Abstract
The effects of a static magnetic field (MF) on the electricity production and anodic bioelectrochemical activity in Geobacter sulfurreducens-inoculated microbial fuel cells (MFCs) were investigated. The results showed that application of a MF of 100, 200, and 400 mT improved the electricity generation considerably, with an increase in the maximum power density by 27–100 %. The maximum power density of 0.030 mW cm−2 was obtained at field strength of 100 mT, representing the best MFC performance with the lowest internal resistance of 453.6 Ω. Electrochemical impedance spectroscopy results showed that the MFC activation resistance was decreased under a MF. The impact of a MF on the bioelectrochemical activity of Geobacter-based biofilm was analyzed by cyclic voltammetry (CV). The redox peak currents of the voltammetric behavior increased in the presence of a MF, indicating enhanced bioelectrochemical catalyzed activity. In addition, evidence from CV revealed higher density of electroactive species in the anodic biofilm with MF than without, leading to a more efficient direct electron transfer process by G. sulfurreducens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cell (MFC) technology enables renewable bioelectricity generation from biodegradable material using bacteria as catalysts [1]. Its most promising application is treatment of organic wastewater with simultaneous energy recovery [2, 3]. One of the main issues related to MFCs is the inefficient electron transfer process from the exoelectrogen cells to the anode, which results in low power efficiency performance. Many efforts have been made to improve the electricity production performance of MFCs [4, 5]. Application of a static magnetic field (MF) has been investigated as a new method to affect the bioelectrochemical activity and growth of bacteria [6, 7]. Liu et al. [8] demonstrated that the anaerobic ammonium oxidizing activity of anammox consortium was improved under a MF of 60 mT. In addition, it has been proved that application of a MF is a promising approach to enhance biological wastewater treatment without extra energy input [9]. Zhou et al. [10] prepared novel magnetic Fe3O4/polyurethane foam composites as carriers for immobilized microorganisms to increase the biomass and enhance the chemical oxygen demand (COD) removal efficiency of toluene-containing wastewater. Moreover, researchers found that a static MF can be effective in numerous instances to improve the performance of MFCs [11]. Li et al. [12] reported that application of a MF of 100 mT increased the electricity production by Shewanella-inoculated MFCs, with the maximum voltage promoted by about 20–27 %. Our previous study proved the positive impact of a MF on the electricity production by dual-chamber MFCs inoculated with equalization tank effluent from a sewage treatment plant [13]. However, the mechanism of this magnetic–biologic stimulation effect on the anode biofilm and exoelectrogenic electron transfer process is still not clearly understood.
Cyclic voltammetry (CV) is a powerful electrochemical method used in MFC research [14, 15]. It offers a first view on electrode/electrolyte systems in the potential window of interest [16, 17]. CV can be used to determine the catalysis effect of modified electrodes [18, 19] or to detect mediators corresponding to microorganism metabolic products [20]. Moreover, anodic biofilm characterization using CV can determine the level of exoelectrogenic growth and the bioelectrochemical activity of the exoelectrogen [21]. Many CV studies on Geobacter sulfurreducens have proved that the electricity generation by this typical exoelectrogen can be attributed to a direct electron transfer process by this bacterium attached to an electrode surface [22, 23]. For Geobacter sp., electroactive species in a conductive biofilm matrix (e.g., cytochrome) act as electron transfer mediators in this direct electron transfer process. Through analysis of CV data, the type of electrode reaction can be identified, and even the surface concentration of electroactive species can be quantified [24, 25].
To provide further evidence for the effect of a magnetic field and shed light on the underlying mechanism, the electricity production by and anodic bioelectrochemical activity of dual-chamber MFCs inoculated with G. sulfurreducens under different MF intensities were investigated in this study. The change of the internal resistance of the MFC was determined by polarization curve and electrochemical impedance spectroscopy (EIS) methods. CV was used to explore the influence of the magnetic field on the bioelectrochemical activity and extracellular electron transfer process of the biofilm. The results could provide valuable information for further investigation on the mechanism of MFCs.
Experimental
MFC configuration and operation
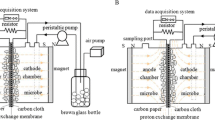
A demountable H-type reactor (Fig. 1) was used throughout this study. The reactor consisted of two 140-mL chambers separated by a proton exchange membrane (Nafion TM117, Ø1.5 cm; Dupont Co., Delaware, USA) and fixed by socket flanges with a clamp in the middle of the glass tube (inner diameter 0.8 cm). For both the anode and cathode, we adopted plain carbon cloth electrodes (3 × 3, 9 cm2) connected by titanium wires with separation of 9 cm. The setups used in the study are denoted as MFC0, MFC1, MFC2, and MFC3, having a static MF of 0, 100, 200, and 400 mT, respectively. To apply the MF, magnets were bound onto the outside of the anode chamber, parallel to the anode. All reactors were autoclaved at 0.1 MPa and 121 °C before the experiments. MFCs were inoculated with 30 % (v) G. sulfurreducens during the startup phase.
The anolyte contained 1.5 g L−1 ammonium chloride (NH4Cl), 0.1 g L−1 potassium chloride (KCl), 0.82 g L−1 sodium acetate (NaAc), vitamin solution (10/1000 v/v), mineral solution (10/1000 v/v), and phosphate buffer solution (PBS, 50 mM) [26]. The anolyte was sparged with mixed N2:CO2 gas (volume ratio 4:1) for about 10 min before injection into the anodic chamber. The catholyte was 100 mM K3Fe(CN)6 in PBS (50 mM, pH 7.2). All experiments were conducted at temperature of 28 ± 2 °C.
MFC analysis
The MFCs started up with fixed resistance of 1000 Ω, and the voltage output was recorded every 5 min using a data acquisition system (Keithley 2701, Tektronix, Inc., USA). Polarization and power density curves were determined by the steady-state method to investigate the influence of a static MF on the electricity production by the MFC after startup was completed. The steady-state method was applied using a variable-resistance box to change the external resistance from 2000 to 20 Ω while obtaining the voltage U (mV) and current density I (mA m−2). The power density (mW cm−2) was calculated according to the equation P = U × I.
EIS and CV measurements were carried out after MFC startup had finished using an electrochemical workstation (PARSTAT 2273, Ametek, Inc., USA). The two-electrode mode for EIS was adopted to analyze the whole MFC, as mentioned in literature [13]. The three-electrode mode was used to measure the voltammetric behavior of the anodic biofilm over the potential range from −0.7 to +0.7 V at scan rate of 5 mV s−1. CV was conducted in the anodic chamber with PBS as electrolyte. The anode was used as the working electrode with a platinum counterelectrode. A saturated calomel electrode (SCE) was used as the reference electrode, placed in the anode chamber. All experiments were performed under strictly anoxic conditions. CV curves were analyzed using PowerSuite software (Ametek, Inc., USA).
Results and discussion
Startup performance of MFCs
The voltage generation by the four reactors during incubation is shown in Fig. 2. The trends of these curves are consistent with the typical startup process of an MFC inoculated with G. sulfurreducens [27]. After circuit connection was established, the voltage increased slowly, indicating the adaptation period of the bacteria, then increased sharply to a stable phase. The voltage jump for MFC2 occurred on day 3, and startup finished on day 5, representing the fastest startup with this MF of 200 mT. For MFC1, under 100 mT, the voltage achieved stability on day 6, compared with about 8 days for MFC0 and MFC4. Such an acceleration effect of a magnetic field on the startup time was also found for MFCs inoculated with mixed culture, reflecting promotion of enrichment and proliferation of electrogenesis [13]. In addition, it can be seen from Fig. 2 that the voltage outputs in the presence of the magnetic fields with external resistance of 1000 Ω were higher than for MFC0. It is conjectured that the role of the MF is to improve the bioelectrochemical activity and reduce the anode activation loss and internal resistance.
Polarization and power density curves
Polarization and power density curves were obtained for the examined MFCs and are shown in Fig. 3. As the external resistance was varied from 2000 to 20 Ω, the current density increased while the cell voltage decreased. It can be seen that the current density and closed-circuit voltage of the MFCs under the magnetic fields at different external resistances were all higher than the values for MFC0, indicating lower internal resistance. The polarization curve represents the voltage as a linear function of current density, from which the internal resistance can be obtained as the slope [28]. The maximum power density and internal resistance of the MFCs obtained based on such calculations are shown in Fig. 4. The maximum power density of the reactor with MF of 100, 200, and 400 mT was 0.03, 0.024, and 0.019 mW cm−2, respectively, being increased by 100, 60, and 27 % compared with MFC0. Corresponding to these results, the internal resistance of MFC1, MFC2, and MFC3 was 453.6, 610.5, and 686.1 Ω, respectively, lower than that for MFC0 of 736.9 Ω. The electricity production performance of the MFCs exposed to a MF was obviously enhanced. Nevertheless, it was found that the maximum power density of the G. sulfurreducens-inoculated MFCs was lower compared with mixed culture-inoculated MFCs [13]. This may be due to the poor robustness and lack of biomass communal synergism in this pure culture system. Additionally, the results show that the effects of 400 mT were lesser than those of 100 and 200 mT. These results are consistent with other studies [13, 29], indicating that there is an optimal MF intensity range for microorganisms.
Effect of MF by EIS analysis
After the reactors had finished startup, EIS of the whole cells was carried out; the results are shown in Fig. 5. Two circular arcs appeared in the Nyquist plots for the MFCs. Electrode potential losses originate from three sources: ohmic, activation, and mass transfer losses [1]. Thus, the equivalent circuit R 1(R 2 Q 2)(R 3 Q 3) was applied to analyze the components of the internal resistance, where R 1 represents the ohmic resistance, R 2 and Q 2 in parallel represent the total activation resistance and the double-layer capacitance for the anode and cathode, while R 3 and Q 3 in parallel are related to the finite diffusion. It can be seen that the arcs can be well fit over the whole frequency range. The chi-squared (χ 2) test parameter for MFC0, MFC1, MFC2, and MFC3 was 1.26 × 10−4, 5.24 × 10−5, 2.86 × 10−5, and 2.84 × 10−5, respectively, implying that the equivalent circuit is suitable for EIS analysis.
The resistance components were obtained by fitting and are presented in Table 1. We chose the activation resistance to investigate the influence of a MF on the microbial catalytic reaction. It can be seen that the activation resistance of MFC1, MFC2, and MFC3 was much lower than for MFC0, being reduced by 70.58, 91.81, and 78.95 %, respectively. The lowest value was 46.29 Ω for MFC2 with MF of 200 mT. In this work, all the MFCs exhibited similar cathodic behavior. Therefore, it can be conjectured that the decreased activation resistance can be attributed to the differences in the anode. This finding of considerably decreased activation resistance in the presence of a static MF is the same as in our previous study on mixed culture-inoculated MFCs, indicating that the key effect of a MF on the MFC performance is stimulation of the anodic bioelectrochemical process.
Cyclic voltammetric test of anodic biofilm
Cyclic voltammetry tests were conducted to further investigate this change in the bioelectrochemical activity of the anodic biofilm. In Fig. 6, an oxidation peak over the range from 450 to 550 mV in the forward (positive) direction and a reduction peak over the range from −50 to −150 mV in the reverse (negative) direction are present in the voltammograms of the anodic biofilm. It is believed that these redox couple peaks in the voltammetric behavior of the anodic biofilm indicate the bioelectrochemical activity of the electroactive species in the Geobacter-based biofilm. The electroactive species plays a critical role in transferring electrons from the substrate to the anode. It can be seen that MFC2 with MF of 200 mT showed the highest redox peak current, demonstrating enhanced bioelectrochemical activity for this appropriate MF intensity.
To further understand the voltammetric behavior of the anodic biofilm, CV tests of the MFCs were carried out at different scan rates (taking MFC2 as an example). In Fig. 7, it can be seen that the redox peak current obviously increased with the scan rate. The plot of peak oxidation current versus scan rate (Fig. 7, inset) reveals a linear correlation (R 2 = 0.998), indicating typical surface-controlled electrochemical processes [19]. This voltammetric behavior is similar to that of metalloproteins or redox enzymes adsorbed on an electrode surface [30]. Thus, Laviron’s model can be used to estimate the surface concentration (surface coverage Γ) of electroactive species and the electron transfer number of the electrode reaction from the CV curves [31, 32]. According to Laviron’s equation,
from which equations for n and Γ can be deduced as follows:
where i p is the peak current (μA), Q is the quantity of charge (mC) calculated from the oxidation peak area of the voltammograms, n represents the electron number of the electrode reaction, A is the electrode area (cm2), v represents the scan rate (mV s−1), F stands for the Faraday constant, R stands for the ideal gas constant, and T is absolute temperature. The electron transfer number n and the concentration of electroactive species on the electrochemical surface Γ were thus obtained and are presented in Table 2. The concentration of electroactive species on the electrode surface was 3.60 × 10−10, 4.58 × 10−10, 9.01 × 10−10, and 7.36 × 10−10 mol cm−2, respectively. It was found that the concentration of redox species in the biofilm under MF exposure was greater than that in the biofilm of MFC0. The MFC with MF of 200 mT showed the highest level among the four MFCs.
Based on the EIS analysis results, it can be seen that a static magnetic field can decrease the activation loss of the MFC. This is attributed to promotion of the bioelectrochemical activity of electrogenesis by the MF, as conjectured in many literature studies. In this study, we proved this inference and estimated the surface coverage of electroactive species in Geobacter-based biofilm by CV. It is known that the electron transfer process of G. sulfurreducens relies on extracellular direct electron transfer. The electroactive species plays an important electron shuttle role in the anodic reaction. Our results reveal a higher density of electroactive species in the presence of a MF, which is beneficial to increase the exchange current density of the catalyzed reaction, leading to a more efficient direct electron transfer process [33]. This implies that application of a MF can accelerate the electron transfer process, thereby decreasing the activation loss and improving the power generation. However, for exoelectrogens dependent on a mediated electron transfer process, such as Shewanella, the concentration and activity of the soluble mediator are the key factors. It has been previously reported that secretion of flavins was slightly enhanced in a Shewanella-inoculated MFC [12], while no distinct change of mediator secretion was found under MF exposure in a mixed culture-inoculated MFC [11]. Thus, the influence of a MF on the mediated electron transfer process also needs to be explored further to comprehensively understand the mechanism of stimulation by the MF.
Conclusions
The power production by a dual-chamber microbial fuel cell inoculated with G. sulfurreducens was investigated under different MF intensities. With a MF, higher voltage output and maximum power density were obtained. The internal resistance with MF application was obviously reduced, and the MFC with a MF of 200 mT showed the lowest value of 453.6 Ω. Furthermore, the activation resistance was reduced for a certain MF intensity. Based on CV analysis, the application of a MF promoted the bioelectrochemical activity of the anodic biofilm and enhanced the concentration of electroactive species on the electrode surface. This implies that the electricity production by Geobacter-inoculated MFCs can be promoted in the presence of a MF due to a more efficient direct electron transfer process.
References
B.E. Logan, Microbial Fuel Cells (Wiley, New York, 2008)
A.E. Franks, K. Nevin, Energies 3, 3899 (2010)
A. Domínguez-Garay, A. Berná, I. Ortiz-Bernad, A. Esteve-Núñez, Environ. Sci. Technol. 47, 2117 (2013)
M. Zhou, M. Chi, J. Luo, H. He, T. Jin, J. Power Sources 196, 4427 (2011)
B.E. Logan, Nat. Rev. Microbiol. 7, 375 (2009)
L. Strašák, V. Vetterl, Electromagn. Biol. Med. 29, 177 (2010)
S. Dunca, D.E. Creanga, O. Ailiesei, E. Nimitan, J. Magn. Magn. Mater. 289, 445 (2005)
S. Liu, F. Yang, F. Meng, H. Chen, Z. Gong, J. Biotechnol. 138, 96 (2008)
A. Tomska, L. Wolny, Desalination 222, 368 (2008)
L.C. Zhou, G.Y. Li, T.C. An, Y.F. Li, Res. Chem. Intermed. 36, 277 (2010)
Z.H. Tong, H.Q. Yu, W.W. Li, Y.K. Wang, M. Sun, X.W. Liu, G.P. Sheng, Ecotoxicology 24, 2175 (2015)
W.W. Li, G.P. Sheng, X.W. Liu, P.J. Cai, M. Sun, X. Xiao, Y.K. Wang, Z.H. Tong, F. Dong, H.Q. Yu, Biosens. Bioelectron. 26, 3987 (2011)
Y. Yin, G. Huang, Y. Tong, Y. Liu, L. Zhang, J. Power Sources 237, 108 (2013)
S.K. Chaudhuri, D.R. Lovley, Nat. Biotechnol. 21, 1229 (2003)
D.R. Lovley, Nat. Rev. Microbiol. 4, 497 (2006)
A. Pozio, M. De Francesco, A. Cemmi, F. Cardellini, L. Giorgi, J. Power Sources 105, 13 (2002)
P.T. Kissinger, W.R. Heineman, J. Chem. Educ. 60, 702 (1983)
X. Gao, Y. Zhang, X. Li, J. Ye, Mater. Lett. 105, 24 (2013)
Y. Yuan, H. Shin, C. Kang, S. Kim, Bioelectrochemistry 108, 8 (2016)
S. Yang, F. Du, H. Liu, Biomass Bioenergy 46, 531 (2012)
P. Liang, H.Y. Wang, X. Xia, X. Huang, Y.H. Mo, X.X. Cao, M.Z. Fan, Biosens. Bioelectron. 26, 3000 (2011)
K.P. Katuri, P. Kavanagh, S. Rengaraj, D. Leech, Chem. Commun. 46, 4758 (2010)
S. Matsuda, H. Liu, S. Kato, K. Hashimoto, S. Nakanishi, Environ. Sci. Technol. 45, 10163 (2011)
Y. Yuan, S. Zhou, N. Xu, L. Zhuang, Colloids Surf. B 82, 641 (2011)
L.J.C. Jeuken, A.K. Jones, S.K. Chapman, G. Cecchini, F.A. Armstrong, J. Am. Chem. Soc. 124, 5702 (2002)
X. Cao, X. Huang, P. Liang, K. Xiao, Y. Zhou, X. Zhang, B.E. Logan, Environ. Sci. Technol. 43, 7148 (2009)
D.R. Bond, D.R. Lovley, Appl. Environ. Microbiol. 69, 1548 (2003)
Y. Fan, E. Sharbrough, H. Liu, Environ. Sci. Technol. 42, 8101 (2008)
Q. Tao, S. Zhou, Appl. Microbiol. Biotechnol. 98, 9879 (2014)
F.A. Armstrong, H.A. Heering, J. Hirst, Chem. Soc. Rev. 26, 169 (1997)
E. Laviron, J. Electroanal. Chem. Interfacial Electrochem. 101, 19 (1979)
A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980)
K. Rabaey, L. Angenent, U. Schroder, J. Keller, Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application, 1st edn. (IWA Publishing, London, 2009)
Acknowledgments
The authors thank Professor Xia Huang (Tsinghua University) for donating the Geobacter sulfurreducens culture. This study was supported financially by the National Natural Science Foundation of China (51078144) and the Fundamental Research Funds for the Central Universities (22A201514061).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, Y., Huang, G., Di, M. et al. Increased electroactive species concentration in anodic biofilm of Geobacter-inoculated microbial fuel cells under static magnetic field. Res Chem Intermed 43, 873–883 (2017). https://doi.org/10.1007/s11164-016-2670-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2670-0