Abstract
The aim of this study was to investigate the effects of coal mining activities on the community structures of woody and herbaceous plants. The response of individual plants of community to defilement caused by coal mining was also assessed. Air monitoring, soil physico-chemical and phytosociological analyses were carried around Jharia coalfield (JCF) and Raniganj coalfield. The importance value index of sensitive species minified and those of tolerant species enhanced with increasing pollution load and altered soil quality around coal mining areas. Although the species richness of woody and herbaceous plants decreased with higher pollution load, a large number of species acclimatized to the stress caused by the coal mining activities. Woody plant community at JCF was more affected by coal mining than herbaceous community. Canonical correspondence analysis revealed that structure of herbaceous community was mainly driven by soil total organic carbon, soil nitrogen, whereas woody layer community was influenced by sulphur dioxide in ambient air, soil sulphate and soil phosphorus. The changes in species diversity observed at mining areas indicated an increase in the proportion of resistant herbs and grasses showing a tendency towards a definite selection strategy of ecosystem in response to air pollution and altered soil characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the historically recent period of worldwide industrialization, the level of human population has been closely related to the amount of energy we have used (Krausmann et al. 2009). Currently energy is mostly produced by burning of fossil fuel such as coal (Veziroglu and Sahin 2008). In order to encounter the energy requisite, the overall coal production and coal mining have staggeringly increased in India, which ranks third amongst top ten coal producing countries (World Coal Association 2011). Mining operations have the potential to demolish flora and fauna and contaminate soil, air and water in the surrounding areas (Ejaz et al. 2014; Si et al. 2010). As a result, deterioration of ecosystem may take place with the loss of sensitive species.
The harmful effects of air pollutants on vegetation have been reported widely (Agrawal and Agarwal 2000; Emberson et al. 2001). Narayan et al. (1994) reported the effects of fluoride pollution on plant community structure at different downwind directions of an aluminium factory. Singh et al. (1994) also showed negative effects of coal fired thermal power plant emissions on herbaceous community structure. Soil nutrient and physico-chemical properties also affect plant communities (de Deyn et al. 2004; Dutta and Agrawal 2003; Hernández and Pastor 2008). Coal mining and active mine fires, vehicular emissions and windblown dust through unpaved roads and overburdens are the main contributors of air pollutants around coal mining areas (Pandey et al. 2014). Sulphur dioxide (SO2), nitrogen dioxide (NO2) and total suspended particulates (TSP) are the important air pollutants around coal mining areas (Pandey et al. 2014). In coal mining areas, the top soil gets blended with overburden materials and loosely bound soil of overburden through wind may also affect the soil physico-chemical properties (Rai et al. 2010).
Plant community and their species carry out variety of biological services in an ecosystem viz., protecting areas from soil erosion (Trabucchi et al. 2013), defending from floods and other destructive weather conditions (Depietri et al. 2012), reducing the risk of local and global climate change (Mori et al. 2013), recycling nutrients (Jones et al. 2013), pollination and biological control of diseases (Kremen et al. 2007), controlling pollutants and of enormous economic values (Grimm et al. 2008). Plant community structure depends on various environmental factors such as climatic and edaphic factors (Narayan et al. 1994).
Plant species are distributed in variable habitats, but within the limits of the area, species are most abundant in places which represent their ecological optimum, and hence the composition of plant communities is a function of changing habitat conditions along the environmental gradients (Bello et al. 2012). Different air pollutants and altered soil physico-chemical properties affect individual plants and their population via various mechanisms of action, and thus influence the plant community structure, because the optimum value of environmental gradient is different for different species. Plant community structure of an area tends to become established by the selection pressures of the environment. The superimposition of severe pressures on plant community sometimes occurs to allow for feedback mechanisms to operate for selection of resistant species. There is now much concern that the stability of various plant communities has been seriously threatened by increasing concentrations of air pollutants and disturbed soil quality (Gupta et al. 2013; Ulrich et al. 2013).
We studied the air pollution status, soil quality, woody and ground layer plant diversity parameters around Jharia (Jharkahnd, India) and Raniganj (West Bengal, India) coal mining areas. We hypothesized that plant community structure in the woody and ground layer is strongly differentiated between coal mining and non-mining areas owning to contrasting air and soil conditions. The site far away from coal mining area will support greater plant diversity because of low loading of air pollutants and better soil quality. We used species distribution curve and constrained ordination (Canonical correspondence analysis—CCA) (Ter Braak 1986) to provide a quantitative assessment of the importance of the air and soil factors in controlling community composition and diversity. Our specific objectives were to determine the effects of coal mining on plant communities and to test the hypothesis that mixed stress (air+soil) affects differently the individuals of plant community.
Materials and methods
Study area
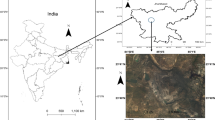
The present study was carried out in Jharia coalfield (JCF) (latitudes 23°39′ to 23°48′N, longitudes 86°11′ to 86°27′E, 222 m above mean sea level), located in Dhanbad district of Jharkhand state and Raniganj coalfield (RCF) (latitudes 23°25′ to 23°55′N, longitudes 86°40′ to 87°25′E, 117 m above mean sea level), located in Bardhaman district of West Begal state of India (Fig. 1a) during 2010–2011. JCF and RCF are the most exploited coalfields because of available metallurgical grade coal reserves. A reference site, Central Institute of Mining and Fuel Research (CIMFR) (latitudes 23°47′ to 23°49′N, longitudes 86°23′ to 86°25′E, 172 m above mean sea level), was also selected for comparing the air quality and plant community structure, situated about 6 km in north direction from Jharia and 55 km west from RCF (Fig. 1a). The climate is sub-tropical in the study region. The cool winter season is from November to February and May is the hottest month. It remains warm until the monsoon outbreaks towards the middle of June, when the rain starts and the temperature falls and humidity rises. The rainy season continues from July to October. The dominant wind directions in the area are SE/SSE with low calm conditions (5.3 %). Generally the dominant wind speed ranges between 4 and 6 m h−1 (Fig. 1b). The variations in meteorological parameters in the area are given in Fig. 1c.
Air monitoring
The air quality was monitored for TSP matter (High volume method), SO2 (improved West and Gaeke 1956) NO2 (modified Jacobs and Hochheiser 1958) once in a fortnight at each site for 8 h from 09:00 to 17:00 h during 2010–2011. The methods in detail are given in Pandey et al. (2014).
Soil analysis
Analyses of soil samples were carried out for pH, total porosity (TP), bulk density (BD), water holding capacity (WHC), total organic carbon (TOC), total nitrogen (N), available phosphorus (P) and sulphate (S) contents. At each site, five sub sites were selected and the soil samples were collected up to 15 cm depth with the help of an auger from three places of each sub site, every 3 months for two consecutive years from 2010 to 2011. Sites for soil sampling were the same where air monitoring was done. Three replicate samples of soil were collected from five sub sites of each selected site. The soil from three replicate sites of sub sites were mixed together to form a composite soil sample and three replicates were used for various analyses. Moisture content and soil pH were determined just after collection of samples, and rest of the soil was air dried and ground to pass through 2 mm sieve. For bulk density and water holding capacity, soil was collected up to 15 cm depth using the soil corer (6.5 cm diameter). Soil organic carbon was determined following modified method of Walkley and Black (1947). Total N in soil was determined using Gerhardt Automatic N Analyzer (Model KB8S, Frankfurt, Germany). Available P was extracted following Olsen et al. (1954) and estimated by the method of Dickman and Bray (1941). Available sulphur in soil was estimated following Williams and Steinbergs (1959).
Phytosociological analysis
Biodiversity analysis of the flora was conducted at three study sites (JCF, RCF and CIMFR) at the time of peak canopy biomass during October 2011. The sites for biodiversity analyses were the same where air monitoring and soil analyses were done. At each site, 25 sub sites were stabilized and 1 × 1 m2 size quadrats were used for herbaceous layer and 20 × 20 m2 size quadrats were used for sampling woody layer community. Presence and absence of species, number of the individual of species and their basal area in each quadrat were recorded. Plant species were identified through Flora of Bihar, India (Singh et al. 2001). Relative frequency, relative density, relative dominance, important value index (IVI), species diversity, β-diversity, species richness, similarity coefficient, equitability and concentration of dominance were calculated. The basal area of the species was taken as an index of dominance for tree species.
Statistical analyses
Air monitoring data and soil physico-chemical parameters were processed for one-way univariate analysis of variance (ANOVA) for examining the effects of site on different parameters. Duncan’s multiple range tests (DMRT) were performed as post hoc for various measurements after subjecting to one way ANOVA. To best explore the available data, we conducted the multivariate statistical analysis, CCA using XLSTAT-Pro (Version 2013.1, Addinsoft, Inc., Brooklyn, NY, USA) software for Windows, which can relate the quantitative changes of plant community to environmental gradient directly. For CCA, the five subplots were considered as separate site (15 sites). Eleven environmental variables related with air (TSP, SO2 and NO2) and soil quality (soil pH, TP, TOC, N, BD, P, WHC and S) were included. The statistical significance (at the 5 % level) of relationships between species data (species density) and environmental variables were assessed using the Monte Carlo test on 999 random permutations to test the null hypothesis that plant community were unrelated to environmental variables.
Results
Air monitoring
The average concentrations of air pollutants varied significantly between sites, and higher concentrations at JCF and RCF were recorded compared to CIMFR for both the years of monitoring (Online Resource 1). The trend of mean concentrations of SO2 and NO2 was JCF > RCF > CIMFR, while TSP showed a trend of RCF > JCF > CIMFR.
Soil physico-chemical analyses
Soil pH, TP, WHC, N and P were higher at CIMFR compared to coal mining sites (JCF and RCF) (Online Resource 2). Soil BD, TOC and S were lower at CIMFR than JCF and RCF (Online Resource 2). Significant variations were observed for all soil physico-chemical parameters between the sites except WHC (Online Resource 2).
Community analyses
The total number of herbaceous species was 23, 19 and 19 at CIMFR, JCF and RCF, respectively. Alternanthera paronychioides showed highest relative frequency at JCF followed by RCF, whereas the relative density, relative dominance and IVI were highest at CIMFR. Cynodon dactylon showed highest RF, RDo and IVI at CIMFR. RF and RDo of C. dactylon at RCF were higher than at JCF, while RD and IVI were higher at JCF (Table 1).
The number of woody species was 22, 18 and 20 at CIMFR, JCF and RCF, respectively (Table 2). Tree density and canopy cover were higher at CIMFR compared to JCF and RCF. Butea monosperma was the most dominant species (IVI = 44.4 and 35.9) followed by Ficus benghalensis (IVI = 38.0, 35.2), Ficus religiosa (IVI = 35.4, 32.2) and Psidium guajava (IVI = 28.9, 29.3) at JCF and RCF. These four species contributed 49.8 and 37.2 % of the total tree density at JCF and RCF, respectively (Table 2). At CIMFR, F. benghalensis (IVI = 31.3), F. religiosa (IVI = 29.4), Acacia catechu (IVI = 20.1) and Syzygium cuminii (IVI = 19.2) were dominant species accounting for 28.3 % of the total tree density. RF, RD and RDo for B. monosperma were highest at JCF followed by RCF. Alstonia scholaris, Buchanania lanzan, Delonix regia and Ficus tomentosa were absent at most polluted sites (Table 2 and Fig. 2).
The Shannon–Wiener diversity index was highest at CIMFR followed by RCF and JCF both for the herbaceous and woody vegetation (Table 3). β-diversity was 1.89, 1.75 and 1.72 for herbaceous vegetation, while 3.11, 2.64 and 2.87 for woody layer at CIMFR, JCF and RCF, respectively. The maximum concentration of dominance and minimum species richness were recorded at the most polluted site i.e. JCF (Table 3).
Species richness was 6.022, 5.061 and 5.004 for herbaceous vegetation and 8.97, 7.36 and 8.22 for woody layer at CIMFR, JCF and RCF, respectively. Evenness for herbaceous species was highest at RCF followed by JCF and CIMFR. Evenness for tree species was highest at CIMFR followed by JCF and RCF (Table 3). JCF and RCF have similarity coefficient of 0.947, while similarity coefficients for RCF with CIMFR and JCF with CIMFR were 0.857 for herbaceous species. For woody layer, the similarity coefficient for JCF–RCF, RCF–CIMFR and JCF–CIMFR were 0.947, 0.857 and 0.850, respectively (Online Resource 3).
Within the CCA eigenvector analysis, we observed that most of the inertia was carried by the first axis. With the second axis, we obtained 86.5 and 75 % of the total inertia for herbaceous and tree species, respectively. Therefore, the relationships between the species and the variables were analyzed by two-dimensional CCA map. The synthetic gradient extracted by CCA ordination diagram consists the points showing species and classes for qualitative environmental variables, and arrows for quantitative environmental variables (Fig. 3a, b). The total inertia (the summation of canonical and non-canonical eigenvalues) was 5.521 for ground vegetation and 4.593 for woody layer. The eigenvalues for two axes were 0.026 and 0.011 for herbaceous vegetation, and 0.027 and 0.026 for woody layer.
Ordination of the herbaceous (a) and woody species (b) species by canonical correspondence analysis. TOC, total organic carbon; WHC, water holding capacity; Bd, Bulk density; TP, total porosity; N, total nitrogen; P, total phosphorus; S, Sulphate; Aa, Achyranthes aspera; Ac, Ageratum conyzoides; Ap, Alternanthera paronychioides; As, Amaranthus spinosus; Bod, Boerhaavia diffusa; Ca, Cassia alata; Cha, Chrysopogon aciculatus; Coa, Convolvulus alsinoides; Cd, Cynodon dactylon; Cr, Cyperus rotundus; Dt, Desmodium trifolium; Dia, Dichanthium annulatum; Ds, Digitaria sanguinalis; Ea, Eclipta alba; Ec, Eragrostis cynosuroides; Eva, Evolvulus alsinoides; Hc, Heteropogon contortus; Ob, Ocimum basilicum; Ph, Parthenium hysterophorus; Sg, Setaria glauca; Sc, Sida cordifolia; Sn, Solanum nigrum; Sx, Solanum xanthocarpum; Si, Sphaeranthus indicus; Tp, Tephrosia purpurea; Aa, Acacia Arabica; Ac, Adina cordifolia; Ah, Artocarpus heterophyllus; Ai, Azadirachta indica; Al, Albizia lebbeck; Am, Aegle marmelos; As, Alstonia scholaris; Aca, Acacia catechu; Bc, Bombax ceiba; Bf, Borassus flabellifer; Bl, Buchanania lanzan; Bm, Butea monosperma; Cc, Corymbia citriodora; Dr, Delonix regia; Fb, Ficus benghalensis; Fra, Ficus racemosa; Fr, Ficus religiosa; Ft, Ficus tomentosa; Hi, Holoptelea integrifolia; Mi, Madhuca indica; Pg, Psidium guajava; Sc, Syzygium cuminii; St, Saccopetalum tomentosum; Ta, Terminalia arjuna
Discussion
Air quality
The concentration of SO2 at JCF and RCF exceeded the prescribed limit of National Ambient Air Quality Standards (NAAQS) India (80 µg m−3; CPCB(Central Pollution Control Board 2009). High SO2 at JCF and RCF sites may be ascribed to active mine fires in these mines (Wang and Xiao, 2004). Concentration of NO2 was below NAAQS (80 µg m−3; CPCB, 2009) guidelines at all the three sites. High concentrations of NO2 at JCF and RCF may be mainly due to combustion processes, including vehicle exhaust, coal, oil and natural gas, with some emissions during blasting. TSP concentration exceeded the prescribed limit of NAAQS (500 µg m−3; CPCB 1995) at JCF and RCF. As compared to the present study, lower concentrations of TSP (368 µg m−3) and SO2 (65 µg m−3) and higher concentration of NO2 (52 µg m−3) were recorded by Ghose and Majee (2000) at JCF and higher TSP (598 µg m−3), but lower SO2 (48 µg m−3) and NOx (37 µg m−3) concentrations in coal mining area of Sambalpur, India by Chaulya (2004). The sources of fugitive dust at coal mining areas include overburden removal, blasting, mineral haulage, mechanical handling operations, minerals stockpiles and site restoration (Appleton et al. 2006).
Soil characteristics
The extensive coal mining in the area has reduced the soil pH at JCF and RCF. Coal mining typically exposes sulphur-containing pyrites that oxidize to sulphuric acid when exposed to oxygen, water and certain aerobic bacteria, leaving low soil pH (Gitt and Dollhopf 1991). Increase in soil sulphur (10–100 times) and low pH near coal mining area at Lisbon, Columbiana have also been reported (Liu and Lal 2013). Use of heavy vehicles during coal mining for transportation of coal and overburden material exerts high ground pressure. Therefore compaction takes place, and results in high bulk density, low porosity and low water holding capacity of the soil in coal mining areas (Bradshaw 1997; Maiti 2013). The low N content in soil of JCF and RCF as compared to CIMFR may be due to lack of adequate amount of mineralisable organic nitrogen, lower mineralization and nitrification rates, reduced vegetation cover and lack of microbial activity (Maiti 2013). In acidic soil, phosphorous forms insoluble Fe and Al phosphate (dos Santos et al. 2013), so its available form reduced in soil near JCF and RCF. High TOC value is probably due to fossil carbon present in the soil of coal mining areas (Kriibek et al. 1998). Low inorganic N and low available P near the site of stripe mining have been reported at Ohio, USA (Boerner et al. 1998).
Vegetation structure
In the present study, A. paronychioides and C. dactylon were the most dominant species of the herbaceous community at all the sites (Table 1). A. paronychioides is not a native of the area, but become very prevalent (Tanveer et al. 2013). High RF and RDo of A. paronychioides and C. dactylon suggested its growth under a wide range of environmental variables. It has also been suggested that wide distribution of some species is a result of evolution rather than innate physiological tolerance and pollution level (Bradshaw 1993). Higher plasticity of invasive species than native is reported at low resource systems (Sun et al. 2010).
The polluted sites, JCF and RCF were species poor compared to reference site CIMFR (Table 1). Achyranthes aspera, Convolvulous alsinoides, Dichanthium annulatum, Eclipta alba and Solanum nigrum are most sensitive as these were present at CIMFR and completely absent from the coal mining areas (Table 1). Eragrostis cynosurides and Setaria glauca are polluphilic species as these were only present at the polluted sites (JCF and RCF). Ageratum conyzoides, Chrysopogon aciculatus, Evolvulus alsinoides, Parthenium hysterophorus and Tephrosia purpurea also showed lower IVI at CIMFR compared to JCF and RCF, and therefore are also tolerant to coal mining activities (Table 1).
Alstonia scholaris, B. lanzan and F. tomentosa are described as native species in the area (Bauri 2013).Their lower IVI suggests their sensitivity to altered air and soil conditions, due to excessive coal mining activities in JCF and RCF. Species such as F. beghalensis, F. religiosa, P. guajava, M. indica, A. indica, A. marmelos and C. citriodora showing highest IVI at polluted sites are resistant (Table 2).
Although some species showed resistance or polyphallic behaviour, but the numbers of species participated in community structure as dominants or co-dominants were higher at CIMFR compared to JCF and RCF. This result suggests that a large number of species acclimatize to stress conditions originated due to coal mining activities. As variations in environmental factors tax the adaptive abilities of organisms, only those species which are adapted to new conditions or those which can become accustomed to the new conditions, participate in the community formation (Agrawal and Agarwal 2000). The species vulnerable to the air pollution and altered soil properties are removed and more tolerant species become widespread (Buma and Wessman 2013). Air pollutants and altered soil properties in the area due to coal mining activities modified the response of the vegetation either by acting synergistically or antagonistically.
Species diversity
Shannon–Wiener diversity index suggests that the coal mining sites are suffered due to heavy pollution load and the most sensitive species have disappeared leaving only tolerant species represented by a large number of individuals. The stability of the community is related to the species diversity, the higher the value of the diversity index, the greater will be the stability of community structure and function. Species diversity and concentration of dominance are inversely related. The plant community is heterogeneous at CIMFR because of low pollution load at this site, favouring the growth, survival and regeneration of natural vegetation and new arrivals. However, the vegetation closest to coal mining subsequently lost sensitive plant species, which created a niche that became available to opportunistic and more tolerant species, which became abundant at polluted sites. Evenness of community increased, whereas species richness declined at the sites having higher pollution load. Evidently species richness had greater influence on species diversity than evenness observed in this study. Coal mining activities had a negative influence on species composition as shown by the higher similarity coefficient value between JCF and RCF and lower similarity coefficient with CIMFR for both woody and herbaceous communities. Similarity coefficient of the study areas reflects that effects of the coal mining were more pronounced at JCF. The results also suggest that tree community at JCF was more affected by coal mining than herbaceous community.
The curve of IVI versus species sequence in decreasing order of IVI showed a log normal pattern at CIMFR (Fig. 2). It is known that undisturbed stable and equilibrium communities follow a lognormal pattern of distribution (Cepelova and Munzbergova 2012). The shape of the distribution curve slipped from a lognormal at JCF and RCF sites, having altered air and soil characteristics. The slipping of curves towards geometric series was less pronounced for herbaceous compared to woody layer. The species distribution curve clearly suggests that both herbaceous and woody plant communities at JCF and RCF are negatively affect by coal mining activities (Fig. 2).
Canonical correspondance analysis
The relative importance of a single parameter is depicted in the CCA ordination diagramme by the length of their corresponding lines (Ter Braak 1987, Ter Braak and Verdonschot 1995). CCA analysis revealed that structure of herbaceous community was mainly driven by soil TOC and N, whereas woody layer was influenced by SO2, soil sulphate and P. For herbaceous and woody layer, the first axis showed strong positive correlation with N and TP and strong negative correlation with soil sulphate, TSP and SO2 (Fig. 3a, b). The second axis showed only strong positive correlation with TOC.
Amaranthus spinosus, Cassia alata, Digitaria sanguinalis, Setaria glauca, Desmodium trifolium and Sida cordifolia were common where TOC, NO2, SO2 and soil sulphate were high and soil N and P were low. These species seem to be resistant to coal mine emission and low nutrients. While A. aspera, C. alsinoides, D. annulatum, E. alsinoides and T. purpurea, common at sites where soil N was high and TOC, NO2, SO2 and soil sulphate were low, are sensitive to coal mine emissions. Abundance of A. aspera, C. alsinoides and D. annulatum was low at all study sites, as these species are found distant to both axes in CCA biplot (Fig. 3a). B. diffusa, C. rotundus, D. trifolium, H. contortus, S. xanthocarpum and S. indicus were found near the first axis and are also resistant to air emission and consequent altered soil qualities around coal mines (Fig. 3a).
For woody vegetation, the CCA showed that SO2 and TSP in air, soil P, N and S were important environmental factors (Fig. 3b). F. religiosa, F. benghalensis, P. guajava, B. monosperma, C. citriodora, M. indica and T. arjuna seemed to be resistant species as their density at coal mining areas were high. F. religiosa, F. benghalensis, P. guajava and B. monosperma showed very close relations towards TSP, SO2 and NO2. A. scholaris, B. lanzan and F. tomentosa seemed to be sensitive to coal mining emissions. The other sensitive species such as A. heterophyllus, S. cumini and A. cordifolia showed very close relation to soil P (Fig. 3b).
Conclusion
Distinct changes in the community structure were recorded at coal mining areas. The changes in species diversity observed at mining areas indicated an increase in the proportion of resistant herbs and grasses showing a tendency towards a definite selection strategy of ecosystem in response to air pollution and altered soil characteristics. According to canonical correspondence analysis, sulphate, phosphate, total N and total organic carbon concentrations in soil and TSP, SO2 and NO2 in ambient air were major factors governing the community structure at the coal mining areas. Large scale and long term biodiversity assessment programme should be taken around coal mining areas to understand the community composition changes and development of resistance in herbaceous plants undergoing low level stresses in such areas. Natural reestablishment of herbaceous vegetation on coal mined dumps should be studied to understand the natural recolonization of degraded land. Assessment of herbaceous vegetation for its socio-economic input to the inhabitant of the area should also be conducted. Since trees are good in absorbing air pollutants and mitigating the climate of the area, efforts should be taken to conserve the tree species still existing in the area and more plantations of resistant species should be tried to reduce the cost of health burden on the community.
References
Agrawal SB, Agarwal M (2000) Environmental pollution and plant responses. CRC Press, Boca Raton
Appleton TJ, Kingman SW, Lowndes IS, Silvester SA (2006) The development of a modelling strategy for the simulation of fugitive dust emissions from in-pit quarrying activities: a UK case study. Int J Min Reclam Environ 20:57–82
Bauri T (2013) Phytosociology and pedological characteristics of selected beats of durgapur forest range, west bengal, India. Commun Plant Sci 3:37–45
Bello FD, Lavorel S, Lavergne S, Albert CH, Boulangeat I, Mazel F, Thuiller W (2012) Hierarchical effects of environmental filters on the functional structure of plant communities: a case study in the french alps. Ecography 36:393–402
Boerner RE, Scherzer AJ, Brinkman JA (1998) Spatial patterns of inorganic N, P availability, and organic C in relation to soil disturbance: a chronosequence analysis. Appl Soil Ecol 7:159–177
Bradshaw AD (1993) Understanding the fundamentals of succession. In: Miles J, Walton DH (eds) Primary succession on land. Blackwell, Oxford
Bradshaw A (1997) Restoration of mined lands—using natural processes. Ecol Eng 8:255–269
Buma B, Wessman CA (2013) Forest resilience, climate change, and opportunities for adaptation: a specific case of a general problem. For Ecol Manag 306:216–225
Cepelova B, Munzbergova Z (2012) Factors determining the plant species diversity and species composition in a suburban landscape. Landsc Urban Plan 106:336–346
Chaulya SK (2004) Assessment and management of air quality for an opencast coal mining area. J Environ Manag 70:1–14
Central Pollution Control Board (CPCB) (1995) National Ambient Air Quality Standards (NAAQS), Gazette Notification, New Delhi
Central Pollution Control Board (CPCB) (2009) National Ambient Air Quality Standards (NAAQS), Gazette Notification, New Delhi
de Deyn GB, Raaijmakers CE, Van der Putten WH (2004) Plant community development is affected by nutrients and soil biota. J Ecol 92:824–834
Depietri Y, Renaud FG, Kallis G (2012) Heat waves and floods in urban areas: a policy-oriented review of ecosystem services. Sustain Sci 7:95–107
Dickman SR, Bray RH (1941) Replacement of adsorbed phosphate from kaolinite by fluoride. Soil Sci 52:263–274
dos Santos JV, de Melo Rangel W, Guimaraes AA, Jaramillo PMD, Rufini M, Marra LM, Lopez MV, da Silva MAP, Soares CRFS, de Souza Moreira FM (2013) Soil biological attributes in arsenic-contaminated gold mining sites after revegetation. Ecotoxicology 22:1526–1537
Dutta RK, Agrawal M (2003) Restoration of opencast coal mine spoil by planting exotic tree species: a case study in dry tropical region. Ecol Eng 21:143–151
Ejaz S, Camer GA, Anwar K, Ashraf M (2014) Monitoring impacts of air pollution: PIXE analysis and histopathological modalities in evaluating relative risks of elemental contamination. Ecotoxicology 23:357–369
Emberson LD, Ashmore MR, Murray F, Kuylenstierna JCI, Percy KE, Izuta T, Zheng Y, Shimizu H, Sheu BH, Liu CP, Agrawal M, Wahid A, Abdel-Latif NM, van Tienhoven M, de Bauer LI, Domingos M (2001) Impacts of air pollutants on vegetation in developing countries. Water Air Soil Pollut 130:107–118
Ghose MK, Majee SR (2000) Assessment of the impact on the air environment due to opencast coal mining—an Indian case study. Atmospheric Environ 34:2791–2796
Gitt MJ, Dollhopf DJ (1991) Coal waste reclamation using automated weathering to predict lime requirement. J Environ Qual 20:285–288
Grimm NB, Foster D, Groffman P, Grove JM, Hopkinson CS, Nadelhoffer KJ, Pataki DE, Peters DP (2008) The changing landscape: ecosystem responses to urbanization and pollution across climatic and societal gradients. Front Ecol Environ 6:264–272
Gupta DK, Huang HG, Nicoloso FT, Schetinger MR, Farias JG, Li TQ, Razafindrabe BHN, Aryal N, Inouhe M (2013) Effect of Hg, As and Pb on biomass production, photosynthetic rate, nutrients uptake and phytochelatin induction in Pfaffia glomerata. Ecotoxicology 22:1403–1412
Hernández AJ, Pastor J (2008) Relationship between plant biodiversity and heavy metal bioavailability in grasslands overlying an abandoned mine. Environ Geochem Health 30:127–133
Jacobs MB, Hochheiser S (1958) Continuous sampling and ultramicrodetermination of nitrogen dioxide in air. Anal Chem 30:426–428
Jones DL, Cross P, Withers PJ, DeLuca TH, Robinson DA, Quilliam RS, Harris IM, Chadwick DR, Edwards-Jones G (2013) Nutrient stripping: the global disparity between food security and soil nutrient stocks. J Appl Ecol 50:851–862
Krausmann F, Gingrich S, Eisenmenger N, Erb KH, Haberl H, Fischer-Kowalski M (2009) Growth in global materials use, GDP and population during the 20th century. Ecol Econ 68:2696–2705
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R, Packer L, Ricketts TH (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
Krıibek B, Strnad M, Bohacek Z, Sykorovaa I, Cejka J, Sobalik Z (1998) Geochemistry of Miocene lacustrine sediments from the Sokolov coal basin (Czech Republic). Int J Coal Geol 37:207–233
Liu R, Lal R (2013) An empirical study on improving quality of coal-mining refuse for re-vegetation using amendments. J Sustain Dev 6:44
Maiti SK (2013) Ecorestoration of the coalmine degraded lands. Springer, India
Mori AS, Spies TA, Sudmeier-Rieux K, Andrade A (2013) Reframing ecosystem management in the era of climate change: issues and knowledge from forests. Biol Conserv 165:115–127
Narayan D, Agrawal M, Pandey J, Singh J (1994) Changes in vegetation characteristics downwind of an aluminium factory in India. Ann Bot (Lond) 73:557–565
Olsen SR, Cole CV, Watanable FS (1954) Estimation of available phosphorus in soil by extraction with sodium bicarbonate. USDA Circular 939
Pandey B, Agrawal M, Singh S (2014) Assessment of air pollution around coal mining area: Emphasizing on spatial distributions, seasonal variations and heavy metals, using cluster and principal component analysis. Atmos Pollut Res 5:79–86
Rai AK, Paul B, Singh G (2010) Assessment of top soil quality in the vicinity of subsided area in Jharia coalfield, Dhanbad. Jharkhand. Rep opin 2:9
Si H, Bi H, Li X, Yang C (2010) Environmental evaluation for sustainable development of coal mining in Qijiang, Western China. Int J Coal Geol 81:163–168
Singh J, Agrawal M, Narayan D (1994) Effect of power plant emissions on plant community structure. Ecotoxicology 3:110–122
Singh NP, Mudgal V, Khanna KK, Srivastava SC, Sahoo AK, Bandyopadhyay S, Aziz N, Das M, Bhattacharya RP, Hajra PK (2001) Flora of Bihar—analysis. Botanical Survey of India, Ministry of Environment and Forests
Sun Y, Ding J, Frye MJ (2010) Effects of resource availability on tolerance of herbivory in the invasive Alternanthera philoxeroides and the native Alternanthera sessilis. Weed Res 50:527–536
Tanveer A, Khaliq A, Siddiqui MH (2013) A review on genus Alternanthera weeds implications. Pak J Weed Sci Res 19(1):53–58
Ter Braak CJ (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Ter Braak CJ (1987) The analysis of vegetation–environment relationships by canonical correspondence analysis. Theory and models in vegetation science. Springer, Netherlands, pp 69–77
Ter Braak CJ, Verdonschot PF (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289
Trabucchi M, Comín FA, O’Farrell PJ (2013) Hierarchical priority setting for restoration in a watershed in NE Spain, based on assessments of soil erosion and ecosystem services. Reg Environ Change 13:911–926
Ulrich W, Piwczyński M, Zaplata MK, Winter S, Schaaf W, Fischer A (2013) Soil conditions and phylogenetic relatedness influence total community trait space during early plant succession. J Plant Ecol-UK. doi:10.1093/jpe/rtt048
Veziroglu TN, Sahin S (2008) 21st century’s energy: hydrogen energy system. Energy Convers Manag 49:1820–1831
Walkley A, Black IA (1947) Determination of organic matter in the soil by chromic acid digestion. Soil Sci 63:251–264
Wang SW, Xiao F (2004) AHU sensor fault diagnosis using principal component analysis method. Energy Build 36:147–160
West W, Gaeke GC (1956) Fixation of sulphur dioxide on disulfitomercurate II and subsequent colorimetric estimation. Anal Chem 28:1816–1819
Williams CH, Steinbergs A (1959) Soil sulphur fractions as chemical indices of available sulphur in some australian soils. Crop Pasture Sci 10:340–352
World Coal Association (2011) Coal Facts (2011), http://www.worldcoal.org/resources/coal statistics. Accessed 29 Apr 2011
Acknowledgments
We thank, Head, Department of Botany, Banaras Hindu University and Director, Central Institute of Mining and Fuel Research, Dhanbad for providing all the necessary laboratory facilities during the research work. Financial assistance in the form of a research project (EE/39) funded by Ministry of Coal, Government of India, New Delhi is also acknowledged.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandey, B., Agrawal, M. & Singh, S. Coal mining activities change plant community structure due to air pollution and soil degradation. Ecotoxicology 23, 1474–1483 (2014). https://doi.org/10.1007/s10646-014-1289-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1289-4