Abstract
Estuaries are subjected to continual environmental impacts from activities in the catchment areas. This research assessed the quality of two estuarine habitats located in Ilha do Maranhão, Brazil, through histological and genotoxic biomarkers in Centropomus undecimalis, comparing the data obtained to metal, physical, and chemical concentrations of water samples. The gill histological alterations were analyzed by the histological alteration index and genotoxic lesions in erythrocytes were detected by the Micronucleus Test. The analysis of metals revealed that all water samples contained at least two elements with concentrations higher than that allowed by the current Brazilian law. For gill histological analysis, snook of both areas assessed exhibited moderate lesions, indicating that the local fish are affected by environmental stress. Micronucleus analysis of snook showed that the Bacanga river basin is the most affected. In addition to assessing the health of commercial fish populations, the information about the biomarkers used for the species can serve to contribute to the preparation and/or application of health assessment models and implementation of environmental recovery policies for coastal aquatic environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are water bodies that occupy the transition region between the oceans and rivers. They have great ecological importance, as vital microcosms of growth and reproduction for various biological species, such as mollusks, fish, and crustaceans, among other groups (Santos et al. 2006). They also are economically important in that they support industries such as fishing, agriculture, tourism, navigation, and port operations.
Rapid urbanization on the banks of estuaries has altered the dynamics of these wetland habitats. Domestic and industrial effluents, garbage, landfill, and destruction of vegetation, among others, have largely contributed to the pollution of these water bodies. Given the environmental issues, programs are needed for restoring and preserving these ecosystems.
The species of an ecosystem can be used to indicate changes in local environmental conditions, and the responses of the organisms form the basis of biological indicators of environmental degradation (Buss et al. 2003). These responses may be adaptive to stress, evidenced as cellular, biochemical, histological, physiological, or behavioral alterations, currently defined as biomarkers (de Jesus and de Carvalho 2008).
Biomarkers in fish are promising tools in the evaluation of degraded aquatic ecosystems, as these organisms are directly exposed to environmental pollutants, reacting sensitively to any alterations in the environment (Lemos et al. 2007). Among the biomarkers, emphasis is placed on histological and genotoxic alterations.
Histological alterations in fish tissues are used to document and quantify both exposure and the effect of pollutants (Winkaler et al. 2001; Pacheco and Santos 2002; Veiga et al. 2002; Meletti et al. 2003). Genotoxic lesions allow for assessing the effects of mutagenic, clastogenic, and/or aneugenic pollutants in the genetic material of organisms (Kendall et al. 2001).
Taking this into consideration, the present study examined the histological and genotoxic alterations in Centropomus undecimalis (Pisces, Centropomidae) as aquatic contamination biomarkers in assessing the environmental quality of two estuaries of Ilha do Maranhão, Brazil.
Materials and methods
Study area and sample collection
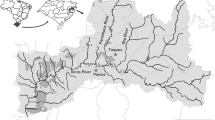
The study was conducted in two estuaries of Ilha do Maranhão: the Bacanga river basin (S1) and the Pau Deitado estuary (S2) (Fig. 1). The Bacanga river basin, located northwest of the Island, is one of the largest green areas in the State of Maranhão. It is important for the set of its basins and ecological diversity, which led the government to establish the Bacanga State Park (Maranhão State Decree no. 7545/1980) in 1980. The basin also houses the Environmental Protection Area (APA) of Maracanã, established by State Decree no. 12.103/1991 (Maranhão 1991).
Intense urbanization on the Bacanga riverbank has altered the dynamics of the site, affecting biodiversity and quality of the water body through discharge of domestic sewage and garbage.
The Pau Deitado estuary, located in the town of Pau Deitado, Municipality of Paço do Lumiar, Maranhão, flows into the Curupu bay and encompasses an extensive area of mangroves. It is located far from the urban center; however, there is a presence of bars and residences.
In both study areas, the Bacanga river basin (2° 32′ 53″ S and 44° 18′ 15″ W) and the Pau Deitado estuary (2° 31′ 49″ S and 44° 5′ 23″ W), specimens of C. undecimalis, popularly known as seabass, were collected in 2015 during the rainy season, in the months of April and May, and during the months of September to November in the dry season. The number of fish collected in Bacanga River was determined by the State Secretary of Environment (SEMA), in accordance with the environmental law for protected areas.
Samples were collected through artisanal fishing using cast nets. The standard lengths (SLs) of fish were measured in the field as follows: the total distance between the mouth and the end of the tail (TL) and the distance between the mouth and the beginning of the tail (PL). The sex and gonadal stage (GS) of the samples taken were determined according to Vazzoler (1996) at the Animal Morphophysiology Laboratory, Department of Chemistry and Biology, State University of Maranhão. The GS was classified as follows: I, immature; II, under maturation; III, under advanced maturation; and IV, mature.
The samples for water analyses were collected in plastic bottles and stored in an isothermal box with ice. The parameters were compared to resolution no. 357/2005, of the National Environmental Council (CONAMA; Brasil 2005). The physicochemical parameters analyzed were temperature, pH, dissolved oxygen, and salinity, obtained on site by a HANNA multiparameter with GPS HI 9828. The metals analyzed were zinc (Zn), copper (Cu), iron (Fe), aluminum (Al), cadmium (Cd), mercury (Hg), and lead (Pb) detected by atomic absorption spectrometry technique, in the Soil Laboratory of the State University of Maranhão.
Histopathological analysis of gills
The right gill arch of fish was removed and fixed in 10 % formaldehyde. The second gill arch was decalcified in nitric acid at 10 % for 6 h and then dehydrated in ascending series of alcohols (70, 80, and 90 %), diaphonized in xylene, impregnated, and embedded in paraffin in the Animal Morphophysiology Laboratory of the Chemistry and Biology Department, State University of Maranhão. Slides were made with 5-μm-thick cross sections, stained with hematoxylin and eosin (Luna 1968), and subsequently analyzed for gill lesions.
Histological alterations were semiquantitatively evaluated for each fish by calculating the histological alteration index (HAI), according to Poleksic and Mitrovic-Tutundzic (1994), based on the severity of each lesion and classified in progressive stages of tissue damages using the formula HAI = 1 × ∑ I + 10 × ∑ II + 100 × ∑ III (Table 1).
Genotoxic analysis in erythrocytes
Blood samples were collected with the aid of insulin-type syringes through gill puncture of the left arch, and blood smear slides were prepared. The slides were dried for 24 h in the laboratory and further stained with modified Rosenfield (Ranzaini-Paiva et al. 2013).
Two thousand cells per fish were analyzed under an optical microscope for counting the micronuclei (MN) and detecting nuclear abnormalities (NA) according to Fenech et al. (2003).
Data of MN between seasonal periods and between areas were compared by non-parametric test, Kruskal-Wallis, and Dun Method, using the BioEstat 2.0 software. The results were considered positive if a significant difference of at least p ≤ 0.05 was observed between data analyzed.
Results
In all, 23 specimens were collected in the Bacanga river basin dam, 14 and 9 during the rainy and dry seasons, respectively. In the Pau Deitado estuary, 16 samples were taken, 6 during the rainy season and 10 during the dry season. The number of males was equal to the number of females in the fish sample collected from both areas during the rainy season, while during the dry season the number of males collected was greater (Table 2).
Most samples for both sexes in both areas were in the early stage of gonadal maturation during the rainy season. During the dry season however, the fish sampled from the Bacanga river basin showed higher frequency of individuals, both sexes in stage I; in the Pau Deitado estuary, most males were immature, while most females were in gonadal maturation stage (Table 3).
Water analysis
The results of physicochemical analyses of the samples are shown in Table 4. Among the parameters examined, the concentration of dissolved oxygen in all the collections in the two areas of study was below the value established by CONAMA resolution 357/2005. The other parameter that was not in compliance with the standards set by the resolution was the pH, but only in the rainy season in the samples collected from the Bacanga river basin.
Analysis of metals revealed mercury and lead levels above those permitted by the resolution in the Bacanga river basin, both in the rainy and dry seasons. In the Pau Deitado estuary also, most elements were not in agreement with the resolution in both sample periods (Table 5).
Histopathology of gills
The gill structure of C. undecimalis is the same as that of teleost fish. Each gill arch is cartilaginous with a double row of gill filaments divided into lamellae, through which gas exchange occurs. The gill filaments have a stratified epithelium, comprising several types of cells such as chloral and mucous cells. The lamellae lining of the epithelium consists of a single layer of squamous cells, the basal blade of which is supported by the pillar cell system. Figure 2 shows normal gill of C. undecimalis (Santos et al. 2014).
The histological alterations found in the seabass specimens studied included detachment of the epithelium lining of the secondary lamella, disorganization of lamellae, hyperplasia of epithelial cells, complete or incomplete fusion of the lamellae, proliferation of mucus cells mainly in the filament apex, parasite presence, vascular alterations such as congestion of the pillar cell system, dilation of the venous sinus, and lamellar aneurysm (Fig. 3, Table 6).
Histological alterations in gills of Centropomus undecimalis collected during the rainy and dry seasons of 2015. a Detachment of the lamellar epithelium (1); hyperplasia of epithelial cells (2). b Hyperplasia of mucous cells in the filament apex (1). c Parasitic cisty (1). d Complete fusion of all lamellae (1)
Stage I alterations, in the Bacanga river basin, showed hyperplasia of epithelial cells in all fish sampled in both seasonal periods, while in the Pau Deitado estuary, the most common lesion was vascular congestion of the lamellae. For stage II alterations, the breaking of the pillar cell system was more frequent in the Bacanga river basin dam and, in the Pau Deitado estuary, the hyperplasia of mucus cells predominated.
The frequency of gills’ alterations and the average value of the histological alteration index per area is shown in Table 7, indicating moderate lesions in the gills of fish sampled.
In the different categories, most of the fish in both areas showed mild to moderate alterations (Table 8).
Genotoxic analysis
The blood smears of the specimens revealed MN and NA in the form of notched, lobed, blebbed, and binucleated cells (Fig. 4).
Table 9 contains averages of MN and NA frequency observed in erythrocytes of the sampled fish. Values for MN presented significant differences between the two locations in the rainy season. Here, the Bacanga river basin emerged as the more impacted site. Comparing the frequency of MN by seasonal period per area, the Bacanga river basin presented a significant difference in the results, with the most alterations being found in the rain. As for NA, the highest frequency in the sampled fish was during the rains in the Bacanga river basin, while in the Pau Deitado estuary, it was during the dry season.
Discussion
Histological lesions in gills are widely used for assessing the environmental quality of aquatic ecosystems, both in natural environments (Nogueira et al. 2008; Flores-Lopes and Thomaz 2011; Nogueira et al. 2011; Santos-Filho et al. 2014) and toxicological assays (Breseghelo et al. 2004). These biomarkers have been used in fish in Maranhão, Brazil, to assess the water quality of natural environments and fish farms (Carvalho-Neta et al. 2012; Pinheiro-Sousa et al. 2013; Pereira et al. 2014; Santos et al. 2014; Carvalho-Neta et al. 2015). Cantanhêde et al. (2014) were the first to apply these biomarkers in the species C. undecimalis in São Luís, Maranhão, Brazil, to assess the pollution of an estuarine environment, in an environmental protection zone.
The micronucleus test is also widely used in eco-toxicological biomonitoring, mainly for detecting alterations in cells after division. The micronuclei formation mechanism depends on the occurrence of cell division after exposure to the genotoxic agent. The time required for cell division depends on the tissue type, the species, and the environmental conditions. Centropomus and other fish species have already been used for this purpose successfully (Al-sabti and Metcalfe 1995; Kirschbaum et al. 2009; Sharma and Chadha 2016; Hussain et al. 2016).
The results obtained highlight the health of the environment, in particular, the health of the fish included in the study, because snooks are considered sentinels of environmental monitoring (Kirschbaum et al. 2009; Souza et al. 2013). Being carnivorous, snooks tend to accumulate high levels of contaminants in their diet. Carnivores are commonly used in the evaluation of environmental contamination since they have an intrinsic relationship with the entire lower food chain, indicating chronic, cumulative, and persistent effects on the chain level, in addition to direct effects on an individual level (Dórea et al. 2004; Durrieu et al. 2005; Kehring et al. 2009; Cui et al. 2011). The biological response to the exposure to contaminants can be assessed through histological and genotoxic alterations, as discussed above. Our tests indicated that fish investigated from both areas were affected.
One of the causes can be the high levels of metals recorded in the areas, particularly high mercury and lead, in all sampling periods, and the presence of high levels of aluminum and iron in S2, with values above that are allowed by the CONAMA resolution 357/2005, the rule that regulates the qualities for the water use in Brazil, considering estuarine water is used for food production and for recreation purposes. These results were also higher than the values reported for drinking water by the WHO—World Health Organization (2011).
Metals have peculiar atomic characteristics, conferring them high resistance to chemical, physical, and biological degradation in the aquatic system. This leads them to persist in the aquatic environment for several years, even after the ban on their use or disposal in watercourses (Moraes and Jordão 2002; Ikem et al. 2003). By persisting in the aquatic system, the metal concentration is gradually increased, which facilitates its highest concentration in the water and higher intake by organisms (Rodrigues et al. 2005; Rodrigues and Formoso 2006; Arai et al. 2007).
Among the metals, arsenic (As), cadmium (Cd), lead (Pb), chromium (Cr), copper (Cu), iron (Fe), nickel (Ni), manganese (Mn), mercury (Hg), and zinc (Zn) are the major elements studied in fish contamination (Lima Junior et al. 2002; Canli and Atli 2003; Ikem et al. 2003; Costa and Hartz 2009; Pereira et al. 2010; Cui et al. 2011; Gomes and Sato 2011; Muto et al. 2011; Yi et al. 2011; Yi and Zhang 2012). These metals have been reported to be responsible for disorders in fish, including low fertility, reduced immune defense, reduced growth rate, and pathologies that can be fatal (Queiroz 2006; Meneses 2008).
The linkage between metal and fish health previously recorded in literature enables us to discuss our data showing alterations in histopathology and genotoxicity. In gills, histological lesions were found in both areas and were more frequent in the rainy season. Nuclear alterations were also more common during the rainy season in the S1 area, the most anthropic-impacted region. These alterations may be associated with exposure to contaminants, as metals that are suspended and dissolved in the water can be absorbed by fish through diffusion through the gills (Miranda 2006; Muto et al. 2011; Kehring et al. 2011, Dragun et al. 2016). Lesions in gills have been reported earlier when fish were exposed to metals: Pantunga et al. (2008) for Clarias macrocephalus × Clarias gariepinus after Cd exposure; Figueiredo-Fernandes et al. (2007) for Oreochromis niloticus after Cu exposure; and Bomfim de Jesus et al. (2011) for Hoplias malabaricus after HgCl2 exposure. This kind of damage affects the organ functions, since the gill epithelium cells respond directly or indirectly to environmental factors and internal alterations of the organism (Lupi et al. 2007). Metals observed as genotoxic agents (HERAG 2007) act in redox cycles, producing reactive oxygen species (Stohs and Bagchi 1995); the oxidative stress affects several metabolic pathways, including those involved in the repair of DNA damage, known as genotoxic effects (Prá et al. 2006).
Mercury in particular, found in all the water samples, can be related to observed damages because it is a proven carcinogenic, mutagenic, and teratogenic element (Banerjee and Bhattacharya 1995; Hylander et al. 2000; Yokoo et al. 2001; Porto et al. 2004; Weech et al. 2004). Chemical and biological alterations and interactions in the aquatic environment can determine the ionization of mercury and facilitate the formation of methyl mercury (Hylander et al. 2000; Ravichandran 2004). Methyl mercury, in addition to being toxic, has high potential for bioaccumulation in the tissues (Souza Lima et al. 2000) and biomagnification in organisms through the food chain (Weech et al. 2004), besides posing a hazard for public health.
In our study, mercury level was higher in the rainy season; for both areas, the same trend was observed for lesions in the gills and nuclear alterations in the S1 area. This might be explained by the high flow of water during the rains, which in turn causes the maximum dilution of waste, providing higher rates of bioavailability of metals in the water (Bambic et al. 2006; Silva et al. 2009; Pizarro et al. 2010). The source of the pollutants in both areas is in all probability the discharge of domestic effluents and garbage, primarily in the Bacanga river basin due to its being surrounded by peripheral neighborhoods.
For gill histology, observed stages of damages were between I and II, considered mild to middle lesions. Stage I alterations allow recovery of structure and function of the gill tissues on improving environmental conditions. However, these can progress to the second stage when environmental conditions remain unchanged and there is long-term exposure to pollutants (Poleksic and Mitrovic-Tutundzic 1994). Stage I is related to response to contamination since it reduces the gill surface area and increases the diffusion barrier to pollutants. It is characterized by increased functions of cells and tissue, caused by alteration of their physiological activities; however, it can cause fusion of several lamellae and jeopardize the gas exchange in the organ (Takashima and Hibiya 1995; Erkmen and Kolankaya 2000; Winkaler et al. 2001; Thophon et al. 2003).
Stage II alterations are more severe and compromise the gill function; however, on improved water quality, these lesions may be reversible. However, if the pollution levels continue to increase, it can progress to the third stage. Aneurysm, a stage II alteration found in samples of the fish analyzed mainly in the Pau Deitado estuary, has been recorded in high frequency in the presence of toxic substances (Stentiford et al. 2003), usually resulting from collapse of the pillar cell system, which impairs vascular integrity and releases large amounts of blood, pushing the lamellar epithelium out (Hinton and Laurén 1990), which can cause disruption of the epithelium and consequently bleeding.
During the observation of the gills, we also found alterations caused by parasites. These indicate a broken equilibrium among the host-parasite environment, which may cause inflammation and cysts, affecting the health of the organism (Schalch et al. 2006; Campos et al. 2011).
In the micronucleus test analysis, fish specimens of the Bacanga (S1) river basin presented a higher frequency of micronuclei in erythrocytes when compared to Pau Deitado estuary (S2), in both seasonal periods. The fish in the S2 area showed less genotoxic damage. It is important to note here that, despite the metal contamination, S2 is far from the urban center and presents well-preserved vegetation. It is known that mangroves are rich in organic matter and sediment and that factors such as particulate matter and the presence of large amounts of organic matter contribute to balance the concentration of metals in the environment (Obasohan 2008; Kpee et al. 2009). The low concentration of dissolved oxygen in both areas is an indication that the amount of organic matter is high and promotes the multiplication of microorganisms, increasing oxygen consumption (Fiourucci and Benedetti-Filho 2005).
The level of dissolved oxygen can affect the health of the fish, because it is one of the most important parameters that express the quality of water of an aquatic environment (Ostrensky and Boeger 1998; Masser et al. 1993; Brasil 2006). The solubility of oxygen in water depends on different environmental factors such as temperature, atmospheric pressure, and salinity (Sipaúba-Tavares 1994), and the need of oxygen varies according to the species and its stage in life (Ostrensky and Boeger 1998).
In addition, a high concentration of lead is detrimental for the organisms, owing to its high toxicity (Bilandzic et al. 2011; Ersoy and Celik 2010). At high concentrations, lead causes behavioral deficits in fish, in addition to reduced growth and development, changes in metabolism, and increase in mucus formation (Meletii et al. 2003; Cestari et al. 2004; Ferraro et al. 2004; Martinez et al. 2004; Schmitt et al. 2007).
The fish specimens analyzed in the survey showed a proliferation of mucus cells in secondary lamellae. The proliferation and hypersecretion of mucous cells in the gills under stressful situations can be seen as a defense mechanism; however, this may compromise the gill function, depending on the severity (Leonardo et al. 2001; Fracácio et al. 2003).
Since the effects of lead are attributed to reduced development, the observed values of size of the individuals, the majority of which were young, could be related to the presence of this metal. However, because Centropomus is a migratory species, found in the estuarine region in the young age (Kirschbaum et al. 2009; Oliveira et al. 2014), this may not hold true. This migration may have also determined the sex ratio recorded with most being males (Vazzoler 1996). Snooks are protandric hermaphroditic organisms (Taylor et al. 2000); thus, one expects to find an increased proportion of male individuals in the smaller sizes and an increased proportion of females in larger sizes. Therefore, most of the sampled snooks were males in both areas. The proportion of males larger than females in a population of youths is a reproductive strategy of protandric hermaphrodites (Taylor et al. 2000; Perera-García et al. 2011).
The data discussed here draw attention to the impact of pollutants in Brazilian estuarine regions and highlight the urgency of actions to be taken to minimize the disposal of domestic sewage in these areas (Greene et al. 2014; Lazarus et al. 2015) It is well known that estuaries are among the richest and most important global ecosystems. They have a significant and important role in food production. The impacts on these ecosystems threaten the sustainability of estuarine resources all across the world (Chagnon et al. 2015; Mitra and Zaman 2016). Because estuaries are the nurseries for several species, the impact on estuarine areas may affect much larger regions of fauna.
Finally, the impacts on the health of the Centropomus from the estuarine coast of Maranhão need to be considered in the public health context. Since it is one of the most consumed fish worldwide, the metal accumulation by snooks can affect human health through its consumption as food (Yokoo et al. 2001; Mergler 2002), which is a major source of human exposure to heavy metals (Lebel et al. 1997; Tao et al. 2012).
Conclusion
Of the biomarkers analyzed, both genotoxic and histological markers proved as valuable tools for assessing the environmental quality of estuarine ecosystems using the species Centropomus undecimalis. The two estuarine ecosystems studied presented contamination with heavy metals. The fish exhibited gill damage, probably as a consequence of the metal contamination, which was more predominant in the rainy season. Genetic damage was also prevalent in the area, especially in Bacanga river (S1), that receives larger amount of domestic effluents and garbage.
In addition to assessing the health of commercial fish populations, the information generated on the biomarkers used in this study will serve to contribute to the preparation and/or application of health assessment models and implementation of environmental recovery policies for coastal aquatic environments. Here, we emphasize the importance of protecting the estuarine habitat which is one of richest ecosystems on earth.
References
Al-sabti K, Metcalfe CD (1995) Fish micronuclei for assessing genotoxicity in water. Mutat Res 343:121–135
Arai T, Ohji M, Hirata T (2007) Trace metal deposition in teleost fish otolith as an environmental indicator. Water Air Soil Pollut 179:255–263
Bambic DG, Alpers CN, Green PG, Fanelli E, Silk WK (2006) Seasonal and spatial patterns of metals at a restored copper mine site. I. Stream copper and zinc. Environ Pollut 144:774–782
Banerjee S, Bhattacharya S (1995) Histopathological changes induced by chronic nonlethal of elsan, mercury, and ammonia in the small intestine of Channa punctatus (Bloch). Ecotoxicol Environ Saf 31:62–68
Bilandzic N, Dokic M, Seda M (2011) Metal content determination in four fish species from the Adriatic Sea. Food Chem 124:1005–1010
Bomfim de Jesus T, Almeida PGA de, Vergílio CS, Machado ALS, Carvalho CEV (2011) Acute Intraperitoneal Mercury Chloride Contamination and Distribution in Liver, Muscle and Gill of a Neotropical Fish Hoplias malabaricus (BLOCK, 1794). Braz arch biol technol 54:379–386
Brasil—Ministério do Meio Ambiente—Conselho Nacional do Meio Ambiente (2005) Resolução n° 357, de 17 de março de 2005. Diário Oficial Da União, Brasília. http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=459 Accss 10/01/2016
Brasil—Ministério da Saúde—Secretaria de Vigilância em Saúde (2006) Vigilância e controle da qualidade da água para consumo humano. Ministério da Saúde, Brasília (Portuguese)
Breseghelo L, Cardoso MP, Borges-de-Oliveira R, Costa MF, Barreto JCB, Saboia-Morais SMTS, Yamada AT (2004) Efeitos do fluoreto de sódio no epitélio da brânquia do peixe guaru (Poecilia vivipara). Braz J Vet Res Anim Sci 41:274–280 Portuguese
Buss DF, Baptista DF, Nessimian JL (2003) Bases conceituais para a aplicação de biomonitoramento em programas de avaliação da qualidade da água de rios. Cad Saúde Pública 19:465–473 Portuguese
Campos CM, Moraes JRE de, Moraes FR de (2011) Histopathology of gills of Piaractus mesopotamicus (Holmberg, 1887) and Prochilodus lineatus (Valenciennes, 1836) infested by monogenean and myxosporea, caugth in Aquidauana River, State of Mato Grosso do Sul, Brazil. Rev Bras de Parasitol Vet 20:67–70
Canli M, Atli G (2003) The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ Pollut 121:129–136
Cantanhêde SM, Medeiros AM, Ferreira FS, Ferreira JRC, Alves LMC, Cutrim MVJ, Santos DMS (2014) Uso de biomarcador histopatológico em brânquias de Centropomus undecimalis (Bloch, 1972) na avaliação da qualidade da água do Parque Ecológico Laguna da Jansen, São Luís—MA. Arq Bras Med Vet Zoo 66:593–601 Portuguese
Carvalho-Neta RNF, Torres-Jr AR, Abreu-Silva AL (2012) Biomarkers in catfish Sciades herzbergii (Teleostei: Ariidae) from polluted and non-polluted areas (São Marcos’ Bay, Northeastern Brazil). Appl Biochem Biotechnol 166:1314–1327
Carvalho-Neta RNF, Sousa DBP, Macêdo-Sobrinho IC, Horton EY, Almeida ZS, Tchaicka L, Sousa AL (2015) Genotoxic and hematological parameters in Colossoma macropomum (Pisces, Serrasalmidae) as biomarkers for environmental impact assessment in a protected area in northeastern Brazil. Environ Sci Pollut Res 22(20)
Cerqueira VR, Machiavello JAG (1994) Comparação do crescimento de juvenis do robalo (Centropomus undecimalis) alimentados com uma dieta experimental e uma dieta comercial para truta. Simpósio Brasileiro De Aqüicultura, 7; Encontro Brasileiro De Patologia De Organismos Aquáticos, 3 (ed) Anais, Peruíbe, São Paulo (Portuguese)
Cestari MM, Lemos PMM, Ribeiro CAO, Costa JRMA, Pelletier E (2004) Genetic damage induced by trophic doses of lead in the neotropical fish Hoplias malabaricus (Characiformes, Erythrinidae) as revealed by the comet assay and chromosomal aberrations. Genet Mol Biol 27:270–274
Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, Sluijs JPV (2015) Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ Sci Pollut Res Int 22:119–134
Costa SC, Hartz SM (2009) Evaluation of trace metals (cadmium, chromium, copper and zinc) in tissues of a commercially important fish (Leporinus obtusidens) from Guaíba Lake, Southern Brazil. Braz Arch Biol Technol 52:241–250
Cui B, Zhang Q, Zhang K, Liu X, ZHANG H (2011) Analyzing trophic transfer of heavy metals for food webs in the newly-formed wetlands of the Yellow River Delta, China. Environ Pollut 159:1297–1306
Dórea JG, Barbosa A, Souzade J, Fadini P, Jardim WF (2004) Piranhas (Serrasamus spp.) as markers of mercury bioaccumulation in Amazon ecosystems. Ecotoxicol Environ Saf 59:57–63
Dragun Z, Tepić N, Krasnići N, Teskeredžić E (2016) Accumulation of metals relevant for agricultural contamination in gills of European chub (Squalius cephalus). Environ Sci Pollut Res Int 1–4. http://www.ncbi.nlm.nih.gov/pubmed/27194015 Access: June 16, 2016
Durrieu G, Maury-Brachet R, Boudou A (2005) Goldmining and mercury contamination of piscivorous fish Hoplias aimara in French Guiana (Amazon basin). Ecotoxicol Environ Saf 60:237–352
Erkmen B, Kolankaya D (2000) Effects of water quality on epithelial morphology in the gill of Capoeta tinca living in two tributaries of Kizilirmak River, Turkey. B Environ Contam Tox 64:418–425
Ersoy B, Celik M (2010) The essential and toxic elements in tissues of six commercial demersal fish from Eastern Mediterranean Sea. Food Chem Toxicol 48:1377–1382
Fenech M, Cheng WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Ferraro MVM, Fenocchio AS, Mantovani MS, Ribeiro CO, Cestari MA (2004) Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genet Mol Biol 27:103–107
Figueiredo-Fernandes A, Ferreira-Cardoso JV, Garcia-Santos S, Monteiro SM, Carrola J, Matos P, Fontaínhas-Fernandes A (2007) Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesq Vet Bras 27:103–109
Fiorucci AR, Benedetti-Filho E (2005) A importância do oxigênio dissolvido em ecossistemas aquáticos. Química Nova na Escola. (Portuguese) http://qnesc.sbq.org.br/online/qnesc22/a02.pdf Access: June 16, 2016
Flores-Lopes F, Thomaz AT (2011) Histopathologic alterations observed in fish gills as a tool in environmental monitoring. Braz J Biol 71:179–188
Fracácio R, Verani NF, Espíndola ELG, Rocha O, Rigolin-Sá O, Andrade CA (2003) Alterations on growth and gill morphology of Danio rerio (Piscis, Ciprinidae) exposed to the toxic sediments. Braz Arch Biol Technol 46:685–695
Gomes MVT, Sato Y (2011) Avaliação da contaminação por metais pesados em peixes do Rio São Francisco à jusante da represa de Três Marias, Minas Gerais, Brasil. Rev Saúde Amb 6:24–30 Portuguese
Greene CM, Blackhart K, Nohner J, Candelmo A, Nelson DN (2014) A national assessment of stressors to estuarine fish habitats in the contiguous USA. Estuaries and Coasts May 38:782–799
HERAG—HEALTH RISK ASSESSMENT GUIDANCE FOR METALS (2007) Mutagenicity. EBRC (European Business Reliance Centre), Luxembourg
Hinton DE, Laurén DJ (1990) Integrative histopathological approaches to detecting effects of environmental stressors on fishes. In: Adams SM (ed) Biological indicators of stress in fish. American Fisheries Society Symposium, Bethesda, pp. 51–66
Hylander LD, Meili M, Oliveira LJ, Castro-Silva E, Guimarães JRD, Araujo DM, Neves RP, Stachiw R, Barros AR, SILVA GD (2000) Relationship of mercury with aluminium, iron, and manganese oxy hydroxides in sediments from the Alto Pantanal, Brazil. Sci Total Environ 260:97–107
Hussain B, Sultana T, Sultana S, Mahboob S, Al-Ghanim KA, Nadeem S (2016) Variation in genotoxic susceptibility and biomarker responses in Cirrhinus mrigala and Catla catla from different ecological niches of the Chenab River. Environ Sci Pollut Res Int. http://www.ncbi.nlm.nih.gov/pubmed/27068917 Access: June 16, 2016
Ikem A, Egiebor NO, Nyavor K (2003) Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut 149:51–75
de Jesus TB, de Carvalho CEV (2008) Utilização de biomarcadores em peixes como ferramenta para avaliação de contaminação ambiental por mercúrio. Oecol Bras 12:680–693 Portuguese
Kehring HA, Fernandes KWG, Malm O, Seixas TG, Di Benedito APM, Souza CMM (2009) Transferência trófica de mercúrio e selênio na costa norte do Rio de Janeiro. Quim Nov. 32:1822–1828 Portuguese
Kehring HA, Malm O, Palermo EFA, Seixas TG, Baeta AP, Moreira I (2011) Bioconcentração e biomagnificação de metilmercúrio na baía de Guanabara, Rio de Janeiro. Quim Nov. 34:377–384 Portuguese
Kendall RJ, Anderson TA, Baker RJ, Bens CM, Carr JA, Chiodo LA, Cobb GP, Dickerson RL, Dixon KR, Frame LT, Hooper MJ, Martin CF, McMurry ST, Patino R, Smith EE, Theodorakis CW (2001) Environmental ecotoxicology. In: Klaassen CD (ed) Casarett and Doull’s toxicology: the basic science of poisons, 6th edn. Mcgraw-Hill, New York, p. 236
Kirschbaum AA, Seriani R, Pereira CDS, Assunção A, Abessa DMS, Rotundo MM, Ranzani-Paiva MJT (2009) Cytogenotoxicity biomarkers in fat snook Centropomus parallelus from Cananéia and São Vicente estuaries, SP, Brazil. Genet Mol Biol 32:151–154
Kpee F, Ozioma E, Ihunwo L (2009) Seasonal variation of Cd, Ni, Cu and Pb in catfish, sediment and water samples from Ipo Stream in Ikwerre District of Rivers State, Nigeria. J Appl Sci Environ Manag 13:63–67
Lazarus RS, Rattner BA, McGowan PC, Hale RC, Schultz SL, Karouna-Renier NK, Ottinger MA (2015) Decadal re-evaluation of contaminant exposure and productivity of ospreys (Pandion haliaetus) nesting in Chesapeake Bay regions of concern. Environ Pollut 205:278–290
Lebel J, Roulet M, Mergler D, Lucotfe M, Larribe F (1997) Fish diet and mercury exposure in a riparian Amazonian population. Water Air Soil Pollut 97:31–44
Lemos CT, Rodel PM, Terra NT, Oliveira NCD, Erdtmann B (2007) River water genotoxicity evaluation using micronucleus assay in fish erythrocytes. Ecotoxicol Environ Saf 66:391–401
Leonardo JMLO, Vargas L, Ribeiro RP, Moreira HLM, Natali MRM, Volski T, Cavichiolo F (2001) Histologia das brânquias de larvas de tilápia do Nilo, Oreochromis niloticus (L.) de origem tailandesa, submetidas a diferentes níveis de vitamina C. Acta Sci 23:863–870 Portuguese
Lima Junior RGS, Araújo FG, Maia MF, Pinto ASSB (2002) Evaluation of heavy metals in fish of the Sepetiba and Ilha Grande bays, Rio de Janeiro, Brazil. Environ Res Sect 89:171–179
Luna LG (1968) Manual of histologic staining methods of Armed Forces Institute of Pathology. McGraw-Hill Book Company, New York
Lupi C, Nhacarini NI, Mazon AF, Sá OR (2007) Avaliação da poluição ambiental através de alterações morfológicas das brânquias de Oreochromis niloticus (tilápia) nos córregos Retiro, Consulta e Bebedouro, município de Bebedouro-SP. Rev Fafibe on line 3:1–6 Portuguese
MARANHÃO. Decreto 12.103 de 01 de outubro de 1991. São Luís: D.O.E, de 01.10.1991, Ano LXXXV, n. 189 (Portuguese)
MARANHÃO. Decreto n° 7.545 de 07 de março de 1980. São Luís: D.O.E, de 21.03.1980, Ano LXXIII, n. 56 (Portuguese)
Martinez CBR, Nagae MY, Zaia CTBV, Zaia DAM (2004) Acute morphological and physiological effects of lead in the neotropical fish Prochilodus lineatus. Brazi J Biol 64:797–807
Masser MP, Cichra E, Gilbert RJ (1993) Fee-fishing ponds: management of foodfish and water quality. SRAC 480:1–8
Meletti PC, Rocha O, Martinez CBR (2003) Avaliação da degradação ambiental na bacia do rio Mogi-Guaçu por meio de testes de toxidade com sedimento e de análises histopatológicas em peixes. In: Brigante J, Espíndola ELG (ed) Limnologia Fluvial: um estudo no rio Mogi – Guaçú. São Carlos, São Paulo, pp 149–180 (Portuguese)
Meneses TS (2008) Fauna, pesca e contaminação por metais pesados em pescado no litoral de Sergipe. Dissertação, Universidade Tiradentes (Portuguese)
Mergler D (2002) Review of neurobehaviroral deficits and river fish consumption from the Tapajós (Brazil) and St. Lawrence (Canada). Environ Toxicol Phar 2:93–99
Miranda ALC (2006) Bioacumulação de poluentes organopersistentes (pops) em traíra (Hoplias malabaricus) e seus efeitos in vitro em células do sistema imune de carpa (Cyprinus carpio). Dissertação, Universidade Federal do Paraná (Portuguese)
Mitra A, Zaman S (2016) Threats to marine and estuarine ecosystems. Basics of Marine and Estuarine Ecology 12:365–417
Moraes DSL, Jordão BQ (2002) Degradação de recursos hídricos e seus efeitos sobre a saúde humana. Rev Saude Publica v: 370–374 (Portuguese)
Muto EY, Soares LSH, Sarkis JES, Hortellani MA, Petti MAV, Corbisier TN (2011) Biomagnificação de mercúrio na teia trófica marinha da baixada Santista (SP). Ocean Politicas Publicas 43:12–17 Portuguese
Nogueira DJ, Castro SC, Vieira RCA, Rigolin-Sá O (2011) Utilização das brânquias de Pimelodus maculatus (Lacèpéde, 1803) (Siluriformes; Pimelodidae) Como biomarcador de poluição no reservatório da UHE Marechal Mascarenhas de Moraes, Minas Gerais, Brasil. Rev Biotemas 24:51–58 Portuguese
Nogueira DJ, Castro SC, Sumaya C, Sá OR (2008) Avaliação da qualidade da água no reservatório UHE Furnas—MG, utilizando as brânquias de Pimelodus maculatus (LACÈPÉDE, 1803) como biomarcador de poluição ambiental. Rev Ciencia et Praxis 1:15–20 Portuguese
Obasohan EE (2008) Bioaccumulation of chromium, copper, maganese, nickel and lead in a freshwater cichlid, Hemichromis fasciatus from Ogba River in Benin City, Nigeria. Afr J Agric Res 4:141–152
Oliveira BL, Fernandes LFL, Bianchini A, Chippari-Gomes AR, Silva BF, Brandão GP, GOMES ALC (2014) Acute copper toxicity in juvenile fat snook Centropomus parallelus (Teleostei: Centropomidae) in sea water. Neotrop ichthyol 12:845–852
Ostrensky A, Boeger W (1998) Piscicultura: fundamentos e técnicas de manejo. Agropecuária, Guaíba (Portuguese)
Pacheco M, Santos MA (2002) Biotransformation, genotoxic, and histopathological effects of environmental contaminants in European eel (Anguilla anquilla L.). Ecotoxicol Environ Saf 53:331–347
Pantunga N, Helander KG, Helander HF, Cheevaporna Pantunga (2008) Histopathological Alterations of Hybrid Walking Catfish (Clarias macrocephalus × Clarias gariepinus) in Acute and Subacute Cadmium Exposure. Environ Asia 1:22–27
Pereira DP, Santos DMS, Carvalho Neta AV, Cruz CF, Carvalho Neta RNF (2014) Alterações morfológicas em brânquias de Oreochromis niloticus (Pisces, Cichlidae) como biomarcadores de poluição aquática na Laguna da Jansen, São Luís, MA (Brasil). Biosci J 30:1213–1221 Portuguese
Pereira P, Pablo H, Pacheco M, Vale C (2010) The relevance of temporal and organ specific factors on metals accumulation and biochemical effects in feral fish (Liza aurata) under a moderate contamination scenario. Ecotoxicol Environ Saf 73:805–816
Perera-García MA, Mendoza-Carranza M, Contreras-Sánchez WM, Huerta-Ortíz M, Pérez-Sánchez E (2011) Reproductive biology of common snook Centropomus undecimalis (Perciformes: Centropomidae) in two tropical habitats. Rev Bio Trop 59:669–681
Pinheiro-Sousa DB, Almeida ZS, Carvalho-Neta RNF (2013) Integrated analysis of two biomarkers in Sciades herzbergii (Ariidae, Siluriformes), to assess the environmental impact at São Marcos’ Bay, Maranhão. Lat Am J Aqua Res 41:305–312
Pizarro J, Vergara PM, Rodríguez JÁ, Valenzuela AM (2010) Heavy metals in northern Chilean rivers: spatial variation and temporal trends. Jo Hazard Mater 181:747–754
Poleksic V, Mitrovic-Tutundzic V (1994) Fishi gills as a monitor of sublethal and chronic effects of pollution. In: Muller R, Lloyd R (eds) Sublethal and chronic effects of pollutants on freshwater fish. Fishing News Books, Oxford, pp. 339–352
Porto JIR, Araujo CSO, Feldberg E (2004) Mutagenic effects of mercury pollution as revealed by micronucleus test on three Amazonian fish species. Environ Res. http://www.sciencedirect.com
Prá D, Guecheva T, Frank SIR, Knakievi CZT, Erdtmann B, Henriques JAP (2006) Toxicidade e genotoxicidade do sulfeto de cobre em planárias de água doce e camundongos. J Braz Ecotox 2:171–176 Portuguese
Queiroz MTA (2006) Bioacumulação de metais pesados no Rio Piracicaba, Minas Gerais, aplicando a análise por ativação Neutrônica Instrumental. Dissertação, Centro Universitário do Leste de Minas Gerais (Portuguese)
Ranzani-Paiva MJT, Pádua SB, Tavares-Dias M, Egami MI (2013) Métodos para análise hematológica em peixes. EdUEM, Maringá (Portuguese)
Ravichandran M (2004) Interations between mercury and dissolved organic matter—a review. Chemosphere 55:319–331
Rodrigues LS, Duarte RM, Val AL (2005) Determinação da sensibilidade ao cobre para a espécie de peixe amazônica Paracheirodon axelrodi, schultz 1956. In: Sociedade De Ecologia Do Brasil (ed) Anais. Congresso de Ecologia do Brasil, Caxambu, pp 3–5 (Portuguese)
Rodrigues MLK, Formoso MLL (2006) Heavy metals in recent sediments and bottom-fish under the influence of tanneries in south Brazil. Water Air Soil Pollut 176:307–327
Santos CHA, Lourenço JA, Braga Neto FHF, Costa OR, Igarashi MA (2006) Características dos ecossistemas estuarinos brasileiros e as atividades Antrópicas. In: IV Semana do Meio Ambiente da UFC (ed) Anais. UFC, Fortaleza, pp 71–85 (Portuguese)
Santos DMS, Melo MRS, Mendes DCS, Rocha IKBS, Silva JPL, Cantanhêde MC, Meletti PC (2014) Histological changes in gills of two fish species as indicators of water quality in Jansen Lagoon (São Luís, Maranhão State, Brazil). In J Environ Res Publ Health 11:12927–12937
Santos-Filho FM, Rezende KFO, Emerenciano AK, Moreira LM, Vila VB, Borges RM, Pressinotti LN (2014) Avaliação de biomarcadores histológicos em peixes coletados a montante e a jusante da mancha urbana. Atas Saúde Ambient 2:9–22 Portuguese
Schalch SHC, Moraes FR, Moraes JRE (2006) Efeitos do parasitismo sobre a estrutura branquial de Leporinus macrocephalus GARAVELLO E BRITSK, 1988 (Anastomidae) e Piaractus mesopotamicus HOLMBERG, 1987 (Osteichthyes: Characidae). Rev Bras de Parasitol Vet 15:110–115
Schmitt CJ, Brumbaugh WG, May TW (2007) Accumulation of metals in fish from lead–zinc mining areas of southeastern Missouri, USA. Ecotoxicol Environ Saf 67:14–30
Sharma M, Chadha P (2016) Study on DNA damaging effects of 4-nonylphenol using erythrocytes from peripheral circulation, gill and kidney of fish Channapunctatus. J Environ Biol 37:313–318
Silva DS, Lucotte M, Paquet S, Davison R (2009) Influence of ecological factors and of land use on mercury levels in fish in the Tapajós River basin, Amazon. Environ Res 109:432–446
Sipaúba-Tavares LH (1994) Limnologia aplicada à aquicultura. FUNEP, São Paulo (Portuguese)
Souza Lima AP, Müler RCS, de Sarkis JE S, Alves CN, Bentes MHS, Brabo E, Santos EO (2000) Mercury contamination in fish from Santarém, Pará, Brazil. Environ Res 83:117–122
Souza IC, Duarte IAND, Pimentel NQ, Rocha LD, Morozesk M, Bonomo MM, Azevedo VC, Pereira CDS, Monferrán MV, Milanez CRD, Matsumoto ST, Wunderlin DA, Fernandes MN (2013) Matching metal pollution with bioavailability, bioaccumulation and biomarkers response in fish (Centropomus parallelus) resident in neotropical estuaries. Environ Pollut 180:136–144
Stentiford GD, Longshaw M, Lyons BP, Jones G, Green M, Feist SW (2003) Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar Environ Res 55:137–159
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metals ions. Free Radic Biol Med 2:321–336
Takashima F, Hibiya T (1995) An atlas of fish histology. Normal and pathological features, 2a ed. Kodansha LTD, Tokyo
Tao Y, Yuan Z, Xiaona H, Wei M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicol Environ Saf 81:55–64
Taylor RG, Whittington JA, Grier HJ, Crabtree RE (2000) Age, growth, maturation, and protandric sex reversal in the common snook, Centropomus undecimalis, from South Florida waters. Fish Bull 98:612–624
Thophon S, Kruatrachue M, Upatham ES, Pokethitiyook P, Sahaphong S, Jaritkhuan S (2003) Histological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environ Pollut 121:307–320
Vazzoler AEM (1996) Biologia da reprodução de peixes teleósteos: teoria e prática. EDUEM/SBI/CNPQ/NUPELIA, Maringá (Portuguese)
Veiga ML, Rodrigues EL, Pacheco FJ, Ranzani-Paiva MJT (2002) Histopathologic changes in the kidney tissue of Prochilodus lineatus Valenciennes, 1836 (Characiformes, Prochilodontidae) induced by sublethal concentration of trichlorfon exposure. Braz Arch Biol Technol 45:171–175
Weech AS, Scheuhammer AM, Elliott JE, Cheng KM (2004) Environ Pollut 131:275–286
Winkaler EU, Silva AG, Galindo HC, Martinez CBR (2001) Biomarcadores histológicos e fisiológicos para o monitoramento da saúde de peixes de ribeirões de Londrina, Estado do Paraná. Acta Sci 23:507–514 Portuguese
WHO—World Health Organization (2011) Guidelines for drinking-water quality. 4th ed. WHO Library Cataloguing-in-Publication Data
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Yi Y, Zhang S (2012) Heavy metal (Cd, Cr, Cu, Hg, Pb, Zn) concentrations in seven fish species in relation to fish size and location along the Yangtze River. Environ Sci Pollut Res 19:3989–3996
Yokoo EM, Valente JG, Sichieri R, Silva EC (2001) Validation and calibration of mercury intake through self-referred fish consumption in riverine populations in Pantanal Mato-grossense, Braz. Environ Res 86:88–93
Acknowledgments
The authors acknowledge Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão—FAPEMA (Foundation for Research and Scientific and Technological Development of Maranhão) for granting the scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Cantanhêde, S.M., da Silva Castro, G., Pereira, N.J. et al. Evaluation of environmental quality of two estuaries in Ilha do Maranhão, Brazil, using histological and genotoxic biomarkers in Centropomus undecimalis (Pisces, Centropomidae). Environ Sci Pollut Res 23, 21058–21069 (2016). https://doi.org/10.1007/s11356-016-7294-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7294-9