light/8h-dark. The H.azteca used were about 7–11 days old. Before performing the toxicity test, the quality assurance and quality control (QA/QC) test was performed using sodium chloride (NaCl) and potassium chloride(KCl)for the H.azteca.
Test solution
In this study, the PAHs that were to be exposed to the organisms were dissolved in water using dimethyl sulfoxide (DMSO). After the PAHs were dissolved in DMSO, the DMSO solution was dissolved in water. Once the PAHs were dissolved in water, the 50, 25, 12.5 and 6.25% PAHs solutions were prepared with a serial dilution of 100%. The water used to dilute the solutions of the PAHs was diluted with the culture medium used for the cultivation of Daphnia magna or Hyalella azteca. After the PAH solutions were prepared, their PAH concentration was measured using GC-MS. All the test solutions to be analyzed by GC-MS were extracted by the liquid–liquid extraction method with methyl chloride (DCM) as the solvent. Analytical samples extracted with DCM were concentrated using nitrogen gas. The concentrated DCM solution was then replaced with hexane solvent. Replacing the concentrated solution with hexane solvent was to rid the solution of impurities. The extracts were analyzed using GC-MS (Agilent 6890/HP 5973) with splitless mode injection on a bonded phase fused silica capillary column DB5-MS ((30 m × 0.25 mm) i.d. × 0.25 μm film thickness). The column temperature was 70 °C for 4 min, and ramped at 10 °C/min to 300 °C, which was held for 10 min. The injector and detector temperatures were kept at 260 and 300 °C, respectively. The flow rate of helium was 1 mL/min.
Acute toxicity test (Daphnia magna)
The acute toxicity tests on the Daphnia magna was performed according to US EPA standard methods. D.magna are neonates for less than 24 h after birth. In this study, five neonates were exposed to 200 mL of different concentrations of PAHs (6.25, 12.5, 25, 50, and 100%) and 200 mL of culture media. The illumination was maintained at 500 lux to 1000 lux and the photoperiod was 16 h a day (followed by 8 h of darkness). The exposure time of the D.magna to the PAHs was 24 h, which was enough time for the PAHs to impose its acute toxicity on the organisms. The effects of the PAHs on the D.magna were observed for 24 h, and the results were used to calculate the median effective concentration (EC50) values. The exposure experiment was 3 replicates per concentration of PAHs. EC50 values and 95% confidence intervals were calculated using the Spearman-Karber method (Hamilton et al. 1977) of ToxCalc 5.0.
Acute toxicity test (Hyalella azteca)
The method for conducting the acute toxicity tests on the Hyalella azteca was based on standard toxicity test procedures of the United States Environmental Protection Agency(Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms). The illumination was maintained at 500 to 1000 lux, and the photoperiod was 16 h a day (followed by 8 h of darkness). The temperature of the experiment was 23 °C. The PAH test solutions were dissolved in water using DMSO. Each test vial of the H.azteca contained seven H.azteca and 400 mL of PAH test solution. The H.azteca used in this study were selected from amphipods 7 to 10 days old. A total of 5 H.azteca groups were exposed to PAHs for 96 h (4 days) each. The exposure experiment was three replicates per concentration of PAHs. During the experimental period, the H.azteca were not fed and their developments were recorded. EC50 values and 95% confidence intervals were calculated using the Spearman-Karber method (Hamilton et al. 1977) of ToxCalc 5.0.
Target lipid model
The target lipid model predicts the target lipid of the organism from the critical body burden which utilizes the same value of the median effective concentration (EC50) as the toxic endpoint (Di Toro et al. 2000; Di Toro and McGrath 2000). These values are calculated with the target lipid-water partition coefficient, KLW. The TLM makes the following two assumptions: the species-specific critical target lipid body burden (CTLBB) CL is the same for all narcotic chemicals and the chemical-specific target KLW is the same for all aquatic organisms. The validity of the two assumptions has been validated by comparing the endpoint’s predicted and actual observed results. In this study, the QSAR model was constructed by applying the following TLM equation:
In the above equation, m is the slope and b is the intercept in the relationship between log (EC50) and log(Kow). The relationship between the logKow and logEC50 value of PAHs using was confirmed through the use of a biosensor, and the intercept value was confirmed by applying the universal slope of −9.936 to slope m. When the universal slope was applied, the intercept value was compared with the result of the preceding study and the validity of the QSAR model was verified through the result of this experiment. The validation test was conducted using the ECOSAR program and the QSAR model created in this study. ECOSAR (v2.0) is a model for predicting the ecotoxicological values of international standard test species based on certain chemicals manufactured by the US EPA. The validity of the model was justified by comparing the toxicity value of the QSAR model created in this experiment to the toxicity value of the existing model. The toxicity values of the chemicals tested in this study were verified through ECOSAR.
Results
Characterization of the assay
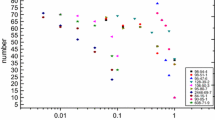
The Fig. 1 and Table 1 shown display basic information of the involved PAHs. Dimethyl sulfoxide (DMSO) solution was used to dissolve the PAHs and the test solutions were prepared by dissolving the solutions in water. The control solution was prepared through the same method except for the use of the PAHs. When the PAHs were dissolved in water using DMSO, the concentrations of the PAHs was measured by GC-MS in the solution of the highest concentration in the test solution. The concentration of PAHs in the test solution is displayed on the shown Fig. 2 and Table 4. The PAHs of the test solution used in this study were found to be dissolved in ranges not exceeding their solubility. Since the control solution did not show the concentration of the PAHs, it was confirmed that the preparation of the test solution using the DMSO did not affect the concentration of PAHs.
Effects of the PAHs
The endpoints of PAHs exposed to the organisms were defined as either death or immobilization of the organisms. The median effective concentration (EC50) values for the endpoints were calculated using ToxCalc 5.0. The EC50 concentrations of the 8 kinds of PAHs affecting the neonates of Daphnia magna and Hyalella azteca are shown on the Table 5. The control solution, in which only the DMSO was dissolved, was found to not affect both the D.magna and the H.azteca.
The dose-response relationships of the 8 different types of PAHs that the D.magna and the H.azteca were exposed to are shown on the Figs 3 and 4. The concentration at which 50% of the test organisms survived was confirmed by the median effective concentration (EC50) value of the experimental endpoint.
QSAR
The correlation between the logEC50 value and the logKow value of the PAHs affecting the two organisms used in the experiment is shown in the Figs 5 and 6. As shown, the logEC50 value decreases as the logKow value increases. A decrease in the logEC50 value indicates that an increase in toxicity; this phenomenon has been observed in both organisms used in the experiment. The intercept values of Hyalella azteca and Daphnia magna were 1.661 and 1.612, respectively, when universal slope of −0.936 was applied to the equation. The two intercept values are unique to the organisms. When applying the universal slope, the RMSE(Root Mean Square Error) values of the linear regression were 6.0049 for the D.magna and 5.9980 for the H.azteca, respectively. The QSAR model was compared with the ECOSAR data (Fig. 7) to confirm its validity. The correlation between D.magna results of ECOSAR and D.magna results of this study was found. The R2 value of correlation between the predicted and observed results was 0.9356.
Discussion
The toxic mechanisms of the PAHs
Through many studies, PAHs are known as representative narcotic chemicals. Historically, it has been known that narcotic effects are influenced by the ability of compounds to act on the cellular membrane by n-octanol: water partition coefficient (Kow). It is known that narcotic effects occur as a result of noncovalent interactions involving the disruption of the van der Waals interaction between the lipid and/or protein components in cellular membranes (Franks and Lieb 1990). The toxicity of narcotic chemicals has been shown to be influenced by the interaction of biomembranes with lipids. Thus, it was found that the toxicity of narcotic chemicals can be calculated from n-octanol: water partition coefficient, logKow. The toxicity of the narcotic chemical type is also called baseline toxicity (Schultz et al. 1998; McFarland 1970; Liao et al. 1996; Cronin and Schultz 1997).
PAHs are narcotic chemicals that accumulate in the lipid bilayer in cells and can be toxic if they meet certain criteria. Enzymatic activation is the precursor to DNA mutation. The induction of the cytochrome P450 family enzymes is an important step before the activation phase of carcinogens (Kennedy and Jones 1994; Szklarz and Paulsen 2002). Many studies have shown that PAHs are associated with many illnesses such as cancers, renal disease, circulatory disorders, and immune system dysfunction (ATSDR 1990). The harmful effects of the PAHs are initiated by the oxidation of members of the cytochrome P450 family, such as the CYP1A1 enzyme. The induction process of PAH adsorption begins with the systemic circulation of cells. When PAH metabolism is initiated, PAHs in the cytosol attach to aryl hydrocarbons receptors (AHR) and fall into two heat shock proteins (Hsp90). The AHR-ligand complex is phosphorylated by tyrosine kinase, which allows the AHR and the ligand to enter the nucleus and attach to the AHR-nuclear translocator protein (ARNT). The PAH then attaches to the xenobiotic response element in the upstream region of a gene that promotes transcription (Parkinson 1996). The process of absorption and transport of the PAHs into the cytosol of cells can limit the metabolic activities of cells (Parkinson 1996).
Similarly, the structure of the PAH molecule can serve as active intermediates to direct the path of cell metabolism. Mutagenic activity is affected by structural factors such as isomeric positioning, conformation, steric hindrances, physical dimension, and the ability to stabilize electrophilic metabolites of the PAHs (Ames et al. 1975; Analogues, C. o. P. a. S. and Hazards, B. o. T. a. E. H. 1983; Ashurst et al. 1983; Warshawsky and Landolph 2006). Structure activity relationships can be a good precedent for toxic endpoints associated with carcinogenesis and can be found in the results of previous experiments.
QSAR
Generally, the higher the logKow value (the value that shows how well the PAHs can resist from dissolving in water), the EC50 concentration of the PAH is decreasing. The lowering of the EC50 value indicates an increase in toxicity. In this study, the relationship between the logKow value and the EC50 concentration in the Hyalella azteca when they were exposed to the PAHs showed a general correlation. The Daphnia magna, however, when exposed to the Naphthalene, showed a very strong correlation between the two values.
The toxic effects of the Naphthalene were more delayed when compared to the effects of the other harmful compounds that the D.magna were exposed to. This phenomenon can be explained by the slower bioconcentration kinetics of Naphthalene, which is due to the differences in time that hydrophilic chemicals have to reach equilibrium partitioning concentrations when they affect aquatic different organisms (Sijm et al. 2007). Risk assessments are usually performed using short-term toxicity tests based on 24 or 96 h experiments (ECETOC 1996).
The PAHs, when exposed to the Hyalella azteca, had sufficient time to affect the organisms during 96 h exposure periods, although the PAHs varied in solubility to water depending on the type. However, the D.magna only underwent PAH exposure for 24 h as per experiment standards. This allowed for the exceptional results of the Naphthalene exposure to be obtained. These results can be attributed to the fact that the actual toxicity of the PAHs is often underestimated due to hydrophobic chemicals not having enough time to concentrate in sufficiently large amounts internally in aquatic organisms. Therefore, if an experiment to evaluate the toxicity of hydrophobic chemicals is performed in a relatively short period of time, one must consider such a factor.
Based on the results of this study, the correlation between the toxicity value (EC50) and the logKow value of PAHs was investigated using the RMSE(Root Mean Square Error) value. The RMSE values of D.magna and H.azteca were 6.0049 and 5.9980, respectively. The lower the value of the RMSE value, the higher the precision. Therefore, this study confirms the RMSE of the QSAR model created by exposing PAHs to living organisms, thus constituting a precise model. In order to verify the predictions of the QSAR model constructed in this study, we compared those results to the results estimated by ECOSAR (v2.0) (Fig. 7). Through this comparison, an R2 value of 0.9356 was concluded, which would indicate high correlation between the two values.
Conclusion
In this study, ecotoxicity tests of 8 kinds of PAHs were carried on Daphnia magna and Hyalella azteca. With the results obtained, a QSAR model of the PAHs was constructed for each test organism. When the logKow value, an index of PAH solubility in water, showed a correlation to the EC50 value, the PAH concentration required to harm the organisms, the EC50 value showed a tendency to decrease as the logKow value increased. In other words, as the logKow value increased, the toxicity of the PAHs to D.magna and H.azteca was found to increase, and this result was used to create QSAR models. The R2 value was found to be 0.9356 through the comparison of the QSAR model created through the D.magna experiment with the toxicity data in the existing ECOSAR. Therefore, the QSAR model developed in this study can accurately provide data for predicting the toxicity of PAHs and may be helpful for use in the toxicity prediction for other kinds of PAHs. As in this study, it is necessary to further study modeling methods such as QSAR by comparing predicted toxicity values with existing data from actual experimentation.
References
Agency for Toxic Substances and Disease Registry (1990) Toxicological profile for polycyclic aromatic hydrocarbons. U.S. Department of Health and Human Services, ATSDR/TP-Draft, Washington, DC
Amirhossein M, Ming XL, Ka-Wy Ng, Tech SAM (2016) The fate and toxicity assessment of polycyclic aromatic hydrocarbons (PAHs) in water streams of Malaysia. Procedia Eng 148:806–811
Ames BN, McCann J, Yamasaki E (1975) Proceedings: carcinogens are mutagens: a simple test system. Mutat Res 33:27–28
Analogues, Committee on Pyrene and Selected, and Hazards, Board on Toxicology and Environmental Health (1983) Polycyclic aromatic hydrocarbons—Evaluation of sources and effects. National Academies Press, Washington, DC
Ashurst SW, Cohen GM, Nesnow S, DiGiovanni J, Slaga TJ (1983) Formation of benzo(a)pyrene/DNA adducts and their relationship to tumor initiation in mouse epidermis. Cancer Res 43:1024–1029
Cronin MTD, Schultz TW (1997) Validation of Vibrio fischeri acute toxicity data: mechanism of action-based QSARs for non-polar narcotics and polar narcotic phenols. Sci Total Environ 204:75–88
Chen Y (2007) Gaseous and particulate polycyclic aromatic hydrocarbons (PAHs) emissions from commercial restaurants in Hong Kong. J Environ Monit 9(12):1402–1409
Di Toro D, McGrath J, Hansen D (2000) Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. I. Water and tissue. Environ Toxicol Chem 19:1951–1970
Di Toro DM, McGrath JA (2000) Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. II. Mixtures and sediments. Environ Toxicol Chem 19:1971–1982
European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) (1996) Aquatic toxicity testing of sparingly soluble, volatile and unstanble substances. Mono no. 026 Brussels, Belgium
Franks NB, Lieb WR (1990) Mechanisms of general anesthesia. Environ Health Persp 87:199
G. Producers (2005) Fate and effects of naturally occurring substances in produced water on the marine environment. International Association of Oil & Gas Products 364:18–20
Hamilton MA, Russo RC, Thurston RV, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Kennedy SW, Jones SP (1994) Simultaneuos measurement of cytochrome P4501A catalytic acitivity and total protein concnetration with a fluorescence plate reader. Anal Biochem 222:217–223
Lee BK (2010) Sources, distribution and toxicity of polyaromatic hydrocarbons (PAHs) in particulate matter, Air Pollution. 5 (Vanda Villanyi (Ed.)): 99-101
Lehto KM, Vuorimaa E, Lemmetyinen H (2000) Photolysis of polycyclic aromatic hydrocarbons (PAHs) in dilute aqueous solution detected by fluorescence, J. Photochem. Photobiol. A Al36(53):53–60
Liao YY, Wang LS, He YB, Yang H (1996) Toxicity QSAR of substituted benzenes to yeast Saccharomyces cerevisiae. Bull Environ Contam Toxicol 56:460–466
Mc Farland JW (1970) On the parabolic relationship between potency and hydrophobicity J Med Chem 13:1092–1196
Parkinson A (1996) Biotransformation of xenobiotics. In: Klaassen CD, Amdur MO, Doull J Eds. Casarett and Doull’s toxicology: The basic science of poisons. McGraw-Hill, New York, NY, p 133–224
Schultz TW, Sinks GD, Bearden AP (1998) QSAR in aquatic toxicology: a mechanism. Of action approach comparing toxic potency to pimephales promelas, Tetrahymena pyriformis, and Vibrio fischeri. In: Devillers J (Ed.) Comparative QSAR. Taylor&, Francis, New York, NY, USA, p 51–109
Seo JS, Keum YS, Li QX (2009) Bacterial degradation of aromatic compounds. J Environ Res Public Health 6:278–309
Sijm DTHM, Rikken MGJ, Rorije E, Traas TP, Mclachlan MS, Peijnnburg WJGM (2007) Transport, accumulation and transformation processes. In: van Leeuwen CJ, Vermeire TG (Eds.) Risk Aassessment of Chemicals, second ed. Springer, Netherlands
Szklarz GD, Paulsen MD (2002) Molecular modeling of cytochrome P450 1A1: Enzyme–substrate interactions and substrate binding affinities. J Biomol Struct Dyn 20:155–162
US EPA (1996) Ecological Effects Test Guidelines, United States Environmental Protection Agency OPPTS 850. 1735 Whole Sediment Acute Toxicity Invertebrates, Freshwater
Warshawsky D, Landolph JR eds (2006) Molecular carcinogenesis and the molecular biology of human cancer. CRC Press, Boca Raton, FL
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ha, H., Park, K., Kang, G. et al. QSAR study using acute toxicity of Daphnia magna and Hyalella azteca through exposure to polycyclic aromatic hydrocarbons (PAHs). Ecotoxicology 28, 333–342 (2019). https://doi.org/10.1007/s10646-019-02025-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02025-1