Abstract
We compared zinc, copper and cadmium concentrations and the operationally defined geochemical partitioning of the three metals in sediments enriched with biofilm versus sediments without obvious biofilm present (reference) sampled from five locations within the Fraser River Delta, British Columbia, Canada. Two-way ANOVA’s with site and biofilm (enriched or reference) as the two factors were applied to determine if metal concentrations or the partitioning of the metal was dependent on the two factors. Sediment enriched in biofilm contained greater amounts of aqua regia extracted zinc and copper and tended to have greater amounts of reducible cadmium as compared to reference sediments. By contrast, reference sediments had greater concentrations of easily reducible copper suggesting differences in speciation between the two sediment types. Greater concentrations of reducible cadmium within biofilm may provide a route of contaminant exposure to shorebirds whose diet is dependent on biofilm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intertidal regions of the world’s coastlands are critical habitat for a number of shorebirds such as the Western Sandpiper (Calidris mauri) and Dunlin (Calidris alpina). Estuarine muddy embayments for example, the Fraser River Delta (FRD), British Columbia, Canada, are used as key migratory stop over sites where shorebirds enroute either north to breeding grounds or south to their overwintering sites, feed to gain the energy required to make the journey. A primary food source of the Western Sandpiper and to a lesser extent the Dunlin at their stop over site within the FRD is biofilm which is produced within the nutrient rich sediments of the delta (Elner et al. 2005; Kuwae et al. 2008). It has been estimated that biofilm can account for close to 60 % of their total diet, or 50 % of their required daily energy budget (Kuwae et al. 2008).
Biofilm is a thin (0.01–2 mm), dense layer of microbes, organic detritus and sediment in a mucilaginous matrix of extracellular polymeric substances together with non-carbohydrate components secreted by microphytobenthos and benthic bacteria (reviewed in Decho 2000). Decho (2000) highlighted the importance of biofilms within intertidal environments in both acting as a sediment stabilizer as well as an efficient trophic-transfer vehicle for the entry of contaminants into food webs. These films or coatings have been shown to exhibit high affinities for divalent cationic metals actively participating in the binding of dissolved overlying and pore-water metals in estuarine sediments (Schlekat et al. 1998). Indeed, studies within freshwater environments have indicated that freshwater biofilms accumulate metals at concentrations often greater than in sediments (e.g. Horvath et al. 2013; Ancion et al. 2010; 2013). The partitioning of metals within the biofilm and its relative bioavailability, however, has yet to be well studied, in either freshwater or saline/estuarine conditions.
When feeding on biofilm, the shorebird ingests both sediment and the associated biofilm. The bioavailability of sediment bound metals to sediment ingesting organisms depends on the concentration of the metal and the nature of the geochemical component with which the metal is associated. The most important geochemical components considered to influence the bioavailability of metals to sediment ingesting organisms have been shown to be organic matter, iron oxides, and manganese oxides (e.g., Bendell-Young et al. 2002). For example, trace metals such as zinc or cadmium associated with oxides of manganese and iron are thought to be more available as compared to metals associated with the residual components of surface sediments (e.g., Thomas and Bendell-Young 1998). The partitioning of the metal within a biofilm may also be geochemically dependent which in turn could influence metal availability to higher trophic levels. This would be especially relevant to shorebirds which will ingest biofilm as a major part of their diet (e.g., Elner et al. 2005). Therefore, the objectives of this study were two-fold; (1) to determine if biofilm collected from sediment within the intertidal contained greater metal concentrations as compared to reference sediment (sediment with no obvious biofilm present) and (2) through the application of the simultaneous extraction scheme of Bendell-Young and Harvey (1992), to determine if metals within biofilm are potentially more available as compared to reference sediments.
Greater concentrations of cadmium have been reported in tissues of Western Sandpiper (McFarland et al. 2002) as compared to other shorebirds that derive a smaller part of their diet/energy requirements from biofilm (Clair 2013). Zinc and copper have also been reported to be elevated within intertidal sediments (Thomas and Bendell-Young 1998). Hence, we analyzed reference and biofilm enriched sediments for cadmium, copper and zinc. We hypothesized that sediment enriched in biofilm sampled from the FRD would have greater metal content as compared to sediment without biofilm present and thus provide an important exposure route to the shorebird. We hope that such information will provide a more comprehensive understanding as to the routes of metal exposure to shorebirds and hence the risk that feeding behavior presents with respect to exposure to metals such as cadmium which can be toxic at low doses.
Materials and methods
Study site and sampling
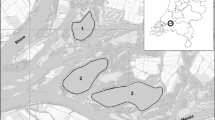
Samples were taken in early summer 2012 from Roberts Bank, B.C., located 20 km south of Vancouver (Fig. 1a ,b). GPS Coordinates are; Transect A: N49°03′34.3″, W123°09′01.2″, Transect B: N49°03′23.8″, W123°08′54.7″ Transect C: N49°03′10.3″, W123°08′32.9″, Transect D: N49°03′14.9″, W123°08′30.6″, Transect E: N49°03′21.5″, W123°08′39.8″ (Fig. 1a, b). Details of Roberts Bank including tides and climate can be found in Bigley (1981). Five randomly selected transects were made approximately 100 meters apart and perpendicular to the shoreline. We assessed biofilm presence visually by noticing what areas had film or coloured growth on top (after Couch 1989). After observing transects for biofilm levels (where dense, brown micro-algal mats were apparent), six samples from each transect (with three representing high, and three representing low biofilm levels) totaling 30 samples were taken. Sediments with biofilm present are referred to as biofilm enriched, sediments with no biofilm obviously present are referred to as reference. Sediment with and without biofilm were collected by gently scraping with toothbrushes previously rinsed with distilled deionized water the upper 1 mm of surface sediment. To prevent microbial and chloroplast activity in response to temperature and light, samples were placed in sealed centrifuge tubes, covered in tinfoil, and into a cooler. After collection, samples were stored at 4 °C for 18 h prior to centrifugation for 20 min at 6,000 rpm (657.4 G) to remove pore water. Samples were refrigerated again for a maximum of 60 h until processing.
Location of Roberts Bank (a) and approximate location of the five study sites (b). RB GPS coordinates; Transect A N49°03′34.3″, W123°09′01.2″, Transect B N49°03′23.8″, W123°08′54.7″, Transect C N49°03′10.3″, W123°08′32.9″, Transect D N49°03′14.9″, W123°08′30.6″, Transect E N49°03′21.5″, W123°08′39.8″. Maps from google maps
Simultaneous extraction
The simultaneous extraction procedure used separates metal from sediment and sediment-biofilm mixtures into three operationally defined components: easily reducible (ER or associated with oxides of Mn), reducible (R or associated with oxides of Fe), and organically bound metal (ORG), and aqua regia (AR) extracted metal (near total dissolution of metals) (see Bendell-Young and Harvey 1992; Thomas and Bendell-Young 1998 for complete details).
Four 0.9 g sub-samples were taken from each sample and subjected to one of four treatments representing either the ER, R, ORG, or AR extraction. Each sub-sample received 13.5 mL of reagent (for a 15:1 solution:solid ratio), and was digested as outlined in Bendell-Young and Harvey (1992) and Thomas and Bendell-Young (1998). Two blanks and two MESS-3 samples (provided by National Research Council of Canada) were included in each extraction. Acid extracts were removed from samples by centrifuging for 20 min at 6,000 rpm (657.4g) and refrigerated in the dark at 4 °C in sealed plastic vials for no more than one month until analysis (with the exception of the ER mixtures which were left out of refrigeration for one night following the extraction with loosely tightened seals to allow off-gassing).
Metal concentrations
Extracts were analyzed for concentrations of cadmium, copper, and zinc with a Perkin–Elmer model 100 Flame Atomic Absorption Spectrometer (AAS) at Simon Fraser University in Burnaby, British Columbia. Because of the high recovery of metal within the AR fraction, extracts were diluted to 5 % with 84.5 mL of distilled deionized water added to 12.5 mL of extract prior to analyses. Metal concentrations in the ER, R, ORG, and AR extracts were determined by standard calibration curves of absorbance versus known metal concentrations. Quality assurance and quality control of the analyses was ensured through inclusion of blanks and standards. No background levels of metals were detected within the blanks and precision of the analysis as indicated by replicate measures of the MESS-3 samples was within 5 %.
Dry weight and %LOI
To convert sediment-biofilm metal concentrations to a dry weight basis, wet/dry weight ratio was first determined by drying 2 g of each sample to a constant weight at 60 °C for 24 h and the wet/dry weight ratio used for standardization. Organic content was measured by heating dried samples to 600 °C for 1 h to burn all organic matter and weight change recorded, allowing calculation of loss on ignition (LOI %).
Chlorophyll a
Chlorophyll a was used as a proxy for biofilm (allowing us to compare metal concentrations against biofilm levels, Underwood and Smith 1998, see also MacDonald et al. 2012). Chlorophyll a extractions were performed according to the methods of Danovaro 2009. Within 6 days of sample collection, 0.1 g MgCO3 and 8 mL 90 % acetone were combined with 1 g sediment in glass tubes. To homogenize the mixture, tubes were shaken using a vortex for 30 s in the dark and sonicated for 3 min to rupture chloroplast membranes and allow pigment to elute. Mixtures were stored overnight in the dark at 4 °C to allow chlorophyll a to extract before centrifugation. 2.5 mL of supernatant was pipetted into cuvettes and spectrophotometric readings taken at 750 and 665 nm (against a blank of 90 % acetone). To measure and correct for phaeo-pigment absorption, 200 μL 0.1 N HCl was added, and the mixture let stand for 20 s before readings were repeated. Chlorophyll a concentration was then calculated as outlined in (Danovaro 2009).
Statistical analysis
All statistical analysis were performed by SigmaPlot© version 12.5. Two-way ANOVA’s were applied to determine if chlorophyll a, %LOI, metal concentrations recovered in the operationally defined fractions, the sum of metals recovered from the three fractions (ER + R + ORG metal) and aqua regia extracted metal, were dependent on treatment, site, or the interaction between treatment and site. Treatment is either reference or biofilm enriched sediment and site is the five sampling locations A–E. If required, data was log-transformed to meet assumptions of normality and equal variances.
Results and discussion
Our objectives were to determine if biofilm collected from sediment within the intertidal contained greater metal concentrations as compared to reference sediment through the application of the simultaneous extraction scheme of Bendell-Young and Harvey (1992). This aimed to determine if metals within biofilm are potentially more available as compared to metals associated just with surficial sediment (reference sediments).
Metal concentrations and partitioning in biofilm versus sediment samples
Sediments selected as reference sediments with no biofilm obviously present were significantly lower in %LOI and chlorophyll a as compared to sediments that were visually enriched in biofilm (Table 1; Fig. 2). While there was no difference in total amounts of metal recovered from the reference and biofilm enriched sediments (Table 1; Fig. 3a–f), there were site differences and significant differences in amounts of metal recovered from the three fractions. Zinc and copper extracted via aqua regia was greater in biofilm enriched sediments (Table 1; Fig. 4a–c), as was copper recovered in the reducible fraction (associated with oxides of iron). Amounts of reducible cadmium also tended to be greater in biofilm enriched sediments as compared to reference sediments (Table 1; Fig. 4d). By contrast, concentrations of copper recovered by the easily reducible extract were greater in reference rather than biofilm enriched sediments (Table 1; Fig. 4e).
Fractions with a significant difference between treatments (reference vs. biofilm enriched sediment; two-way ANOVA p < 0.05). Results are averages with SE (n = 3). See also Table 1
The partitioning of zinc, copper and cadmium within intertidal surficial sediments determined in the current study has been noted in previous studies. For example, Thomas and Bendell-Young (1998) applied the same extraction procedure as the current study and similar to our findings, noted that metal partitioning was highly site dependent. These authors also found that for surficial sediment, very little zinc was associated with the organic fraction and most was recovered from the reducible (associated with oxides of iron) or from the residual fraction (those metals recovered in the aqua regia extract) of sediment. And, in agreement with Davies-Colley et al. (1984) and Kersten and Forstner (1987), cadmium was found mostly associated with oxides of manganese and iron, an important reservoir for cadmium, rather than the residual or organic fraction. However, unlike our findings, Thomas and Bendell-Young (1998) recovered copper only in the reducible fraction, whereas we found copper distributed between both the reducible and organic fractions of sample.
An important difference between our studies and previous studies on trace metal partitioning within intertidal surficial sediments is the method of sampling. Studies of Thomas and Bendell-Young (1998) for example sampled the upper 1–5 mm of sediment by skimming the surface to collect the most “biologically active” portion of surface sediment (Luoma and Bryan 1981). An important aspect of our sampling was to reproduce the feeding behavior of the shore bird. The use of tooth brushes to collect the thin layer of surface sediment is thought to capture the micro-topography of the surface as well as the action of shorebird tongue spines (Kuwae et al. 2012). It is likely our sampling method captures a more organically enriched fraction of sediment and hence metal associated with the organic fraction of sediment as compared to that of Thomas and Bendell-Young (1998). Comparisons of organic matter content of sampled sediments supports this with surface sediments of Thomas and Bendell-Young (1998) being approximately 2–3 % organic matter whereas sediments recovered by toothbrush average approximately twice that amount.
Greater recovery of aqua regia extracted zinc and copper and reducible copper from samples enriched with biofilm may be due to the greater amounts of diatomaceous biofilm as indicated by Chlorophyll a as zinc and copper are required for algae growth (Knauer et al. 2009). Ancion et al. (2013) in their study on the metal content of biofilm from 13 sites within New Zealand estuaries found that concentrations of zinc and copper were generally greater in biofilm than in sediments and the enrichment could be correlated to the differences in composition observed between biofilm and sediments. Horvath et al. (2013) however, found no difference in amounts of cadmium and copper sequentially extracted from biofilm and sediment samples.
Concentrations of easily reducible copper by contrast to zinc and cadmium were greater in all reference sediments as compared to biofilm samples, and in location E, sixfold greater in recovered amounts. This suggests differences in copper associations between sediment and biofilm. Lawrence et al. (2012) recently reported on the fate of copper nanoparticles in river biofilms using scanning transmission X-ray microscopy. These authors noted that when copper nanoparticles were added to the biofilm the nanoparticles formed aggregates which formed association with the diatoms and extracellular polymeric substances of the biofilm. It is likely that this copper would be much more difficult to recover from the biofilm as compared to copper associated with sediment in the absence of biofilm to complex it. This difference in partitioning then may in part explain the greater recovery of ER copper from reference as compared to biofilm enriched sediments.
Implications of metal bioavailability to biofilm ingesting shorebirds
Greater concentrations of cadmium have been reported in tissues of Western Sandpiper (McFarland et al. 2002) as compared to other shorebirds that derive a smaller part of their diet/energy requirements from biofilm (Clair 2013). Greater amounts of reducible cadmium recovered from biofilm enriched samples as compared to reference samples suggest that some of this body burden may in part be attributed to this diet source. Thomas and Bendell-Young (1998) have also noted that the existence of cadmium entirely in the labile fraction of sediment (ER and R) could represent a risk to any exposed organisms. Shorebirds could also be exposed to greater amounts of zinc and copper via biofilm, however, unlike cadmium, zinc and copper are required nutrients and exposure via the ingestion of biofilm may not be of toxicological concern. As shorebirds seem to be able to discern regions of the intertidal that is rich with biofilm (e.g., Elner et al. 2005), the high concentrations of easily reducible copper in sediments without biofilm would likely not pose a toxicological risk unless the birds are directly ingesting surficial sediment independent of biofilm.
In conclusion, the partitioning of zinc, copper and to a lesser extent cadmium were dependent on the presence of biofilm with greater amounts of aqua regia extracted zinc and copper and reducible cadmium being recovered from biofilm as compared to reference sediments. Exposure to cadmium via biofilm could potentially be of toxicological concern as shorebirds such as the Western Sandpiper will ingest biofilm in amounts of close to 50 % of its overall energy requirement.
References
Ancion PY, Lear G, Lewis GD (2010) Three common metal contaminants of urban runoff (zinc, copper & lead) accumulate in freshwater biofilm and modify embedded bacterial communities. Environ Pollut 158:2738–2745
Ancion PY, Lear G, Dopheide A, Lewis G (2013) Metal concentrations in stream biofilm and sediments and their potential to explain biofilm microbial community structure. Environ Pollut 173:117–124
Bendell-Young L, Harvey HH (1992) The relative importance of manganese and iron oxides and organic matter in the sorption of trace metals by surficial lake sediments. Geochim Cosmochim Acta 56:1175–1186
Bendell-Young LI, Thomas CA, Stecko JRP (2002) Contrasting the geochemistry of oxic sediments across ecosystems: a synthesis. Appl Geochem 17:1563–1582
Bigley RE (1981) The population biology of two intertidal seagrasses Zostera japonica and Ruppia maritima, at Roberts Bank, British Columbia. MSc Thesis, University of British Columbia
Couch C (1989) Carbon and nitrogen stable isotopes of meiobenthos and their food resources. Estuar Coast Shelf Sci 28:433–441
Danovaro R (2009) Photosynthetic pigment concentrations in marine sediments. In: Danovaro R (ed) Methods for the study of deep-sea sediments, their functioning and biodiversity. CRC Press, Ancona, pp 45–51
Davies-Colley RJ, Nelson PO, Wilhamson IH (1984) Copper and cadmium uptake by estuarine sedimentary phases. Environ Sci Technol 18:491–500
Decho A (2000) Microbial biofilms in intertidal systems: an overview. Cont Shelf Res 20:1257–1273
Elner RW, Beninger PG, Jackson DL, Potter TM (2005) Evidence of a new feeding mode in western sandpiper (Calidris mauri) and dunlin (Calidris alpina) based on bill and tongue morphology and ultrastructure. Mar Biol 146:1223–1234
Horvath M, Halasz G, Kucanova E, Kucikova B, Fekete I, Remeteiova D, Heltai G, Florian K (2013) Sequential extraction studies on aquatic sediment and biofilm samples for the assessment of heavy metal mobility. Microchem J 107:121–125
Kersten M, Forstner U (1987) Cadmium associations in fresh-water and marine sediment. In: Nriagu JO, Sprague JB (eds) Cadmuim in the aquatic environment. Wiley, Toronto, pp 51–89
Knauer K, Behra R, Sigg L (2009) Effects of free Cu2+ and Zn2+ ions on growth and metal accumulation in freshwater algae. Environ Toxicol Chem 16:220–229
Kuwae T, Beninger PG, Decottignies P, Mathot KJ, Lund DR, Elner RW (2008) Biofilm grazing in a higher vertebrate: the western sandpiper, Calidris mauri. Ecology 89:599–606
Kuwae T, Miyoshi E, Hosokawa S, Ichimi K, Hosoya J, Amano T, Moriya T, Kondoh M, Ydenberg RC, Elner R (2012) Variable and complex food web structures revealed by exploring missing trophic links between birds and biofilm. Ecol Lett 15(347):356
Lawrence JR, Dynes JJ, Korber DR, Swerhone GDW, Leppard GG, Hitchcock AP (2012) Monitoring the fate of copper nanoparticles in river biofilms using scanning transmission X-ray microscopy (STXM). Chem Geol 329:18–25
Luoma SN, Bryan GW (1981) A statistical assessment of the form of trace metal partitioning in oxidized estuarine sediments. Mar Chem 12:159–181
MacDonald EC, Ginn MG, Hamilton D (2012) Variability in foraging behavior and implications for diet breadth among semipalmated sandpipers staging in the upper Bay of Fundy. Condor 114:135–144
McFarland C, Bendell-Young L, Guglielmo C, Williams T (2002) Kidney, liver and bone cadmium content in the Western Sandpiper in relation to migration. J Environ Monit 4:791–795
Schlekat CE, Decho AW, Chandler GT (1998) Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ Toxicol Chem 17:1867–1874
St Clair TC (2013) Trace elements in Pacific Coast Dunlin (Calidris alpina pacifica): patterns of accumulation and concentrations in kidneys and feathers. MSc Thesis, Simon Fraser University, Burnaby BC
Thomas CA, Bendell-Young LI (1998) Linking the sediment geochemistry of an intertidal region to metal bioavailability in the deposit feeder Macoma balthica. Mar Ecol Prog Ser 173:197–213
Underwood GJC, Smith DJ (1998) Predicting epipelic diatom exopolymer concentrations in intertidal sediments from sediment chlorophyll a. Microb Ecol 35:116–125
Acknowledgements
Funding was provided through an NSERC research scholarship to JM and a NSERC discovery grant to LB. The technical assistance of K. Chan for the cholorphyll a analysis is greatly appreciated.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCormick, J., Clair, C.T.S. & Bendell, L.I. Concentration and partitioning of metals in intertidal biofilms: implications for metal bioavailability to shorebirds. Ecotoxicology 23, 229–235 (2014). https://doi.org/10.1007/s10646-013-1166-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1166-6