Abstract
Trace element concentrations were measured in Pacific Dunlin (Calidris alpina pacifica) to identify factors that influence accumulation and to assess toxicity risks. We report concentrations of cadmium, copper, and zinc in kidneys as well as copper, lead, mercury, selenium and zinc in feathers. Relationships between element concentrations and Dunlin age, sex, bill length, habitat preference, trophic level, and sample group were investigated with regression analyses. Stable isotope ratios of carbon and nitrogen in Dunlin muscle tissue were used to determine habitat preference and trophic level, respectively. Cadmium concentrations in kidneys were significantly related to habitat preference: [Cd] in estuarine foragers >[Cd] in terrestrial foragers. Cadmium accumulation was age-dependent as concentrations increased significantly within 10 months of hatch dates but not afterward. Concentrations of cadmium and zinc in kidneys as well as lead and mercury in feathers were below those known to cause deleterious effects in birds. In contrast, selenium concentrations in feathers (range: 2.1–14.0 µg/g) were often at levels associated with toxicity risks (>5 µg/g). Toxicity thresholds are not available for copper in kidneys or copper and zinc in feathers; however, measured concentrations of these elements were within documented ranges for sandpipers. Future studies should assess potential impacts of selenium on embryonic development in Dunlin and other sandpipers. Risk assessments would yield more conclusive results for all elements if impacts under ecologically relevant stresses (e.g. development in the wild, migration, predation) were better understood.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic emissions of many trace elements have grown to exceed those from natural sources (Nriagu 1989) causing concentrations of these elements and subsequent toxicity risks for wildlife to increase worldwide (Eisler 2000). For birds, toxic effects from trace elements include changes in behaviour, as well as negative impacts on development, and reproduction (Scheuhammer 1987; Burger and Gochfeld 1994; Sileo et al. 2003).
Estuarine systems receive constant inputs of trace elements via sediment deposition (Bryan and Langston 1992; Cundy et al. 1997). Suspended fine grain silts, clays and organic matter draw element ions out of solution in freshwater systems as well as in estuarine systems (Luoma and Davis 1983; Tessier and Campbell 1987). Flocculation and settling of such materials at the freshwater-saltwater interface bring trace elements from both marine and freshwater origins into estuarine sediments. Sediment-dwelling organisms in estuarine habitats can accumulate elements in concentrations corresponding to those in sediments (Tessier et al. 1994; Thomas and Bendell-Young 1998). Some bivalves can even bio-concentrate elements in their tissues relative to the waters and sediments in which they live (e.g. cadmium: Burger 2008). Not surprisingly, birds feeding in near shore and shoreline habitats accumulate higher levels of some trace elements than do birds that forage terrestrially (Burger 1993; Wayland and Scheuhammer 2011). On the North American Pacific Coast, coastal and estuarine-feeding birds have been documented with potentially toxic tissue concentrations of selenium, mercury, and cadmium (Hui 1998; Hui et al. 2001; Barjaktarovic et al. 2002).
The Pacific Dunlin (Calidris alpina pacifica) breeds in western Alaska, and migrates to coastal over-wintering sites primarily from southern British Columbia through Mexico (Holmes 1971; Warnock et al. 2004; but see Gill et al. 2013). Dunlin forage in estuarine and agricultural habitat on bivalves, gastropods, annelids and crustaceans, as well as on terrestrial insects, insect larvae, and plant matter (Evans Ogden et al. 2008). Pacific Dunlin and other shorebirds (e.g. Western Sandpipers (Calidris mauri)) also feed on biofilm in intertidal mudflats (Mathot et al. 2010; Kuwae et al. 2012). This biofilm consists of a sediment-bound layer of mucilaginous, extracellular polymeric substances (EPS) that contains and is produced by microphytobenthos (e.g. diatoms) and bacteria (Decho 1990). Consumption of sediment-bound biofilm by Calidris spp. sandpipers results in some of the highest rates of sediment ingestion known in birds and mammals (Beyer et al. 1994; Mathot et al. 2010). EPS, diatoms, and sediment ingested with biofilm are potential exposure vectors for cadmium, lead, and other elements (Schlekat et al. 1998; Franson and Pain 2011; McCormick et al. 2014). Consequently, both the habitat and diet of Pacific Dunlin and other sandpipers place them at risk of toxic exposure to trace elements.
The objectives of this study were to identify factors that influence trace element accumulation in sandpipers and to assess toxicity risks for Dunlin. Diet and physiology are the principal determinants of trace element exposure, absorption, excretion and, consequently, accumulation. Sandpiper physiology differs across age-classes and sexes (McFarland et al. 2002; Stein and Williams 2006). Bill length, habitat preference, and trophic feeding level vary across individual Dunlin and can influence diet. Season and site, which varied across sample groups, also affect diet. All these characteristics (hereafter referred to as factors) were tested for significant relationships with trace element concentrations in Dunlin tissues. Toxicity risks were gauged by comparing measured element concentrations with toxicological benchmarks, as well as with previously reported element concentrations in other sandpipers. Overarching patterns of element accumulation and toxicity risks for sandpipers are discussed.
Materials and methods
Study site and sample collection
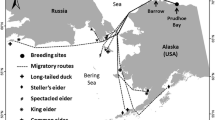
The Fraser River Delta (FRD) hosts 30,000–70,000 Dunlin annually from December through March, and hundreds-of-thousands during fall and spring migrations (Butler and Vermeer 1994; Shepherd 2001). A total of 83 Dunlin were collected from aircraft collisions and by shotgun in the FRD, as well as from incidental mortalities during a separate study in Skagit Bay, WA (Table 1; Fig. 1). Collections were made under the following permits and protocols: U.S. Fish & Wildlife Service Scientific Collection/Import/Export Permit #MB004887-0, Simon Fraser University Animal Care protocol # 946B-09, Environment Canada Scientific Permit # BC-10-0034. An additional fifteen Dunlin were captured by net gun for feather samples at Roberts Bank and at Boundary Bay of the FRD in 2011 (Fig. 1). Following feather collections, Dunlin were tagged with Canadian Wildlife Service bands and released.
Sample collection sites, migration routes, wintering ranges and breeding ranges of Dunlin (Calidris alpina pacifica) collected in the Fraser River Delta (FRD), British Columbia and Skagit Bay, Washington. In the FRD, hatch years were collected during the fall on their first southward migration (2009), second years were collected during their first spring (2010), and after second years were collected in the spring (2010) having already completed the full migration cycle at least once. In Skagit Bay, Dunlin of all age classes were collected during the overwintering period (2007, 2008). Parallel arrows indicate overlapping migration pathways
Factors, tissues, and elements analyzed
Age, sex, bill length, habitat preference (estuarine vs. terrestrial), trophic level, and sample group were identified as factors with potential to influence element accumulation. Stable isotope ratios of carbon and nitrogen in muscle tissues were used as indicators of habitat preference and trophic level, respectively. Bill length was measured from the tip to where the bill meets facial feathers. Most Dunlin collected during the 2010 spring migration could be sexed by examining testes during dissections. All others were sexed by bill length (males <37.7 mm; females >39.8 mm; unknown = 37.7–39.8 mm: Prater 1977). Age was determined by plumage. Dunlin in their first and second calendar years were classified as hatch years (HY) and second years (SY) respectively. Individuals in their third calendar year or older were classified as after second years (ASY). Age-specific migratory histories of Dunlin examined in this study are presented in Fig. 1.
Cadmium is of toxicological interest for Dunlin and other shorebirds due to its abundance in coastal pacific habitats and potential to bio-accumulate and concentrate in their prey (e.g. bivalves and biofilm: Schlekat et al. 1998; Burger 2008). Copper and zinc can influence the bioavailability of cadmium (Fox et al. 1984) and are also potentially toxic in high concentrations. Cadmium, copper, and zinc accumulate in sandpiper kidneys and livers more than other tissues, so kidney tissue was analyzed for concentrations of those elements (Blomqvist et al. 1987; Lucia et al. 2010; Wayland and Scheuhammer 2011). Feathers contain the majority of methyl-mercury in birds and also describe exposure from many other trace elements (Burger 1993; Thompson 1996). Trace element concentrations vary less amongst contour feathers of individual birds than flight feathers so concentrations were measured in back and breast feathers (Furness et al. 1986).
Invertebrate sampling
To confirm stable isotope signatures of Dunlin prey, invertebrates were sampled from estuarine mudflats and agricultural fields within 1 km of intertidal areas at both Roberts Bank and Boundary Bay. Samples were collected with sediment cores (10 cm diameter, 5 cm depth) during winter (Dec–Jan) and spring (Apr–May) of 2010 and 2011. Invertebrates were rinsed from sediments with 2 and 1 mm sieves and sorted according to taxa: polychaetes (Eteone spp), crustaceans (Corophium spp, cumaceans, harpacticoids), mud snails (Batillaria attramentaria).
Stable isotope analyses
Stable isotope ratios in animal tissues can describe diet and, thus, exposure sources for trace elements and other potential toxicants. Ratios of 15N to 14N in biota increase with trophic level. Ratios of 13C and 12C are more stable across trophic levels, but can vary across habitats with distinct dominant photosynthetic pathways (Michener and Lajitha 2007). Carbon and nitrogen stable isotope ratios, hereafter referred to as δ13C and δ15N “signatures”, have tissue-specific turnover rates and reflect diet over a tissue-specific period of time (Hobson and Clark 1992). Of particular interest to this study was time spent at wintering sites where Dunlin habitat preference varies across individuals (Evans Ogden et al. 2005). For Dunlin collected during the spring migration (April 2010), muscle tissue reflected diet for the greatest portion of the non-breeding season (i.e. at wintering sites). Following carcass storage at −20° C for a maximum of 4 weeks, muscle tissues were extracted, homogenized, and treated with a 2:1 chloroform:methanol solvent rinse to remove lipids. Dried muscle tissue (1 mg) and similarly treated invertebrate prey (1 mg) were analyzed for δ13C and δ15N signatures at the Stable Isotope Facility (SIF) of the University of California, Davis (Instrument specifications: PDZ Europa ANCA-GSL elemental analyzer and PDZ Europa 20–20 isotope ratio mass spectrometer). Reference materials with certified δ13C and δ15N values were analyzed alongside samples to verify measurement accuracy. All δ13C and δ15N measurements of reference materials closely paralleled certified values (CV <1 %).
Trace element analyses
Following dissections, kidneys were dried at 60 °C for 48 h and homogenized with a mortar and pestle. Approximately 0.07 g of each kidney sample was digested with 4 ml of 70 % nitric acid solution (adapted from Burger and Gochfeld 1990; McFarland et al. 2002). All sample digestions were accompanied by digestions of a procedural blank (70 % nitric acid) and two reference materials with certified element concentrations (TORT-2, DOLT-2; National Research Council Canada, Ottawa, ON). To evaluate sample homogeneity, two replicate samples from kidneys in each of fifteen individuals were also digested. Samples, reference materials, and blanks were digested on a hot plate at 200 °C in open flasks until 0.5 ml of the digest remained. Digests were diluted with ultra-pure water and transferred to polypropylene tubes. Flasks were rinsed with 2 % nitric acid solution and rinses were added to dilutions. Diluted digests were analyzed for concentrations of cadmium, copper, and zinc with a Perkin–Elmer model 100 Flame Atomic Absorption Spectrometer (AAS) at Simon Fraser University (SFU), Burnaby, British Columbia.
Feathers were cleaned of external debris with an air jet and ultrasonic baths and dried at 60 °C for 48 h (adapted from Norris et al. 2007). Feathers collected in 2010 (15–20 per individual) were digested via microwave with 9 ml nitric acid in closed, acid washed, Teflon vessels to prevent the escape of volatilized mercury (EPA method 3052). Feathers from 2011 (10–25 per individual) were digested on a hot plate at 200 °C in flasks with 5 ml nitric acid (70 %). Once the volume fell below 1 ml, another 1 ml hydrochloric acid (35 %) and 1 ml nitric acid was added and samples were left to digest until less than 1 ml remained. Feather digests were diluted in the same manner as kidney digests. Trace element concentrations in feathers were determined at the Institute for Integrated Research in Materials, Environments, and Society (IIRMES) of California State University at Long Beach. Samples were spiked with iridium, thulium, and rhodium standards, and analyzed along with lab blanks for concentrations of Ag, Al, As, Au, Ba, Ca, Cd, Cr, Cu, Co, Fe, Kr, Mn, Mo, Ni, Pb, Sb, Se, Sn, Sr, Ti, Tl, V, Zn with a Hewlitt Packard (Agilent) 4500 Series Inductively Coupled Plasma Mass Spectrometer (ICP-MS). Digests from 2010 collections were also analyzed for mercury concentrations via cold vapor atomic fluorescence spectroscopy (CVAFS) using a Leeman Labs, Inc. Hydra HF Gold Plus Mercury Analyzer.
Quality control, measurement adjustments, and statistical analyses
Reported concentrations are based on dry-weight mass. Conversions of referenced wet-weight concentrations to dry-weight concentrations were calculated as [wet weight] × 4 as in (Goede et al. 1989) and (Clark and Scheuhammer 2003). Element concentrations in feathers are reported for each element with validated measurements. Validation of measurements required concentrations of an element in reference materials to be within one standard error of certified concentrations and required sample concentrations to be above detection limits. Element concentrations measured in kidneys were adjusted to correct for variation in processing conditions and AAS measurement accuracy across groups of separately processed and analyzed samples. Adjustments were made by multiplying concentration measurements by sample group-specific correction factors. Correction factors (CF) were calculated for each sample group as the inverse of the average ratio of measured reference material concentrations (MRM) relative to certified reference material concentrations \( \left( {CRM} \right):CF\;\; = \;\;1/(\frac{MRM}{CRM}). \) Variances in element concentrations within kidney samples were quantified with a standard deviation calculated from differences between measurements of replicate samples. Limited sample mass prevented variance analyses of element concentrations in feathers. CF applied to kidney AAS measurements were as follows (Mean (SD)): Cd: 0.93 (0.05); Cu: 0.94 (0.05); Zn: 0.94 (0.04). Standard deviations of element concentrations across replicate kidney samples were as follows: Cd: 0.99 μg/g; Cu: 1.39 μg/g; Zn: 3.28 μg/g.
Prior to statistical analyses, all data were tested for normality with Shapiro-Wilks goodness of fit tests. Log transformations were applied to element concentrations with log-normal distributions. Forward stepwise regressions were employed to test factors as explanatory variables of element concentrations in tissues. Separate regressions were conducted for each element in each tissue type. Inclusion of Dunlin characteristics as factors in regression models was contingent upon their independence. All factors with variance inflation factors (VIF) >10 or Pearson’s Product Correlation Coefficients (PPCC) greater than r = 0.6 were considered collinear (i.e. not independent) and were not entered into regression models together (Nielson et al. 2004). Bill length is typically longer in female Dunlin relative to male Dunlin and was used to sex some individuals. Sex and bill length were, therefore, not independent and were considered separately in regression analyses. Categorical factors were also tested for interaction effects on tissue element concentrations.
Analyses of element concentrations in kidneys
Stepwise regressions were conducted within samples collected in April 2010 so the effects of age-class (SY vs. ASY) and sample site (Boundary Bay vs. YVR) could be investigated separately from potentially confounding seasonal effects. Muscle δ15N was not considered in analyses of April 2010 samples because δ15N was correlated with δ13C. Separate regression analyses were conducted within samples of estuarine feeding individuals (muscle δ13C ≥ −15.0 ‰: Fig. 2) to test for relationships between δ15N and kidney element concentrations while controlling variation in δ13C. Additional regressions were conducted to examine differences in kidney element concentrations between HY Dunlin sampled in the fall and after hatch year (AHY) Dunlin sampled in the spring.
Stable isotope signatures of muscle tissue from individual Dunlin (Calidris alpina pacifica) within sample groups collected at Boundary Bay (BB) and Vancouver International Airport (YVR) in the Fraser River Delta (FRD). Boxes describe the range of mean isotopic signatures reported for sandpiper food items in the FRD across years, sites, and seasons (Evans Ogden et al. 2005; Kuwae et al. 2008; Beninger et al. 2011; VAFFC 2012; this study) adjusted by prey to muscle trophic enrichment factors: +1.9 ‰ δ13C, +3.1 ‰ δ15N (Evans Ogden et al. 2004)
Analyses of element concentrations in feathers
Stepwise regressions were conducted separately for elements in feathers collected during 2010 and 2011 to permit consideration of factors that did not vary across sampled individuals in both years. Dunlin were categorized as either terrestrial (muscle δ13C < −20 ‰: Fig. 2) or estuarine (muscle δ13C > −15 ‰) to analyze effects of habitat preference on element concentrations in 2010 feather collections.
The level of statistical significance (α) required to include factors in regression models was 0.05 for analyses of element concentrations in feathers (one regression for each year). Alpha levels were adjusted with bonferroni corrections for analyses of elements in kidneys (three regressions: α = 0.013). Statistical analyses were performed with JMP (ver. 10.0). Figures were constructed with SigmaPlot (ver. 12.3) and ArcGIS (ArcMap 10.0).
Element turnover rates in tissues
Element turnover rates in animal tissues determine the time-period of exposure responsible for element concentrations and stable isotope signatures. In mobile organisms, turnover rates must be known to determine the site or sites responsible for accumulated elements and isotope signatures. Element concentrations in feathers reflect blood and other internal tissue concentrations to varying degrees during feather growth (Bortolotti 2010). A literature review was conducted to determine relevant time periods of exposure for elements measured in feathers and for half-lives of copper and zinc in avian kidneys and carbon and nitrogen in muscle. Data on cadmium in kidneys were sufficient to estimate a half-life specific to Dunlin. Cadmium accumulation rates decrease in Dunlin as kidney concentrations increase and may reflect concentration dependent excretion (Blomqvist et al. 1987, Fig. 3). In a first-order reaction where excretion increases linearly with concentration, half-life is defined as follows:
where k (the reaction rate constant) is the fraction of cadmium reacting per unit of time.
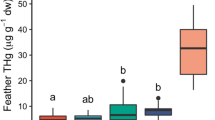
Concentrations of cadmium (a), copper (b), and zinc (c) in kidneys of Dunlin (Calidris alpina pacifica) collected during fall (November 2009) and spring (April 2010) migration periods in the Fraser River Delta, British Columbia. Boxes reflect median concentrations (center line), 25th and 75th percentiles (lower and upper borders), 10th and 90th percentiles (whiskers) and concentrations outside this range (points). Geometric means in upper left text (SD) HY hatch year, SY second year, ASY after second year. Asterisk denotes significantly different mean from other age classes/sample seasons
Cadmium accumulation in kidneys occurred primarily between HY and SY age classes. Subsequent increases in concentration were not significant (Fig. 3). Stable cadmium concentrations in kidneys of AHY Dunlin imply equal rates of input and excretion. Both input and excretion can, therefore, be used to estimate reacting cadmium within a defined period of time for determination of k. Input was estimated from the difference between concentrations in kidneys of AHY and hatchling Dunlin: geometric mean AHY [Cd] − [Cd] in hatchlings. Time for input was defined as 10 months: time from typical hatch dates in June to Dunlin collections in April. Cadmium concentrations in hatchlings were estimated to be 0.5 µg/g based on documented levels in HY Western Sandpipers (ca. 1 µg/g; McFarland et al. 2002) and HY Dunlin (C. alpina) (ca. 0.3 µg/g mean; Blomqvist et al. 1987).
An alternative explanation for greater cadmium accumulation in HY as compared to AHY Dunlin is higher metabolic rates and greater intake rates in HY individuals (Stock et al. 1989). Excretion is not necessarily concentration dependent if age-related changes in metabolic/intake rate control cadmium accumulation. In cases of concentration independent excretion, half-lives are determined using a zero-order reaction model:
where [A]0 is an initial concentration and k is the amount of cadmium reacting per unit of time. Using the following equation, k can be determined with any initial concentration and a second concentration after a defined period of time ([A] t ) while decay (i.e. excretion) is occurring
Considering equal rates of cadmium excretion and input in AHY Dunlin, the equation was altered to estimate k with accumulation
Cadmium concentrations in kidneys of hatchlings (estimated as above) and in AHY Dunlin (geomean) were substituted for [A]0 and [A] t , respectively for determination of k. Accumulation was again estimated to primarily occur within 10 months (t). Estimates of zero-order reaction (concentration independent) half-life also considered sensitivity to intake rate reductions in AHY relative to HY Dunlin. If intake is reduced, input and k would be reduced in AHY Dunlin relative to within the first 10 months of life. Thus, half-lives were also calculated with 10 and 25 % intake reductions in AHY relative to HY Dunlin.
Toxicity risk assessment
The ranges of element concentrations measured in Dunlin tissues are presented in the results and compared to threshold concentrations of potential toxicity as well as previously documented element concentrations in Dunlin and other Calidris sandpipers.
Results
Stable isotope signatures in Dunlin and their prey
Stable isotope ratios of Dunlin estuarine prey in the FRD were enriched in carbon and nitrogen relative to terrestrial prey across sampling sites and years (Fig. 2). Muscle δ13C and δ15N signatures were also positively related and spanned a gradient reflecting mostly terrestrial to predominantly estuarine diet composition.
Patterns of element accumulation in tissues
No significant interaction effects were observed among factors and VIFs were all below ten. However, PPCCs indicated that δ13C and δ15N in Dunlin muscle tissue were collinear (r = 0.82) requiring that these factors be assessed separately in regression analyses. Concentrations of manganese in 2010 and 2011 feathers were inaccurate according to reference material standards, but measurements across reference samples of distinct mass yielded proportionate differences in total manganese. Thus, differences in manganese across samples were considered accurate and measured concentrations were analysed for relationships with the described factors.
Factors and elements considered in regression analyses are summarized along with results in Table 2. In Dunlin collected during April 2010, δ13C was positively related to kidney cadmium concentrations (stepwise regression, p < 0.001, r2 = 0.41). Sample site was significantly related to copper and zinc concentrations in kidneys from April 2010 collections (Cu: p < 0.001, r2 = 0.25; Zn: p < 0.001, r2 = 0.22) with higher concentrations of zinc and copper in Boundary Bay collections relative to YVR collections (Table 6). Regressions including fall HY individuals also found δ13C described a significant amount of the variation in kidney cadmium (p < 0.001, r2 = 0.21), and found inclusion of sample group significantly improved the fit of the model (p = 0.001, r2 = 0.49). Cadmium concentrations in kidneys were lower in HY Dunlin collected in the fall relative to SY and ASY individuals collected during spring (Fig. 3). Higher zinc concentrations were present in HY kidneys from fall collections than in AHY kidneys from spring collections (p < 0.001, r2 = 0.18; Fig. 3). Dunlin from Skagit Bay, Washington had zinc kidney concentrations similar to those of FRD collections, but had relatively lower cadmium and copper concentrations (Table 7). In feathers collected in 2010, manganese concentrations were higher in terrestrially feeding birds as compared to estuarine foragers (p = 0.030, r2 = 0.57). No other factor was significantly related to element concentrations in tissues.
Element turnover rates in tissues
Estimates of k and cadmium half-life in Dunlin kidneys are presented in Table 3. Estimates of carbon and nitrogen half-lives in avian muscle, zinc and copper half-lives in avian kidneys, and time periods of trace element exposure represented in feathers are presented in Table 4.
Toxicity risk assessment
Toxicity thresholds and ranges of validated element concentrations in Dunlin kidneys and feathers are presented in Table 5. Mean element concentrations observed in feathers and kidneys in this study and other studies of sandpipers are presented in Tables 6 and 7.
Discussion
Analyses of the relationships between Dunlin characteristics and element concentrations in tissues revealed several patterns of trace element accumulation. Of all factors investigated, δ13C explained the greatest amount of variation in the concentrations of cadmium in kidneys. A sample group effect could be responsible for this relationship if migratory groups arrived from different sites with distinct isotope signatures and cadmium burdens; however, δ13C has been shown to vary across wintering Dunlin within sites (Evans Ogden et al. 2005) and varied substantially within sample groups from a variety of sites and days in this study (Table 1; Fig. 4). Elevated exposure to trace elements in estuarine habitats is a more likely explanation for the relationship of δ13C with cadmium. A related study analyzed gizzard contents of the same individual Dunlin and found higher concentrations of cadmium in estuarine prey as compared to terrestrial prey within individuals collected from Boundary Bay (C.T. St. Clair 2012). Dunlin residing in saline environments also consume more food, and consequently more trace elements, to accommodate the energetic costs of osmoregulation (Gutiérrez et al. 2011). Thus, agricultural habitat use appears to benefit Dunlin by decreasing cadmium loading and likely provides a similar advantage to other shorebirds. Manganese concentrations in feathers were also significantly related to δ13C. Elevated levels of manganese in feathers of terrestrially foraging Dunlin may result from the use of manganese in fertilizers (e.g. manganese-sulphate, -oxide, -chelate). Although δ13C and δ15N were correlated, the effects of habitat preference and trophic level were not considered confounded. Distinct baseline δ15N signatures in estuarine relative to terrestrial environments have been reported in other estuaries (e.g. Cloern et al. 2002) and are evident in the signatures of estuarine and terrestrial shorebird prey from the FRD (Fig. 2). Thus, the correlation between δ13C and δ15N was considered a result of variation in δ15N across habitats rather than habitat-related differences in the trophic feeding level of Dunlin.
Apart from age class (SY vs. ASY), the only other factor that was significantly related to element accumulation was sample group. To determine whether age class or sample season was responsible for the differences in tissue element concentrations across sample groups, we reviewed age-related trends reported in other studies. Logarithmic cadmium accumulation in avian species is well documented: e.g. Dunlin (Blomqvist et al. 1987), Western Sandpipers (McFarland et al. 2002), Willow Ptarmigan (Lagopus lagopus) (Myklebust and Pedersen 1999), Mallards (Anas platyrynchops) (White and Finley 1978), Oystercatchers (Haematopus ostralegus), and other birds (Stock et al. 1989). Thus, lower cadmium concentrations in fall samples of HY Dunlin were most likely a result of age. Significant age-related trends in zinc have not been reported in other studies (e.g. Blomqvist et al. 1987; Hogstad 1996). However, average concentrations of both zinc and copper are generally higher in kidneys of HY as compared to AHY sandpipers (Table 6). Higher food intake rates in HY Dunlin as compared to older individuals (Stein and Williams 2006) likely contribute to the age–related patterns of cadmium and zinc accumulation. Considering the turnover rates of zinc and cadmium in kidneys (Table 4), increased exposure to HY Dunlin could explain the higher levels of zinc observed in HY birds as well as the increased accumulation of cadmium observed in HY relative to AHY birds.
Studies of other shorebird populations have reported patterns of element accumulation that were not apparent in this study. While neither sex nor bill length was significantly related to cadmium accumulation in Pacific Dunlin, higher cadmium concentrations have previously been documented in male as compared to female Dunlin (Ferns and Anderson 1994) and Western Sandpipers (McFarland et al. 2002). Differing prey preferences of males and females may explain these contradictory findings if diets are similar at some sites, but not others. Insignificant relationships between trophic level (δ15N) and element concentrations suggest that biofilm and associated sediments are not important exposure vectors for trace elements. However, biofilm comprises a larger portion of Western Sandpiper diet as compared to Dunlin (Mathot et al. 2010; Kuwae et al. 2012) and higher concentrations of cadmium occur in Western Sandpipers (range of averages in kidneys: 15–20 μg/g; McFarland et al. 2002). A more estuarine diet in Western Sandpipers may account for some of this difference, but even estuarine-specialist Dunlin have relatively low cadmium concentrations in kidneys (9.0 μg/g ± 3.7(SD)). The relatively high rates of sediment and biofilm ingestion by Western Sandpipers as compared to Dunlin may increase exposure to cadmium, as well as zinc, copper (Schlekat et al. 1998; McCormick et al. 2014) and other elements that do not typically accumulate through the food chain (e.g. Al, Fe, Ti, Pb: Hui and Beyer 1998; Franson and Pain 2011). However, gizzard content analyses also indicate that biofilm and sediment ingestion do not expose Dunlin to high levels of cadmium, copper, or zinc. Concentrations of these elements in sediment dominated gizzard contents of Dunlin sampled in the FRD were within the range of concentrations measured in samples composed of other diet items (C.T. St. Clair 2012).
Sex and bill length were not significantly related to mercury concentrations in Dunlin feathers, and other studies describe inconsistent relationships between sex and mercury in feathers (Burger 1993; Burger et al. 2007). However, consistently higher concentrations of mercury are reported in internal tissues of male as compared to female birds (Eagles-Smith et al. 2009). Feathers primarily contain mercury as methyl mercury (Thompson 1996), so sex-related differences in internal tissue concentrations may reflect patterns of inorganic mercury accumulation.
Finally, other relationships between stable isotope signatures and element concentrations in tissues may have been obscured because tissue concentrations of some elements reflect dietary exposure over dissimilar periods of time relative to δ13C and δ15N. The half-life of cadmium in kidneys is longer than half-lives of δ13C and δ15N in muscle tissue (Table 4). Mercury and lead concentrations in feathers also reflect dietary exposure over a longer period of time than δ13C and δ15N. Consequently, diet shifts occurring within the time responsible for cadmium, mercury and lead concentrations, but before the time responsible for stable isotope signatures could obscure relationships with habitat preference and trophic feeding level. Considering shorter turnover rates of zinc and copper in kidneys relative to δ13C and δ15N in muscle tissue (Table 4), insignificant relationships reported between stable isotope signatures and zinc and copper may also be subject to type II error.
Toxicity thresholds and element concentrations in other sandpiper populations provide measures of toxicity risk as well as additional sample groups across which patterns of accumulation can be observed. Element concentrations measured in Dunlin tissues were generally below toxicity thresholds. Cadmium and copper concentrations in kidneys were intermediate within the range reported for other Calidris sp. populations (Table 7). Zinc concentrations in kidneys and feathers were similar in Dunlin as compared to other sandpiper populations (Tables 6 and 7), but high relative to concentrations in other avian taxa (Burger 1993). To our knowledge, toxicity thresholds are not available for copper and zinc in feathers, but copper was also found at similar concentrations as reported in other sandpipers. Mercury and lead were also found in apparently non-toxic concentrations as has generally been reported in feathers of sandpipers (Table 6).
The only element observed in concentrations above toxicity thresholds was selenium. Selenium concentrations in sandpiper feathers have been reported at ratios of 1:3 and 1:4 relative to kidney tissue (Goede and Debruin 1985) and 1:6 relative to liver tissue (Goede 1985). Risks of selenium toxicity are present at concentrations exceeding 20–22 μg/g in livers and kidneys (Ohlendorf and Heinz 2011). These ratios and thresholds confirm the 5.0 μg/g threshold suggested for feathers by the (U.S. Department of the Interior 1998). Selenium concentrations in tissues of Dunlin and other sandpipers consistently approach and exceed these thresholds (Table 6; White et al. 1980; Braune and Noble 2009). Birds are most sensitive to selenium toxicity during egg development and, hence, during the breeding season (Spallholz and Hoffman 2002). Goede et al. (1989) found that concentrations of selenium in Dunlin (C. alpina) at a breeding site in northern Norway were low relative to levels observed in Dunlin at non-breeding sites in Western Europe. Selenium burdens in sandpipers may be broadly reduced at breeding sites as a result of widespread shifts from estuarine to terrestrial diets; however, considering that selenium in sandpipers is widely present at concentrations associated with reduced egg hatchability and embryo deformities, the subject warrants further investigation.
To determine the location(s) of exposure responsible for element concentrations observed in Dunlin tissues, migratory movements and element turnover rates in tissues were taken into consideration. Trace elements diffuse into feathers from the bloodstream during growth and can also be externally deposited onto feathers (Burger and Gochfeld 1999; Bortolotti 2010). Element concentrations in blood, and thus in feathers, are influenced by recent dietary exposure as well as concentrations in other tissues during the time of growth (Burger 1993). Feathers were collected in April 2010 and 2011 during or shortly after the growth of alternate-plumage, so external deposition of elements was expected to be minimal. Mercury accumulates in birds between moults and the majority of the body burden is transferred to feathers as they grow (Lewis and Furness 1991). Accumulated lead can persist in internal tissues for several months following acute exposure and longer when elevated exposure is chronic (Franson and Pain 2011). The previous, basic moult primarily occurs in Dunlin during September and October. Thus, mercury and lead concentrations reflect exposure from April through as far back as October. Copper, manganese, selenium, and zinc are consistently utilized micronutrients with internal tissue half-lives of less than 10 days (Table 4). Concentrations of these elements in feathers therefore reflect dietary exposure during feather growth and several days prior. Dunlin collected in the FRD during migratory periods and prior to the spring migration spent an unknown amount of time if the FRD prior to sampling dates. Consequently, the proportion of accumulated elements in kidneys and feathers of Dunlin collected in the FRD reflect uncertain proportions of local exposure. For Dunlin collected during the fall migration, concentrations of elements may also represent exposure at breeding sites, staging sites, and migratory stopover sites north of the FRD. For Dunlin collected during the spring migration, concentrations of elements may also reflect exposure from other non-breeding sites south of the FRD. Dunlin from Skagit Bay, WA were collected during the winter when Pacific Dunlin are primarily site-faithful over the course of a week (Shepherd 2001). Considering the short half-lives of copper and zinc in kidneys (Table 4), concentrations measured in individuals from Skagit Bay should reflect exposure at that site.
The half-life of cadmium is longer than other elements so the locations of exposure reflected in tissues can be more difficult to determine. Uncertainties regarding the half-life of cadmium in Dunlin can be reduced with an informed estimate of the differences in intake rate between HY and AHY individuals. Small intestines of SY and ASY Western Sandpipers are 8.5–10.0 % smaller than HY individuals until after the first fall migration (Stein and Williams 2006). Mass and length of small intestines are positively related to food and trace element intake (McWilliams and Karasov 2001). Food intake increases 1.4–2.0 fold relative to increases in the gastro-intestinal tract volume of House Wrens (Troglodytes aedon) (Dykstra and Karasov 1992). Thus, assuming that Dunlin undergo similar reductions in digestive tract mass and intake rate after their first fall migration, intake rate should be reduced by 11–20 % in AHY relative to HY individuals. Applying that range of intake rate reduction to the zero-order reaction model yields half-lives of 6.2–7.7 months (Table 3). Finally, all half-life estimates were determined assuming negligible cadmium excretion from HY Dunlin. Half-lives are therefore overestimated to an unknown degree corresponding to the amount of cadmium excreted from Dunlin kidneys before April collections. Regardless of the extent of overestimation, cadmium in Dunlin collected in April 2010 should primarily reflect exposure during the previous 7 months spent on wintering sites. Cadmium in HY Dunlin collected during November 2009 should primarily reflect exposure from breeding and staging areas.
(Blomqvist et al. 1987) estimated a 1–2.5 year half-life for cadmium in Dunlin (C. alpina), but measured cadmium concentrations in SY birds during autumn. Our results indicate that Dunlin accumulate the majority of cadmium several months earlier, by April of their second calendar-year. Later sample acquisition of SY individuals likely led (Blomqvist et al. 1987) to underestimate accumulation rate and over-estimate half-life.
Conclusions
With the exception of selenium, trace element concentrations measured in Dunlin were below established toxicity thresholds. It is important to note that these thresholds are largely based on impacts observable under laboratory conditions. Avian species are likely to be more sensitive to toxicants under stresses of life in the wild. A number of endpoints could be investigated in relation to toxicant concentrations to address this knowledge gap (e.g. failed or prolonged migration, flight speed and stamina, reproductive success, development). Risk assessments would yield more conclusive results for all elements if impacts under ecologically relevant stresses were better understood.
References
Barjaktarovic L, Elliott JE, Scheuhammer AM (2002) Metal and metallothionein concentrations in Scoter (Melanitta spp.) from the Pacific Northwest of Canada, 1989–1994. Arch Environ Contam Toxicol 43:486–491
Beninger PG, Elner RW, Morancais M, Decottignies P (2011) Downward trophic shift during breeding migration in the shorebird Calidris mauri (Western Sandpiper). Mar Ecol Prog Ser 428:259–269. doi:10.3354/meps09050
Beyer WN, Connor EE, Gerould S (1994) Estimates of soil ingestion by wildlife. J Wildl Manag 58:375–382
Blomqvist S, Frank A, Petersson LR (1987) Metals in liver and kidney tissues of autumn-migrating Dunlin Calidris alpina and Curlew Sandpiper Calidris ferruginea staging at the Baltic Sea. Mar Ecol Prog Ser 35:1–13
Bortolotti GR (2010) Flaws and pitfalls in the chemical analysis of feathers: bad news-good news for avian chemoecology and toxicology. Ecol Appl 20:1766–1774
Braune BM, Noble DG (2009) Environmental contaminants in Canadian shorebirds. Environ Monitor Assess 148:185–204. doi:10.1007/s10661-007-0150-0
Bryan G, Langston W (1992) Bioavailability, accumulation and effects of heavy-metals in sediments with special reference to United-Kingdom estuaries—a review. Environ Pollut 76:89–131. doi:10.1016/0269-7491(92)90099-V
Burger J (1993) Metals in avian feathers: bioindicators of environmental pollution. In: Hodgson E (ed) Reviews in environmental toxicology. Toxicology Communications Inc, Raleigh
Burger J (1995) Heavy metal and selenium levels in feathers of Herring Gulls (Larus argentatus): differences due to year, gender, and age at Captree, Long Island. Environ Monit Assess 38:37–50. doi:10.1007/BF00547125
Burger J (2008) Assessment and management of risk to wildlife from cadmium. Sci Total Environ 389:37–45
Burger J, Gochfeld M (1990) Tissue-levels of lead in experimentally exposed Herring Gull (Larus argentatus) chicks. J Toxicol Environ Health 29:219–233
Burger J, Gochfeld M (1994) Behavioral impairments of lead-injected young Herring Gulls in nature. Fundam Appl Toxicol 23:553–561. doi:10.1006/faat.1994.1140
Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172
Burger J, Gochfeld M (1999) Heavy metals in Franklin’s gull tissues: age and tissue differences. Environ Toxicol Chem 18:673–678
Burger J, Seyboldt S, Morganstein N, Clark K (1993) Heavy-metals and selenium in feathers of 3 shorebird species from Delaware Bay. Environ Monit Assess 28:189–198
Burger J, Gochfeld M, Sullivan K, Irons D (2007) Mercury, arsenic, cadmium, chromium lead, and selenium in feathers of Pigeon Guillemots (Cepphus columba) from Prince William Sound and the Aleutian Islands of Alaska. Sci Total Environ 387:175–184. doi:10.1016/j.scitotenv.2007.07.049
Butler RW, Vermeer K (1994) The abundance and distribution of estuarine birds in the Strait of Georgia, British Columbia. Canadian Wildlife Service technical report number 83. Canadian Wildlife Service, Ottawa
Chiou P, Chen K, Yu B (1997) Toxicity, tissue accumulation and residue in egg and excreta of copper in laying hens. Anim Feed Sci Technol 67:49–60. doi:10.1016/S0377-8401(96)01139-X
Clark AJ, Scheuhammer AM (2003) Lead poisoning in upland-foraging birds of prey in Canada. Ecotoxicology 12:23–30
Cloern JE, Canuel EA, Harris D (2002) Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol Oceanogr 47:713–729
Cundy A, Croudace I, Thomson J, Lewis J (1997) Reliability of salt marshes as ‘‘geochemical recorders’’ of pollution input: a case study from contrasting estuaries in southern England. Environ Sci Technol 31:1093–1101. doi:10.1021/es960622d
Decho A (1990) Microbial exopolymer secretions in ocean environments—their role(s) in food webs and marine processes. Oceanogr Mar Biol Ann Rev 28:73–153
Dykstra CR, Karasov WH (1992) Changes in gut structure and function in House Wrens (Troglodytes aedon) in response to increased energy demands. Physiol Zool 65:422–442
Eagles-Smith CA, Ackerman JT, De La Cruz SEW, Takekawa JY (2009) Mercury bioaccumulation and risk to three waterbird foraging guilds is influenced by foraging ecology and breeding stage. Environ Pollut 157:1993–2002
Eisler R (1987) Mercury hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish and Wildlife Service biological report 85(1.10)
Eisler R (2000) Handbook of chemical risk assessment, Vol. 1: metals. Lewis Publishers, Boca Raton
Evans Ogden LJ, Hobson KA, Lank DB (2004) Blood isotopic (delta C-13 and delta N-15) turnover and diet-tissue fractionation factors in captive Dunlin (Calidris alpina pacifica). Auk 121:170–177
Evans Ogden LJ, Hobson KA, Lank DB, Bittman S (2005) Stable isotope analysis reveals that agricultural habitat provides an important dietary component for non-breeding Dunlin. Avian Cons Ecol 1:3
Evans Ogden LJ, Bittman S, Lank DB (2008) A review of agricultural land use by shorebirds with special reference to habitat conservation in the Fraser River Delta, British Columbia. Can J Plant Sci 88:71–83
Ferns P, Anderson J (1994) Cadmium in the diet and body-tissues of Dunlins Calidris alpina, from the Bristol Channel, UK. Environ Pollut 86:225–231. doi:10.1016/0269-7491(94)90194-5
Fox M, Tao S, Stone C, Fry B (1984) Effects of zinc, iron and copper deficiencies on cadmium in tissues of Japanese Quail. Environ Health Perspect 54:57–65. doi:10.2307/3429791
Franson JC, Pain DJ (2011) Lead in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota. CRC Press, Boca Raton
Furness R, Muirhead S, Woodburn M (1986) Using bird feathers to measure mercury in the environment—relationships between mercury content and molt. Mar Pollut Bull 17:27–30. doi:10.1016/0025-326X(86)90801-5
Gasaway W, Buss I (1972) Zinc toxicity in the Mallard duck. J Wildl Manag 36:1107–1117. doi:10.2307/3799239
Gill RE, Handel CM, Ruthrauff DR (2013) Intercontinental migratory connectivity and population structuring of Dunlins from western Alaska. Condor 115:525–534
Goede A (1985) Mercury, selenium, arsenic and zinc in waders from the Dutch Wadden Sea. Environ Pollut Ser A 37:287–309. doi:10.1016/0143-1471(85)90119-9
Goede A, Debruin M (1985) Selenium in a shore bird, the Dunlin, from the Dutch Waddenzee. Mar Pollut Bull 16:115–117. doi:10.1016/0025-326X(85)90535-1
Goede A, Nygard T, Debruin M, Steinnes E (1989) Selenium, mercury, arsenic and cadmium in the lifecycle of the Dunlin, Calidris alpina, a migrant wader. Sci Total Environ 78:205–218. doi:10.1016/0048-9697(89)90034-X
Gutiérrez JS, Masero JA, Abad-Gomez JM, Villegas A, Sanchez-Guzman JM (2011) Understanding the energetic costs of living in saline environments: effects of salinity on basal metabolic rate, body mass and daily energy consumption of a long-distance migratory shorebird. J Exp Biol 214:829–835. doi:10.1242/jeb.048223
Heinz G (1993) Selenium accumulation and loss in Mallard eggs. Environ Toxicol Chem 12:775–778. doi:10.1002/etc.5620120419
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes. 1. Turnover of C-13 in tissues. Condor 94:181–188
Hogstad O (1996) Accumulation of cadmium, copper and zinc in the liver of some passerine species wintering in central Norway. Sci Total Environ 183:187–194. doi:10.1016/0048-9697(95)05060-4
Holmes RT (1971) Latitudinal differences in breeding and molt schedules of Alaskan Red-backed Sandpipers (Calidris alpina). Condor 73:93–99
Hui CA (1998) Metal and trace element burdens in two shorebird species at two sympatric wintering sites in southern California. Environ Monit Assess 50:233–247
Hui CA, Beyer WN (1998) Sediment ingestion of two sympatric shorebird species. Sci Total Environ 224:227–233
Hui CA, Takekawa JY, Warnock SE (2001) Contaminant profiles of two species of shorebirds foraging together at two neighboring sites in south San Francisco Bay, California. Environ Monit Assess 71:107–121
Jackson N, Stevenson MH, Kirkpatrick GM (1979) Effects of the protracted feeding of copper sulphate-supplemented diets to laying, domestic fowl on egg production and on specific tissues, with special reference to mineral content. Br J Nutr 42:253–266
Kim J, Koo T (2008) Heavy metal concentrations in feathers of Korean shorebirds. Arch Environ Contam Toxicol 55:122–128. doi:10.1007/s00244-007-9089-y
Kim J, Park S, Koo T (2007) Lead and cadmium concentrations in shorebirds from the Yeongjong Island, Korea. Environ Monit Assess 134:355–361
Kuwae T, Beninger PG, Decottignies P, Mathot KJ, Lund DR, Elner RW (2008) Biofilm grazing in a higher vertebrate: the Western Sandpiper, Calidris mauri. Ecology 89:599–606. doi:10.1890/07-1442.1
Kuwae T, Miyoshi E, Hosokawa S, Ichimi K, Hosoya J, Amano T, Moriya T, Kondoh M, Ydenberg RC, Elner RW (2012) Variable and complex food web structures revealed by exploring missing trophic links between birds and biofilm. Ecol Lett 15:347–356. doi:10.1111/j.1461-0248.2012.01744.x
Levengood JM, Sanderson GC, Anderson WL, Foley GL, Skowron LM, Brown PW (1999) Acute toxicity of ingested zinc shot to game-farm Mallards. Ill Nat Hist Surv Bull 36:1–36
Lewis SA, Furness RW (1991) Mercury accumulation and excretion in laboratory reared Black-headed gull Larus ridibundus chicks. Arch Environ Chem Toxicol 21:316–320
Lucia M, Andre J, Gontier K, Diot N, Veiga J, Davail S (2010) Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminium, nickel, arsenic, and selenium) in some aquatic birds of the southwest Atlantic Coast of France. Arch Environ Contam Toxicol 58:844–853. doi:10.1007/s00244-009-9393-9
Luoma SN, Davis JA (1983) Requirements for modeling trace metal partitioning in oxidized estuarine sediments. Mar Chem 12:159–181
Mathot KJ, Lund DR, Elner RW (2010) Sediment in stomach contents of Western Sandpipers and Dunlin provide evidence of biofilm feeding. Waterbirds 33:300–306. doi:10.1675/063.033.0305
McCormick J, St. Clair CT, Bendell LI (2014) Concentrations and partitioning of metals in intertidal biofilms: implications for metal bioavailability to shorebirds. Ecotoxicology 23:229–235
McFarland C, Bendell-Young L, Guglielmo C, Williams T (2002) Kidney, liver and bone cadmium content in the Western Sandpiper in relation to migration. J Environ Monit 4:791–795. doi:10.1039/b206045k
McWilliams SR, Karasov WH (2001) Phenotypic flexibility in digestive system structure and function in migratory birds and its ecological significance. Comp Biochem Physiol A 128:579–593
Michener R, Lajitha K (2007) Stable isotopes in ecology and environmental science, 2nd edn. Blackwell Publishing, Singapore
Myklebust I, Pedersen H (1999) Accumulation and distribution of cadmium in Willow Ptarmigan. Ecotoxicology 8:457–465. doi:10.1023/A:1008912003597
Nielson SE, Boyce MS, Stenhouse GB (2004) Grizzly bears and forestry I. Selection of clearcuts by grizzly bears in west-central Alberta. Can Forest Ecol Manag 199(1):51–65
Norris DR, Lank DB, Pither J, Chipley D, Ydenberg RC, Kyser TK (2007) Trace element profiles as unique identifiers of Western Sandpiper (Calidris mauri) populations. Can J Zool 85:579–583. doi:10.1139/Z07-024
Nriagu JO (1989) A global assessment of natural sources of atmospheric trace metals. Nature 338:47–49
Oh S, Nakaue H, Deagen J, Whanger P, Arscott G (1979) Accumulation and depletion of zinc in chick tissue metallothioneins. J Nutr 109:1720–1729
Ohlendorf HM, Heinz GH (2011) Selenium in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota. CRC Press, Boca Raton
Prater AJ, Marchant AJ, Vuorinen J (1977) Guide to the identification and ageing of Holarctic Waders, field guide 17. British Trust for Ornithology, Tring
Scheuhammer AM (1987) The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: a review. Environ Pollut 46:263–295
Schlekat C, Decho A, Chandler G (1998) Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ Toxicol Chem 17:1867–1874. doi:10.1897/1551-5028(1998)017<1867:SOCTBE>2.3.CO;2
Shepherd PCF (2001) Space use, habitat preferences, and time-activity budgets of non-breeding Dunlin (Calidris alpina pacifica) in the Fraser River Delta, B.C. Phd dissertation, Simon Fraser University, BC
Sileo L, Beyer W, Mateo R (2003) Pancreatitis in wild zinc-poisoned waterfowl. Avian Pathol 32:655–660. doi:10.1080/03079450310001636246
Spallholz JE, Hoffman DJ (2002) Selenium toxicity: cause and effects in aquatic birds. Aquat Toxicol 57:27–37
St. Clair CT (2012) Heavy metals, selenium and pacific Dunlin: patterns of accumulation, exposure from prey and toxicity risks. MSc thesis, Simon Fraser University
Stein W, Williams TD (2006) Causes and consequences of post-growth age-dependent differences in small intestine size in a migratory sandpiper (Calidris mauri, Western Sandpiper). Funct Ecol 20:142–150
Stock M, Herber RFM, Geron HMA (1989) Cadmium levels in oystercatcher Haematopus ostralegus from the German Wadden Sea. Mar Ecol Prog Ser 53:227–239
Tessier A, Campbell P (1987) Partitioning of trace-metals in sediments—relationships with bioavailability. Hydrobiologia 149:43–52. doi:10.1007/BF00048645
Tessier A, Buffle J, Campbell PGC (1994) Uptake of trace metals by aquatic organisms. In: Buffle J, Devitre RR (eds) Chemical and biological regulation of aquatic systems. CRC Press, Boca Raton, pp 197–231
Thomas CA, Bendell-Young LI (1998) Linking the sediment geochemistry of an intertidal region to metal bioavailability in the deposit feeder Macoma balthica. Mar Ecol Prog Ser 173:197–213
Thompson DR (1996) Mercury in birds and terrestrial mammals. In: Beyer WN, Heinz GH, Redmond-Norwoods AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations. CRC Press, Boca Raton
U.S. Department of the Interior (U.S. Fish and Wildlife Service, Bureau of Reclamation, Geological Survey, Bureau of Indian Affairs) (1998) R.A. Engberg (ed), Guidelines for interpretation of the biological effects of selected constituents in biota, water, and sediment: national irrigation water quality program, USDOI, BOR, Denver, CO, pp 139–184 (http://www.usbr.gov/niwqp/guidelines/)
Vancouver Airport Fuel Facilities Corporation (VAFFC) (2012) Vancouver airport fuel delivery project, environmental assessment certificate application, supplement 5: Fraser River Delta biofilm: Sensitivity to a jet A fuel spills—summary report
Vermeer K, Castilla J (1991) High cadmium residues observed during a pilot-study in shorebirds and their prey downstream from the El-Salvador copper mine, Chile. Bull Environ Contam Toxicol 46:242–248
Warnock ND, Takekawa JY, Bishop MA (2004) Migration and stopover strategies of individual Dunlin along the Pacific coast of North America. Can J Zool 82:1687–1697. doi:10.1139/Z04-154
Wayland AM, Scheuhammer M (2011) Cadmium in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota. CRC Press, Boca Raton
Wedekind KJ, Titgemeyer EC, Twardock AR, Baker DH (1991) Phosphorus, but not calcium affects manganese absorption and turnover in chicks. J Nutr 121:1776–1786
White D, Finley M (1978) Uptake and retention of dietary cadmium in Mallard ducks. Environ Res 17:53–59. doi:10.1016/0013-9351(78)90060-9
White D, King K, Prouty R (1980) Significance of organochlorine and heavy-metal residues in wintering shorebirds at Corpus Christi, Texas, 1976-77. Pestic Monit J 14:58–63
Acknowledgments
Funding for this research was provided by SFU, a NSERC Discovery grant to L. Bendell, and Environment Canada via the Centre for Wildlife Ecology at SFU. O. Busby, G. Slater, and D. Ball assisted in sample acquisition. L. Evans Ogden and D. Lank provided a foundation of logistical and ecological knowledge. Assistance with permitting, field and lab work, data analysis, and figure production was provided by K. Chan, H. Walling, C. Smith, J. Barrett, P. van Veelen, W. Stein, U. Somjee, C. Palomera, K. Pillay, L. Du Gas, K. Gorman, K. Wegner, G. Leung, and A. Bykov. Comments from two anonymous reviewers also helped to improve the manuscript. We acknowledge the support of IIRMES at Cal. State Long Beach and SIF at UC Davis. Finally, we recognize the sacrifice of the animals examined in this research so that we could learn to better address their needs and stresses in the future.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
St. Clair, C.T., Baird, P., Ydenberg, R. et al. Trace elements in pacific Dunlin (Calidris alpina pacifica): patterns of accumulation and concentrations in kidneys and feathers. Ecotoxicology 24, 29–44 (2015). https://doi.org/10.1007/s10646-014-1352-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1352-1