Abstract
Microbial fuel cells (MFCs) have emerged as a promising technology for wastewater treatment with concomitant energy production but the performance is usually limited by low microbial activities. This has spurred intensive research interest for microbial enhancement. This study demonstrated an interesting stimulation effect of a static magnetic field (MF) on sludge-inoculated MFCs and explored into the mechanisms. The implementation of a 100-mT MF accelerated the reactor startup and led to increased electricity generation. Under the MF exposure, the activation loss of the MFC was decreased, but there was no increased secretion of redox mediators. Thus, the MF effect was mainly due to enhanced bioelectrochemical activities of anodic microorganisms, which are likely attributed to the oxidative stress and magnetohydrodynamic effects under an MF exposure. This work implies that weak MF may be applied as a simple and effective approach to stimulate microbial activities for various bioelectrochemical energy production and decontamination applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The merit of microbial fuel cells (MFCs) for simultaneous electricity generation and wastewater treatment has been widely recognized (Lovley 2008; Pant et al. 2012; Li et al. 2014). Application of MFCs for wastewater treatment offers great advantages because the small-amount of in situ generated electricity can be effectively utilized to enhance biodegradation and biotransformation of toxic chemicals (Luo et al. 2009; Mathuriya 2014). MFC performance can be affected by many factors, such as cell configuration (Di Lorenzo et al. 2010; Li et al. 2011a), microbial community (Borole et al. 2009, 2011), electrode materials (Zhou et al. 2011; Mohanakrishna et al. 2012), and substrate types (Pant et al. 2010). Especially, the electrochemical activity of anodic microorganisms is usually limiting in practical application (Pham et al. 2009; Lovley 2011).
Various physical, chemical and biological approaches have been explored to “activate” the anodic microorganisms in MFCs and improve electricity generation (Sun et al. 2011; Wang et al. 2013; Xiang et al. 2009; Zhang et al. 2015). Especially, magnetic fields (MFs) stimulation offers a potentially low-cost and convenient approach to enhance microbial activity, since a static MF can be continuously and conveniently applied to any biosystem without need for extra energy input (Ji et al. 2010; Filipic et al. 2012). Previous studies have demonstrated that implementation of an appropriate MF could raise microbial activity and accelerate contaminant degradation in wastewater treatment systems (Liu et al. 2008; Kriklavova et al. 2014; Zaidi et al. 2014). MFs have also been found to promote bioelectrocatalytic activities of several enzyme assemblies linked to electrodes through accelerating electron transfer at electrode-solution interfaces (Katz et al. 2005). Our previous study showed that MF could enhance the power generation of Shewanella-inoculated single-chamber MFCs (Li et al. 2011b). However, such pure-culture system has poor robustness and need sterilization of wastewater. For practical application, MFCs with mixed-culture inoculums would be essential (Wang et al. 2012; Solanki et al. 2013). A recent study showed that MF could also exert positive impacts on two-chamber MFCs with activated sludge (Tao and Zhou 2014), but the application range and stimulation mechanisms of such approaches remain unclear. In this study, we provide further evidences to show that such an MF stimulation technology is also applicable for single-chamber MFCs and shed light into the underlying stimulation mechanism. A weak static MF (100-mT) was applied at the anode proximity. Electrochemical and fluorescence spectroscopic analyses were performed to explore the mechanisms of the MF enhancement effects. This work suggests a high potential of applying a weak MF as a cost-effective and convenient strategy to enhance MFC performance.
Materials and methods
MFC assembly and operation
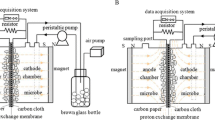
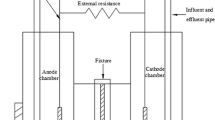
The single-chamber air–cathode MFC had a working volume of 125 mL and was composed of a carbon-paper bioanode and a Pt-coated carbon-cloth cathode separated by a proton exchange membrane (PEM, GEFC-10 N, GEFC Co., China). The effective surface areas of the anode and cathode were 16 and 25 cm2, respectively. The PEM separator was attached to the cathode surface. A 100-mT static MF was applied by binding a magnet onto the external chamber wall near the anode side to constitute an MF-coupled MFC (hereafter denoted as MF system). The schematic and photograph of the MF system are shown in Fig. 1. Another identical MFC without applying MF was run as the control.
Each MFC was inoculated with 10 mL of mixed sludge, collected from a lab-scale upflow anaerobic sludge blanket reactor and an activated sludge reactor. The anodic chamber was filled with 100 mL synthetic wastewater (culture medium) containing 1000 mg L−1 acetate as the substrate. The wastewater composition was as described by Sun et al. (2008). During the experiment, the culture medium was replaced by fresh one once the voltage declined to below 50 mV. All the MFCs were operated in an incubator at 25 ± 0.5 °C. The voltage across a 1000-Ω resistor was measured every 12 h by using a multimeter and the average value of two measurements was used.
Analysis
Polarization curves were recorded by varying the external resistance from 10,000 to 10 Ω at the relatively steady stage of electricity generation. In order to detect the possible redox mediators secreted by the microbes, the culture medium was subjected to electrochemical fluorescence spectral analysis at the end of the experiment. Cyclic voltammetry of the medium was measured using an electrochemical workstation (CHI660C, Shanghai Chenghua Instruments Co., China) at a scan rate of 5 mV s−1 in a beaker. A graphite rod, a Ag/AgCl electrode and a platinum wire were used as the working, reference and counter electrodes, respectively.
The culture medium, after centrifugation (10 min at 5000×g) and filtration through a 0.45-μm acetate cellulose membrane, was analyzed by three-dimensional emission-excision matrix (EEM) fluorescence spectrometry. EEM spectra are a collection of a series of emission spectra over a range of excitation wavelengths, which can be used to identify the fluorescent compounds present in solution (Wang et al. 2009). In this study, the EEM analysis was performed using a luminescence spectrometer (LS-55, Perkin-Elmer Co., USA), following the procedures proposed by Sheng and Yu (2006). The EEM spectra were collected with subsequent scanning emission spectra from 300 to 550 nm at 0.5 nm increments by varying the excitation wavelength from 200 to 400 nm at 10 nm increments. Excitation and emission slits were maintained at 10 nm and the scanning speed was 1200 nm min−1. The EEM data were processed by MatLab 2007b software (MathWorks Inc., USA).
Results and discussion
Electricity generation
The output voltages of the MFCs in four consecutive operating cycles were illustrated in Figure 2. The start-up of the control MFC took 6 days, while the voltage in the MF system increased rapidly to a high level within just 2 days, suggesting that the MFC start up was accelerated by the MF, which was consistent with a previous report (Tao and Zhou 2014). It is suggested that such an acceleration was due to the creation of a more favorable environmental for growth and enrichment of electro-active microorganisms under the MF exposure. Notably, the maximum voltages of both systems were comparable in the first three running cycles, but a much higher voltage of the MF system was observed in Cycle 4. This results indicates that the MF application also led to increased electricity generation, but a relatively long influential time was needed. Considering that an immediate stimulation effect was observed in our previous study with pure culture inoculum (Li et al. 2011a), this result suggests that a longer period of adaptation is required for the mixed culture to establish a high electrochemical activity at the anode. Such an MF effect on other biochemical processes has also been reported previously. For example, an MF with moderate magnetic density was found to promote the bio-removal of toxic Cr(VI) in anaerobic sequencing batch reactor systems (Sun and Xu 2010). Increased biodegradation of formaldehyde by activated sludge was achieved under a static MF exposure (Łebkowska et al. 2011). In addition, an MF was also found to alleviate the toxicological effect induced by cadmium in mungbean seedlings (Chen et al. 2011).
Internal resistance
The MF stimulation effect was also evidenced by the power density and polarization curves of the MFCs. The MF system showed a maximum power density of around 25 mW m−2, which was ~2.5 fold higher than the control (Fig. 3). It can be also seen from the power density curves that the ohm resistances of both MFCs were very close. However, the polarization curves reveal a much smoother initial voltage drop in the MF system over the control, implying a decreased activation loss under the MF (Logan et al. 2006), which agrees well with a previous report (Yin et al. 2013). Given that the influential area of the MF was limited to only the anode vicinity and all the other conditions are identical, here the decreased activation overpotential under MF should be ascribed to enhanced electrochemical activity of the anodic microorganisms (Logan et al. 2006).
Soluble redox mediator secretion
Many bacteria can secrete redox compounds that act as electron mediators to assist electrochemical interaction with an electrode (Torres et al. 2010; Lovley 2008). To find out whether the MF effect work by such a mechanism, we characterized the culture medium at the end of the experiment by voltammetry and three-dimensional EEM spectroscopy.
The EEM spectra of both systems showed only one peak at the excitation/emission wavelength (Ex/Em) of 260/380 nm (Fig. 4), which could be ascribed to the protein-like substances in soluble microbial products (SMPs) (Sheng and Yu 2006). No peak of flavins (a common electron shuttle, typically occurring at Ex/Em of 450/525 nm), was observed in both MFCs, indicating that the improved power generation was not due to increased redox mediator secretion.
The irrelevance of mediator secretion with the MF effect was further proven by the cyclic voltammetry (CV) result (Fig. 5). The media from both MFCs showed a redox couple at the same position (i.e., an oxidation peak at approximately −200 mV and a reduction peak at −290 mV) with similar peak density. Thus, the possibility of MF-induced secretion of redox mediators under MF exposure can be ruled out, and the promotion effects of MF should be ascribed to other mechanisms that enhance the metabolism or extracellular electron transfer of anodic microorganisms.
Possible mechanisms of microbial electrochemical activity simulation by MFs
The MF effects here might be associated with an oxidative stress response of the anodic microorganisms. It has been previously reported that an MF exposure could increase the activity, concentration and lifetime of oxidative metabolites and free radicals (Jones et al. 2007), or improve the activity of some enzymes (Afanasyeva et al. 2006). In addition, a magnetohydrodynamic effect might have also contributed to the improved microbial electrochemical activity (Katz et al. 2005). Specifically, the MF may decrease the thickness of the diffusion double layer at the solution-biofilm interface (Katz et al. 2005), thereby promoting mass transfer and bioelectrocatalytic oxidation of substrate. In all, oxidative stress and magnetohydrodynamic effects could be important mechanisms accounting for the MF stimulation effects in this study, although the exact mechanisms are yet to be clarified.
Conclusions
The implementation of a 100-mT MF at the MFC anode distinctly accelerated the reactor startup and increased electricity generation via enhancing bioelectrochemical activities of the anodic microorganisms. The CV and EEM spectroscopy revealed no distinct differences in concentration of redox mediator in the culture medium, suggesting the MF did not induce redox mediator secretion. Oxidative stress and magnetohydrodynamic effects are likely the major reasons accounting for the elevated bioelectrochemical activity under MF exposure. This study suggests that MF stimulation may offer a simple and cost-efficient approach to stimulate the electrochemical-active microorganisms in various bioelectrochemical energy production and remediation applications.
References
Afanasyeva MS, Taraban MB, Purtov PA, Leshina TV, Grissom CB (2006) Magnetic spin effects in enzymatic reactions: radical oxidation of NADH by horseradish peroxidase. J Am Chem Soc 128:8651–8658
Borole AP, Hamilton CY, Vishnivetskaya T, Leak D, Andras C (2009) Improving power production in acetate-fed microbial fuel cells via enrichment of exoelectrogenic organisms in flow-through systems. Biochem Eng J 48:71–80
Borole AP, Reguera G, Ringeisen B, Wang ZW, Yj Feng, Kim BH (2011) Electroactive biofilms: current status and future research needs. Energy Environ Sci 4:4813–4834
Chen YP, Li R, He JM (2011) Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 20:760–769
Di Lorenzo M, Scott K, Curtis TP, Head IM (2010) Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem Eng J 156:40–48
Filipic J, Kraigher B, Tepus B, Kokol V, Mandic-Mulec I (2012) Effects of low-density static magnetic fields on the growth and activities of wastewater bacteria Escherichia coli and Pseudomonas putida. Bioresour Technol 120:225–232
Ji Y, Wang Y, Sun J, Yan T, Li J, Zhao TT, Yin XH, Sun CJ (2010) Enhancement of biological treatment of wastewater by magnetic field. Bioresour Technol 101:8535–8540
Jones AR, Hay S, Woodward JR, Scrutton NS (2007) Magnetic field effect studies indicate reduced geminate recombination of the radical pair in substrate-bound adenosylcobalamin-dependent ethanolamine ammonia lyase. J Am Chem Soc 129:15718–15727
Katz E, Lioubashevski O, Willner I (2005) Magnetic field effects on bioelectrocatalytic reactions of surface-confined enzyme systems: enhanced performance of biofuel cells. J Am Chem Soc 127:3979–3988
Kriklavova L, Truhlar M, Skodova P, Lederer T, Jirku V (2014) Effects of a static magnetic field on phenol degradation effectiveness and Rhodococcus erythropolis growth and respiration in a fed-batch reactor. Bioresour Technol 167:510–513
Łebkowska M, Rutkowska-Narozniak A, Pajor E, Pochanke Z (2011) Effect of a static magnetic field on formaldehyde biodegradation in wastewater by activated sludge. Bioresour Technol 102:8777–8782
Li WW, Sheng GP, Liu XW, Yu HQ (2011a) Recent advances in the separators for microbial fuel cells. Bioresour Technol 102:244–252
Li WW, Sheng GP, Liu XW, Cai PJ, Sun M, Xiao X, Wang YK, Tong ZH, Dong F, Yu HQ (2011b) Impact of a static magnetic field on the electricity production of Shewanella-inoculated microbial fuel cells. Biosens Bioelectron 26:3987–3992
Li WW, Yu HQ, He Z (2014) Towards sustainable wastewater treatment technologies by using microbial fuel cells–centred technologies. Energy Environ Sci 7:911–924
Liu S, Yang F, Meng F, Chen H, Gong Z (2008) Enhanced anammox consortium activity for nitrogen removal: impacts of static magnetic field. J Biotechnol 138:96–102
Logan BE, Hamelers B, Rozendal RA, Schrorder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Lovley DR (2008) The microbe electric: conversion of organic matter to electricity. Curr Opin Biotechnol 19:564–571
Lovley DR (2011) Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ Sci 4:4896–4906
Luo HP, Liu GL, Zhang RD, Jin S (2009) Phenol degradation in microbial fuel cells. Chem Eng J 147:259–264
Mathuriya AS (2014) Eco-affectionate face of microbial fuel cells. Crit Rev Environ Sci Technol 44:97–153
Mohanakrishna G, Mohan SK, Mohan SV (2012) Carbon based nanotubes and nanopowder as impregnated electrode structures for enhanced power generation: evaluation with real field wastewater. Appl Energy 95:31–37
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543
Pant D, Singh A, Van Bogaert G, Olsen SI, Nigam PS, Diels L, Vanbroekhoven K (2012) Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv 2:1248–1263
Pham TH, Aelterman P, Verstraete W (2009) Bioanode performance in bioelectrochemical systems: recent improvements and prospects. Trends Biotechnol 27:168–178
Sheng GP, Yu HQ (2006) Characterization of extracellular polymeric substances of aerobic and anaerobic sludge using three-dimensional excitation and emission matrix fluorescence spectroscopy. Water Res 40:1233–11239
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresour Technol 131:564–571
Sun SY, Xu YB (2010) Effect of stable weak magnetic field on Cr(VI) bio-removal in anaerobic SBR system. Biodegradation 19:455–462
Sun M, Sheng GP, Zhang L, Liu XW, Xia CR, Mu ZX, Wang HL, Yu HQ, Qi R, Yu T, Yang M (2008) An MEC-MFC-coupled system for biohydrogen production from acetate. Environ Sci Technol 42:8095–8100
Sun M, Mu ZX, Sheng GP, Liu XW, Zhang L, Xia CR, Wang HL, Tong ZH, Yu HQ (2011) Effects of a transient external voltage application on the bioanode performance of microbial fuel cells. Electrochim Acta 55:3048–3054
Tao QQ, Zhou SQ (2014) Effect of static magnetic field on electricity production and wastewater treatment in microbial fuel cells. Appl Microbiol Biotechnol 98:9879–9887
Torres CI, Marcus AK, Lee HS, Parameswaran P, Krajmalnik-Brown R, Rittmann BE (2010) A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev 34:3–17
Wang ZW, Wu ZC, Tang SJ (2009) Characterization of dissolved organic matter in a submerged membrane bioreactor by using three-dimensional excitation and emission matrix fluorescence spectroscopy. Water Res 43:1533–1540
Wang YP, Liu XW, Li WW, Li F, Wang YK, Sheng GP, Zeng RJ, Yu HQ (2012) A microbial fuel cell-membrane bioreactor integrated system for cost-effective wastewater treatment. Appl Energy 98:230–235
Wang HY, Wang GM, Ling YC, Qian F, Song Y, Lu XH, Chen SW, Tong YX, Li Y (2013) High power density microbial fuel cell with flexible 3D graphene-nickel foam as anode. Nanoscale 5:10283–10290
Xiang K, Qiao Y, Ching CB, Li CM (2009) GldA overexpressing-engineered E. coli as superior electrocatalyst for microbial fuel cells. Electrochem Commun 11:1593–1595
Yin Y, Huang GT, Tong YR, Liu YD, Zhang LH (2013) Electricity production and electrochemical impedance modeling of microbial fuel cells under static magnetic field. J Power Sources 237:58–63
Zaidi NS, Sohaili J, Muda K, Sillanpaa M (2014) Magnetic field application and its potential in water and wastewater treatment systems. Sep Purif Rev 43:206–240
Zhang CY, Liang P, Jiang Y, Huang X (2015) Enhanced power generation of microbial fuel cell using manganese dioxide-coated anode in flow-through mode. J Power Sources 273:580–583
Zhou M, Chi M, Luo J, He H, Jin T (2011) An overview of electrode materials in microbial fuel cells. J Power Sources 196:4427–4435
Acknowledgments
The authors wish to thank the Natural Science Foundation of China (51278479 and 21261160489) for the partial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tong, ZH., Yu, HQ., Li, WW. et al. Application of a weak magnetic field to improve microbial fuel cell performance. Ecotoxicology 24, 2175–2180 (2015). https://doi.org/10.1007/s10646-015-1545-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1545-2