Abstract
Recovery of arsenic contaminated areas is a challenge society faces throughout the world. Revegetation associated with microbial activity can play an essential role in this process. This work investigated biological attributes in a gold mining area with different arsenic contents at different sites under two types of extant revegetation associated with cover layers of the soil: BS, Brachiaria sp. and Stizolobium sp., and LEGS, Acacia crassicarpa, A. holosericea, A. mangium, Sesbania virgata, Albizia lebbeck and Pseudosamanea guachapele. References were also evaluated, comprising the following three sites: B1, weathered sulfide substrate without revegetation; BM, barren material after gold extraction and PRNH (private reserve of natural heritage), an uncontaminated forest site near the mining area. The organic and microbial biomass carbon contents and substrate-induced respiration rates for these sites from highest to lowest were: PRNH > LEGS > BS > B1 and BM. These attributes were negatively correlated with soluble and total arsenic concentration in the soil. The sites that have undergone revegetation (LEGS and BS) had higher densities of bacteria, fungi, phosphate solubilizers and ammonium oxidizers than the sites without vegetation. Principal component analysis showed that the LEGS site grouped with PRNH, indicating that the use of leguminous species associated with an uncontaminated soil cover layer contributed to the improvement of the biological attributes. With the exception of acid phosphatase, all the biological attributes were indicators of soil recovery, particularly the following: microbial carbon, substrate-induced respiration, density of culturable bacteria, fungi and actinobacteria, phosphate solubilizers and metabolic quotient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is widely distributed in the Earth’s crust (Kabata-Pendias 2010), but increased mining and industrial activities have changed the geochemical cycle and release of As into the biosphere. Gold (Au) mining sites are characterized by high levels of As (Borba et al. 2003; Lee et al. 2008; Choe et al. 2009), and the most common arsenic mineral is a sulfide, arsenopyrite (FeAsS). The remediation of As-contaminated areas has been a considerable challenge to the scientific community worldwide, particularly mining sites that have dramatically affected plant cover (Huang et al. 2004; Dong et al. 2008). A lack of vegetation in contaminated areas accelerates soil degradation by water and wind erosion, leading to the contamination of water sources and triggering a ripple effect. The recovery of soils degraded by excess As is possible through several processes; of these, revegetation is the most recommended strategy because of its lower cost and the resulting stabilization of the site (Raskin and Ensley 2000; Gratão et al. 2005). In addition, the presence of plants, in particular leguminous species, in mine tailings may enhance the heterotrophic microbial community, which may in turn actively contribute to changing the trace element speciation and assist the plants in overcoming phytotoxicity (Mastretta et al. 2006; Vangronsveld et al. 2009). The contribution of the soil microbiota to plant growth in As-contaminated soil under controlled conditions has previously been reported (Tangahu et al. 2011; Wang and Zhao 2009; Chopra et al. 2007). According to these authors, a higher microbial population in the rhizosphere zone compared to the non-rhizosphere zone may enhance the bioremediation process. Preliminary studies of the biological attributes of the soil, including the microbial biomass and its activities, can provide useful information needed to determine the correct recovery techniques for contaminated sites. High As concentrations can exert a selective effect on the populations, favoring the dominance of more adapted communities. Thus, microbial communities are important indicators of the efficiency of phytoremediation processes, especially when applying in situ remediation (Geets et al. 2008). Although the diverse effects of arsenic on soil microbiota and their processes are being increasingly explored (Edvantoro et al. 2003; Ghosh et al. 2004; Bhattacharyya et al. 2008; Ascher et al. 2009; Wang et al. 2011), no study has evaluated the soil microbiota of revegetation in As-contaminated areas in situ. Therefore, considering that microbial communities are important ecosystem components and are stimulated by vegetation, it is relevant to know the impact of As contamination on the density, biomass and activity of soil microbes in sites degraded by gold mining, both revegetated and without vegetation, which is the object of the present study.
Materials and methods
Description of experimental sites
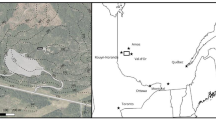
The soil samples analyzed in the present study were collected in a gold mining area located in Morro do Ouro near the Paracatu municipality, in the northwestern region of Minas Gerais State, Brazil. The process involved in the exploration for gold deposits (spoil-B1 layer) involves the removal of natural vegetation and then soil removal to permit gold extraction. This soil removal exposes the sulfide substrate (arsenopyrite and pyrite) to weathering, causing acid mine drainage. The soil removed can be taken to for processing or, if the gold content is low (barren material), it can remain in a stockpile until a certain amount is economically exploitable.
In March of 1997, weathered sulfide substrate (B1) was covered with a compacted sealing layer (30 cm of B1 compacted with a mechanical compactor to maximize compression) (Assis et al. 2011) at two sites that were covered with diverse plant species (Dias et al. 2000). At one of these sites, a B1 soil layer with lime (50 cm) was added after the sealing layer. This site (S 17°11′598″ and W 46°52′299″), called BS, was revegetated with Brachiaria sp. and Stizolobium sp.; these species were observed at the time of soil sampling (December, 2008) in this study. At the other site, a B1 soil layer with lime (25 cm) was also added after the sealing layer. Then, a soil layer (25 cm, B horizon of uncontaminated Oxisol) with lime was added (Assis et al. 2011). At this site (S 17°11′058″ and W 46°52′588″), called LEGS, growth of different leguminous species was observed (Acacia crassicarpa, A. holosericea, A. mangium, Sesbania virgata, Albizia lebbeck and Pseudosamanea guachapele), as well as an invasion of Brachiaria sp. and Stizolobium sp. at the time of soil sampling in this study. These sites were analyzed for their biological attributes as well as the following sites, which were used as references: (1) BM—barren material, economically unusable material due to low Au grade (S 17°12′050″ and W 46°52′481″); (2) B1—rather weathered sulfide substrate without revegetation (S 17°12′121″ and W 46°52′535″); and (3) PRNH—private reserve of natural heritage (S 17°10′628″ and W 46°52′151″) (Fig. 1).
Soil sampling and physicochemical analysis
Soil sampling was conducted in the rainy season (December, 2008). At each one of the five sites, four soil samples composed of five sub-samples (0–20 cm) were collected, resulting in a total of 20 samples. Soil samples were placed in 0.5 kg sterilized bags, transported in styrofoam boxes from the field to the Laboratory of Biology, Microbiology and Soil Biological Processes of the Federal University of Lavras and kept under 4 °C until processing. Soil pH was measured in a soil/H2O suspension (1:2.5 w/v). Available potassium and phosphorus were extracted with Mehlich I solution (Mehlich, 1953). Then, K was determined by flame photometry and P by colorimetry. Exchangeable Ca, Mg and Al were extracted with 1 mol l−1 KCl solution. Both Ca and Mg were measured by atomic-absorption spectrophotometry and Al by titration. Organic carbon (Corg) was determined by titration with a solution of ferrous ammonium sulfate after oxidation of the carbon by potassium dichromate (Walkley and Black 1934). Total arsenic (As-t) was determined according to the USEPA Method 3051A (USEPA 2007), and soluble arsenic (As-s) was determined by Mehlich I extraction. The extracts used to quantify total and soluble arsenic were analyzed in a Perkin 22 Elmer Analyst 800 Atomic Absorption Spectrophotometer with graphite furnace atomization. The soil texture was determined using a hydrometer (Bouyoucos 1951). Chemical and physical properties and arsenic contents of the soil sampling sites are shown in Table 1.
Microbial biomass carbon
The soil microbial biomass carbon (Cmic) was determined by the fumigation-extraction method (Vance et al. 1987). Briefly, moist soil samples equivalent to 20 g (oven dry weight) were extracted with 50 ml of K2SO4 (0.5 M) by shaking for 30 min. Then, the suspension was filtered through filter papers (Whatman No. 42). In addition, other 20-g soil samples were fumigated for 24 h with ethanol-free chloroform. Next, the suspension was extracted with K2SO4 as described above. The Cmic content of the filtrates were determined by dichromate oxidation and subsequent titration with ferrous ammonium sulfate.
Basal and substrate-induced respiration
To measure soil basal respiration (BR), a fresh soil sample corresponding to 20 g dry weight adjusted to 60 % of water holding capacity was placed in a 1 l airtight jar along with 10 ml of NaOH (0.5 M) and incubated for 3 days in the dark at 28 °C. The evolved CO2 was trapped by NaOH solution and subsequently measured by titration with HCl (0.5 M) using a phenolphthalein indicator (Alef 1995). Substrate-induced respiration (SIR) values were obtained using the same method as BR but with the addition of glucose (0.5 %) to the soil sample.
Microbial and metabolic quotient
The ratio between Cmic and Corg was used to calculate the microbial quotient (qMic) (Sparling 1992). The metabolic quotient (qCO2) was calculated by the BR to Cmic ratio and expressed as mg CO2–C mg−1 Cmic h−1 (Anderson and Domsch 1993).
Acid phosphatase
Acid phosphatase activity (Acphos) was quantified according to the methodology proposed by Dick et al. (1996). Briefly, 1 g soil mixed with 0.2 ml of toluene, 4 ml of MUB (modified universal buffer) of pH 6.5 plus 1 ml of p-nitrophenyl phosphatase solution (0.05 M). The flask was placed in an incubator at 37 °C for an hour. Then, the reaction was stopped with 1 ml of 0.5 M CaCl2 and 4 ml of 0.5 M NaOH. The intensity of the yellow color of the filtrate due to p-nitrophenol was determined using a UV–Vis spectrophotometer at a wavelength of 410 nm, and the results were expressed as μg p-nitrophenol g−1 soil h−1.
Microbial enumeration
Sub-samples (10 g) of each soil sample were placed in Erlenmeyer flasks containing 90 ml of sterile saline solution (0.85 %) for shaking (125 rpm, 30 min), and serial decimal dilutions were then prepared for inoculation of 0.1 ml aliquots in different media. Colony-forming units (CFU) of total culturable bacteria were counted in nutrient agar (g l−1) (5 g peptone, 3 g beef extract, 1 g yeast extract, 5 g glucose, 1,000 ml distilled water, 15 g agar, pH 6.8, 0.03 % cycloheximide solution). CFU of fungi was counted in Martin medium (g l−1) (10 g glucose, 5 g peptone, 0.5 g yeast extract, 0.5 g K2HPO4, 0.5 g KH2PO4, 0.5 g MgSO4·7H2O, 0.3 g Rose Bengal, 15 g agar, 0.03 % streptomycin solution, 1,000 ml distilled water). CFU of actinobacteria were counted in starch-casein agar (g l−1) (10 g soluble starch, 1 g NaNO3, 0.3 g K2HPO4, 0.5 g NaCl, 1 g MgSO4·7H2O, 1,000 ml distilled water, 15 g agar, pH 7). The colonies of bacteria, fungi and actinobacteria were counted after incubation at 28 °C for 3, 6 and 8 days, respectively (Martin 1950; Wollum 1982).
Functional groups of microorganisms were also evaluated. CFU of phosphate-solubilizing microorganisms were determined in two different media. The first was GES (g l−1) (10 g glucose, 100 ml soil extract solution, 2 ml 10 % MgSO4·7H2O, 2 ml 1 % CaCl2, 1 ml 10 % NaCl, 2 ml micronutrients solution [0.2 g Ca2MoO4·2H2O, 0.235 g MnSO4·H2O, 0.28 g H3BO3, 0.008 g CuSO4·5H2O, 0.024 g ZnSO4·7H2O, 200 ml distilled water], 4 ml 1.64 % Fe-EDTA, 0.1 g KNO3, 15 g agar, pH 6.8. Calcium phosphate was obtained by adding 50 ml of a 10 % K2HPO4 solution and 100 ml of a 10 % CaCl2 solution to 850 ml of culture medium with 1 l nutrient composition equivalent (all autoclaved separately) to produce an insoluble phosphate precipitate (Sylvester-Bradley et al. 1982). The second media was NBRIP (g l−1) (10 g glucose, 5 g Ca3(PO4)2, 5 g MgCl2·6H2O, 0.25 g MgSO4·7H2O, 0.2 g KCl, 0.1 g (NH4)2SO4, 15 g agar, 1,000 ml distilled water, pH 6.8) (Nautiyal 1999). The designations Ps-GES and Ps-NBRIP, respectively, were adopted for these attributes. Evaluation of CFU was performed 15 days after inoculation at 28 °C.
Most probable numbers (MPN) of ammonium oxidizers (Ammox) were estimated from a dilution series of soil suspensions inoculated into liquid medium according to the method described by Sarathchandra (1978).
Four replicates per dilution were used for bacteria, fungi and actinobacteria, and three replicates per dilution were used to evaluate solubilizing microorganisms.
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and the Scott-Knott mean test (5 %) using the statistical program SISVAR (Ferreira 2011). After the Shapiro–Wilk test, the SIR and qMic data were transformed using a square root transformation of (x + 1). The Cmic, qCO2, CFU of bacteria, fungi, actinobacteria, Ps-NBRIP and Ps-GES CFU and MPN of Ammox were transformed to log(x + 1). The average values of the biological attributes were subjected to Pearson correlation tests by Sigmaplot 11.0. Biological attributes data and Corg, As-t and As-s concentrations were subjected to principal component analysis (PCA) using the program CANOCO for Windows 4.5 (Ter Braak and Smilauer 2002).
Results
Physicochemical analysis of soil
The sites studied exhibited different pH values (Table 1). The lowest pH (3.9) was observed at the barren material (BM) site. The highest pH values (6.5 and 6.0) were observed at the sites with revegetation (BS and LEGS, respectively). The levels of P and total arsenic (As-t) were very different in the revegetated sites. For example, BS had the highest levels of P (40 mg dm−3) and As-t (699.6 mg dm−3), while LEGS had the lowest values, which were 1.7 and 26.3 mg dm−3, respectively. The highest levels of soluble arsenic (As-s) were observed at B1, BM and BS, followed by LEGS and PRNH. As-t and As-s were statistically similar at LEGS and PRNH. Texture varied significantly among sites.
Organic (Corg) and microbial biomass (Cmic) carbon
Corg and Cmic in PRNH were significantly (P < 0.05) higher than at the other sites (Fig. 2a, b). The LEGS site had a Cmic value of 129.9 μg C g−1 soil, which was well below that of the PRNH (619 μg C g−1 soil); however, it was ~130 % higher (P < 0.05) than those values observed for BM, BS and B1. Corg followed the same trends as Cmic (Fig. 2a, b).
Soil biological attributes in different areas [arsenic-contaminated no cover sites (BM, B1), undergone revegetation sites (BS and LEGS) and uncontaminated site (PRNH)]. a Corg—organic carbon, b Cmic—biomass carbon, c CO2 emission—(BR basal respiration, SIR substrate-induced respiration), d qMic—microbial quotient, e qCO2—metabolic quotient f Acphos—acid phosphatase enzymatic activity. Bars with different letters are significantly different at P < 0.05 by the Scott–Knott test. c Uppercase letters compare the BR between sites and lowercase letters compare the SIR. Each value is the mean of four replicates
Basal (BR) and substrate-induced (SIR) respiration
BR and SIR were significantly (P < 0.05) higher at PRNH than the other sites. There were no significant differences (P < 0.05) between the B1, BS and LEGS sites in terms of BR values (Fig. 2c), which averaged ~32.5 mg C–CO2 kg−1 24 h−1 and were half way between those observed at the BM site (13.7 mg C–CO2 kg−1 24 h−1) and PRNH site (62.8 mg C–CO2 kg−1 24 h−1). At the LEGS site, the addition of glucose as a carbon source stimulated soil microbial respiration, reaching values ~260 % higher (P < 0.05) than the sites with high concentrations of arsenic (BM, B1 and BS).
Microbial (qMic) and metabolic (qCO2) quotient
The qMic values ranged from 0.79 to 3.24 % (Fig. 2d). PRNH had the highest value (P < 0.05) compared to the other sites. There were no significant differences among the other sites. The BM, B1 and BS sites had similar qCO2 values (P < 0.05), which were higher than 30 mg C–CO2 mg−1 Cmic h−1. The LEGS and PRNH sites were also significantly similar (P < 0.05) to each other and had the lowest values for this attribute: 10.6 and 4.3 mg C–CO2 mg−1 Cmic h−1, respectively (Fig. 2e).
Acid phosphatase activity (Acphos)
There was higher Acphos activity at the LEGS site (170.05 μg p-nitrophenol g−1 soil h−1) (Fig. 2f). The BM site, which had the highest levels of arsenic contamination, had Acphos activity that was statistically similar (P < 0.05) to PRNH site. The activity of this enzyme was low for both B1 and BS (Fig. 2f).
Colony-forming unit (CFU) counts
There was no bacterial, fungal or actinobacteria growth in the media inoculated with samples from the BM site (Table 2). The CFU counts for bacteria and fungi at the sites with vegetation cover and contamination (BS) or not (PRNH and LEGS) were significantly similar (P < 0.05) to each other and significantly higher (P < 0.05) than B1. There were no significant differences (P < 0.05) in the number of actinobacteria among the B1, BS, LEGS and PRNH sites. Only bacteria grew in both culture media for phosphate solubilizers. The CFU obtained from samples collected at PRNH, BS and LEGS were similar (P < 0.05) to each other for both the NBRIP and GES media and higher than the CFU counts obtained for B1 and BM. The CFU of Ps-NBRIP for BM was lower (P < 0.05) than B1. However, there was no difference (P < 0.05) between these sites for CFU in Ps-GES medium.
There was no growth in ammonium oxidizer (Ammox) medium inoculated with samples from the BM site. The MPN of Ammox was significantly higher (P < 0.05) in the sites that had undergone revegetation (BS and LEGS) compared to the other sites, including the PRNH. There were no significant differences (P < 0.05) between the PRNH and B1 sites for MPN of Ammox.
Correlations between biological attributes and arsenic concentrations
As-s content was negatively correlated (P < 0.01) with SIR, Cmic, Corg, culturable fungi and Ps-GES. As-t concentration was negatively correlated (P < 0.01) only with Cmic, Corg and culturable Ps-GES (Table 3). Organic C was positively correlated with following attributes: BR (P < 0.01), qMic (P < 0.05), culturable fungi (P < 0.01) and culturable Ps-GES (P < 0.05). Microbial quotient was negatively correlated (P < 0.01) with qCO2. Culturable bacteria positively correlated with qCO2 (P < 0.05), qMic (P < 0.05), Ps-NBRIP (P < 0.01) and Ammox (P < 0.01). In addition to the correlations cited, the CFU of culturable fungi was also correlated with (BR) (P < 0.01) and Ps-GES (P < 0.05). Both Acphos and the actinobacteria group were not correlated with any of the attributes studied, including As-s and As-t concentrations.
Principal component analysis
According to PCA, we observed that all attributes except Acphos had higher projections (loading > 0.4 absolute) for the first principal component (PC1), which explained ~53.3 % of the variation (Fig. 3; Table 4). The second principal component (PC2) explained 18.6 % of the variation. Therefore, the first 2 axes together explained 71.9 % of the variations in terms of the chemical characteristics (As-t, As-s and Corg) and biological attributes. Spatial distribution showed that the PRNH and LEGS sites were located near each other, and they had higher Corg, Cmic, SIR and qMic and lower As-t, As-s and qCO2 values. However, the BM and B1 sites formed distinct groups and were characterized as having high values of As-t, As-s and qCO2 and low biological activity. The bacteria, fungi, actinobacteria, phosphate solubilizing and Ammox densities attributed to the BS site were closer to those of the LEGS site, although the BS site also had a high arsenic content and high qCO2 values.
Discussion
Our results show that biological attributes responded rapidly to changes in the environment due to arsenic contamination. However, some attributes were more significant than others. This study can provide useful quality indicators and revegetation strategies for decision makers in terms of environmental protection and the recovery of contaminated areas.
Effects of total (As-t) and soluble (As-s) arsenic contamination on soil biological attributes
Of the 5 study sites, BM, B1 and BS had As-t concentrations above the maximum value established by the Brazilian Ministry of the Environment for industrial intervention, which is 150 mg As kg−1 (CONAMA 2009) (Table 1). According to this governing body, this criterion refers to the concentration of a particular chemical substance in the soil or groundwater above which there is increased potential for a direct or indirect impact on human health. However, As-s that is more readily available to organisms in general demonstrated a higher number of correlations with biological attributes than total arsenic. All the biological attributes evaluated were influenced by arsenic concentrations and/or soil cover conditions. The lower arsenic contents in LEGS sites can be explained by the uncontaminated soil cover layer where soil samples were taken.
Soil organic and microbial biomass carbon
The PRNH site had Corg and Cmic values that were higher than the other sites, which can be explained by the absence of contamination and diversified vegetation cover, which are characteristics of naturally protected environments. Such an environment favors the establishment of an active and efficient microbiota, which is consistent with Silva et al. (2010) and Cardoso et al. (2009) (Fig. 2a, b). Conversely, the LEGS site had Cmic values that were ~79 % lower than the PRNH site. In the BM, B1 and BS sites, the decrease was even more marked, ranging from 90 to 95 %. Edvantoro et al. (2003) studied different types of As-contaminated soils that had total As concentrations of up to 2.941 mg kg−1 and observed reductions in Cmic values ranging from ~50 to 90 % compared to uncontaminated soils. Cmic is one of the most studied soil biological attributes, and its values vary depending on the soil type, coverage conditions, weather, land use system, type of soil management adopted and the presence of contaminants such as trace elements (Feigl et al. 1995; Ghosh et al. 2004; Vásquez-Murrieta et al. 2006). In soils contaminated with these elements, values below 100 μg C g−1 are generally observed, whereas in uncontaminated or preserved sites, Cmic values can be up to or even exceed 1,000 μg C g−1 soil (Feigl et al. 1995; Edvantoro et al. 2003; Wang et al. 2007; Li et al. 2009). Our values are within the range reported in the literature.
The Corg contents of the contaminated sites BM, B1 and BS correspond to ~30 % of that observed in the PRNH, whereas this value was ~42 % for the LEGS site,. Although there was vegetation cover at the BS site, it seems that it was not sufficient to increase the Corg content. In contrast, the LEGS site, where different leguminous species were planted, was clearly aided by the addition of nitrogen to the soil, which results from biological fixation and from carbon compounds introduced via rhizodeposition and necromass (Moreira et al. 2006).
Basal and substrate-induced respiration
The low Corg content affected the respiration rate at the BM, BI, BS and LEGS sites compared to the natural reserve (PRNH) (Table 3; Fig. 2a, c). At the BM site, the highest aluminum content and very low pH were also unfavorable to soil respiration, resulting in the lowest value among the studied sites (Table 1; Fig. 2c). Reductions in CO2 emissions due to a decrease in substrate availability via loss of vegetation and also to an increased contaminant concentration have been reported (Edvantoro et al. 2003; Ghosh et al. 2004; Vásquez-Murrieta et al. 2006; Cycoń and Piotrowska-Seget 2009). However, other characteristics of the soil can affect CO2 emissions (Vásquez-Murrieta et al. 2006).
The strong increase in the SIR after the addition of glucose for the LEGS site indicates the presence of an edaphic microbiota that is capable of responding to stimuli arising from the supply of organic carbon (Fig. 2c). This information is highly useful, especially for the rehabilitation of contaminated sites, because it indicates that when management practices that favor the contribution of carbon are applied, there will be satisfactory soil microbe responses at these sites. Indeed, it is well known that plants influence the activity of the soil microbiota by releasing exudates, particularly sugars, through the rhizodeposition process (Yang et al. 2007; Jones et al. 2009).
Metabolic and microbial quotient
Generally, qMic values fall between 1 and 4 % but can vary depending on management conditions and ground cover (Jenkinson and Ladd 1981; Smith and Paul 1990). In our work, qMic values were within this range (Fig. 2d). Normally, low qMic values are found in contaminated or degraded environments, whereas preserved or non-polluted environments have high values; this has resulted in the frequent use of this biological attribute in studies examining soil quality (Ghosh et al. 2004; He et al. 2005; Wang et al. 2007).
The low qMic values for BM, B1, BS and LEGS indicate that a proportionally larger amount of non-microbial C is present in the organic carbon fraction of the soils of these sites (Fig. 2d). Surprisingly, the qMic for LEGS did not increase proportionately with the deposition of plant residues. This finding is consistent with observations reported by Wang et al. (2004), who studied the effects of different vegetation systems on soil microbial biomass. Conversely, the low values for the BM, B1 and BS sites are justified by the toxic effects of As contamination on microbial biomass, and the correlation between Cmic and As content (total and soluble) supports this assertion (Table 3).
The lowest qCO2 values were found at the PRNH and LEGS sites (Fig. 2e). Lower qCO2 values indicate better conditions for the soil microbial community. Conversely, high qCO2 indicates a stressed microbiota (Anderson and Domsch 1993). Our results indicate that the LEGS site had more favorable conditions for an edaphic microbiota compared to the contaminated sites (BM, BS and B1), which had lower Cmic, BR and SIR values. Therefore, at these sites, the presence of adverse conditions for soil microorganisms, such as high As concentrations, was reflected in the higher qCO2 values. Under such conditions, soil microorganisms tend to direct their metabolism toward biomass maintenance at the expense of increasing their population. qCO2 has been reported as a sensitive indicator of soil disturbances, particularly for toxic contaminants such as arsenic and other heavy metals such as Zn, Cu, Cd and Pb (Brookes 1995; Dias Júnior et al. 1998; Ghosh et al. 2004; Ascher et al. 2009). Although no correlation was found between qCO2 and As content, sites with no As contamination had low qCO2.
Acid phosphatase activity
Phosphatases are enzymes that are induced and synthesized under conditions of low phosphorus availability (Schinner et al. 1996; Allison et al. 2007; Nannipieri et al. 2011; Sarkar et al. 2013). This explains the highest activity of this enzyme for the LEGS site (Fig. 2f), which had the lowest P level in the soil (Table 1). However, other factors also affect the activity of this enzyme; for example, it has been reported that soil physicochemical (soil moisture, clay content, soil depth, organic matter, pH and nutrient content) and biological (microbial density and activity) properties as well as the presence of contaminants influence soil phosphatase activities (Juma and Tabatabai 1977; Tabatabai 1994; Speir and Ross 2002). It is known that low pH decreases the availability of inorganic P, particularly in tropical soils due the formation of iron and aluminum phosphates. In this situation, to compensate for the low availability of phosphorus in BM and B1 (Table 1), populations of surviving microorganisms capable of synthesizing phosphatase increase production of this enzyme to access the P contained in organic matter. This explains why sites such as these, even contaminated and with no cover, exhibited phosphatase activities similar to PRNH and BS, both with vegetation cover (Fig. 2f). Therefore, in our study phosphatase activity was not directly affected by As content, and this was confirmed by the absence of correlation between these parameters (Table 3). Ours results were different that those reported by Speir et al. (1999) and Juma and Tabatabai (1977), who observed strong inhibitory effects of As on phosphatase activities. According to Nannipieri et al. (2011), inhibition of enzyme activity by a pollutant may be masked by the growth of surviving microorganisms that express the genes coding the enzyme.
Colony-forming unit (CFU) counts
In our study, soil cover influenced the densities of culturable microorganisms, given that the highest numbers of CFU of bacteria, fungi, Ps-NBRIP and Ps-GES were observed for the BS, LEGS and PRNH sites (Table 2), which were the sites that had vegetation cover. The lack of fungal, bacterial, actinobacterial and Ammox growth for BM indicates the highly adverse soil conditions characterized by elevated arsenic concentrations and a lack of vegetation, although it is well known that no one medium is able to support growth of all culturable microorganisms and that these microorganisms represent ~1 % of the microbiota. However, growth was observed for other sites but was lower for B1, which was contaminated without vegetation (Table 2). The lack of significant differences for cultivable actinobacteria and Ammox between B1 and sites with vegetation cover (BS, LEGS and PRNH) indicate the greater tolerance of these microbial groups to adverse conditions. This effect can be attributed to low competition among soil microorganisms due the selective pressure, causing resistant groups (in B1 and BS) to increase their populations to the detriment of non-resistant groups (Bååth et al. 1998; Wang et al. 2011). The occurrence of Ps at the BM site and the absence of other microbial groups may be due to incubation time; the NBRIP and GES culture media were incubated for 15 days, whereas bacterial and fungal culture media normally last only up to 7 days (Wollum 1982). This same incubation time effect may also explain why the Ps-GES CFU number was higher than the number of bacteria in the PRNH site, although these media have different compositions that can influence growth. The higher tolerances observed for fungi and actinobacteria had previously been reported by Oliveira and Pampulha (2006) in a study examining multi-element contaminated soil, which included arsenic contamination.
Correlations between biological attributes, arsenic concentrations and the principal component analysis
The sites with higher As concentrations were those with little vegetation cover (BS) or no cover (BM and B1), and consequently these sites had low Corg (Fig. 2a). This effect explains the negative correlations between this attribute and the As-s and As-t concentrations (Table 3). Organic matter had a major influence on soil microbes because several processes performed by microorganisms depend on nutrients and energy from this fraction of the soil (Williamson and Johnson 1994; Kaiser et al. 1995; Powlson et al. 2001). Thus, the factors that negatively affect the supply of carbon in the soil, such as arsenic contamination, also directly or indirectly affect the biological attributes of the soil. For example, Cmic, which is part of living organic matter, was greatly affected by the presence of arsenic in the soil and correlated negatively with the soluble and total fractions of this element (Table 3). Cmic is quite sensitive to changes in the soil environment and thus has been one of the most evaluated biological attributes in studies of soils impacted by trace elements (Edvantoro et al. 2003; Ghosh et al. 2004; He et al. 2005; Renella et al. 2008). Soluble arsenic negatively affected the density of culturable fungi (Table 3). This group represents one of the primary components of the microbial biomass, which can reach ~70–80 % and account for up to 80 % of the activity in acidic soils (Anderson and Domsch 1975). Of the respiratory activities, only SIR was significantly correlated with As-s, and of the solubilizers, Ps-GES was negatively correlated with both As fractions. These two attributes are related to the presence of phosphate groups for cellular activity. It has been reported that arsenate (As+5), which is notably similar to the phosphate group, can replace phosphate in DNA macromolecules, compete for phosphate transporters, or inhibit ATP synthesis (Paivoke and Simola 2001; Rosen 2002). Thus, the metabolic processes for which phosphate is a contributing factor may be partially compromised or completely inhibited due to As contamination. Cmic and Corg had high positive correlations with BR and SIR (Table 3). As these soil carbon fractions were affected by arsenic content, microbial respiration should have been indirectly affected.
In studying the chemical, physical and biological attributes of the soil, we should consider the complex interactions between them. Thus, multivariate analyses are useful tools for obtaining more generalized inferences about the combinations of these factors. In our study, based on the PCA, we observed that all the biological attributes except Acphos were good indicators of As contamination (Fig. 3; Table 4) and had positive loadings ≥ 0.5 (absolute values). These results explain the grouping of the PRNH and LEGS sites, which are characterized by high microbial biomass and activity and also explain the separation of the B1 site from the others, which was due to its low microbial activity caused by the high As concentrations and consequently the high qCO2 emissions. Conversely, the BM site, which also had high levels of contamination and a lack of vegetation, was separated from the other sites because it had notably few culturable microorganisms (bacteria, fungi, actinobacteria, Ps-NBRI, Ps-GES and Ammox).
The grouping of the PRNH and LEGS sites (Fig. 3) is a significant result because it indicates that revegetation, especially by legumes, associated with an uncontaminated soil cover layer effectively contributed to the return of an edaphic microbiota and its processes to conditions that were similar to a natural environment. Considering only loadings greater than 0.8 (absolute values), the most indicative biological attributes to discriminate these sites were as follows: microbial biomass, carbon, substrate-induced respiration, density of culturable bacteria, fungi and actinobacteria and phosphate solubilizers in GES medium.
Conclusions
Arsenic contamination and lack of vegetation cover strongly affected the biomass and microbial activities of the soil; despite this, however, microorganism biomass and activity can be found in these highly contaminated sites. Revegetation using different legumes and an uncontaminated soil cover layer effectively contributed to the improvement of the biological conditions of the soil. The attributes that best explain these results were as follows: microbial biomass carbon, substrate-induced respiration, density of culturable bacteria, fungi and growth of actinobacteria and phosphate solubilizers in GES medium.
Future research endeavors should focus on testing these revegetation strategies in other areas contaminated with arsenic or other contaminants as well as the usefulness of the most sensitive biological attributes as quality indicators. Additionally, extant microorganism populations in areas with high levels of arsenic contamination must be isolated and studied in relation to their tolerance mechanisms and biotechnological potential as plant growth promoters.
References
Alef K (1995) Estimation of soil respiration. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, New York, pp 464–470
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39:1770–1781
Anderson JPE, Domsch KH (1975) Measurement of bacterial and fungal contributions to respiration of selected agricultural and forest soils. Can J Microbiol 21:314–322
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such pH, on the microbial biomass of forest soil. Soil Biol Biochem 25:393–395
Ascher J, Ceccherini MT, Landi L, Mench M, Pietramellara G, Nannipieri P, Renella G (2009) Composition, biomass and activity of microflora, and leaf yields and foliar elemental concentrations of lettuce, after in situ stabilization of an arsenic-contaminated soil. Appl Soil Ecol 41:351–359
Assis IR, Dias LE, Abrahão WAP, Ribeiro ES Jr, Mello JWV (2011) Cover layers to the growth of trees and shrubs over a sulfide spoil from gold mining. Rev Arvore 35:941–947
Bååth E, Díaz-Raviña M, Frostegård A, Campbell CD (1998) Effect of metal-rich sludge amendments on the soil microbial community. Appl Environ Microbiol 64:238–245
Bhattacharyya P, Tripathy S, Kim K, Kim SH (2008) Arsenic fractions and enzyme activities in arsenic-contaminated soils by groundwater irrigation in West Bengal. Ecotox Environ Safe 71:149–156
Borba RP, Figueiredo BR, Matschullat J (2003) Geochemical distribution of arsenic in waters, sediments and weathered gold mineralized rocks from iron quadrangle, Brazil. Environ Geol 44:39–52
Bouyoucos GJ (1951) A recalibration of the hydrometer method for making analysis of soils. Agr J 43:433–437
Brookes PC (1995) The use of microbial parameters in monitoring soil pollution by heavy metals. Biol Fert Soils 19:269–279
Cardoso EL, Silva MLN, Moreira FMS, Curi N (2009) Atributos biológicos indicadores da qualidade do solo em pastagem cultivada e nativa no Pantanal. Pesqui Agropecu Bras 44:631–637
Choe E, Kim KW, Bang S, Yoon IH, Lee KY (2009) Qualitative analysis and mapping of heavy metals in an abandoned Au–Ag mine area using NIR spectroscopy. Environ Geol 58:477–482
Chopra BK, Bhat S, Mikheenko IP, Xu Z, Yang Y, Luo X, Chen H, Van Zwieten L, Lilley R, McC R, Zhang R (2007) The characteristics of rhizosphere microbes associated with plants in arsenic-contaminated soils from cattle dip sites. Sci Total Environ 378:331–342
Conselho nacional do meio ambiente (CONAMA) (2009) Resolução No 420. http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=620. Accessed 03 May 2013
Cycoń M, Piotrowska-Seget Z (2009) Changes in bacterial diversity and community structure following pesticides addition to soil estimated by cultivation technique. Ecotoxicol 18:632–642
Dias Júnior HE, Moreira FMS, Siqueira JO, Silva R (1998) Metais pesados, densidade e atividade microbiana em solo contaminado por rejeitos de indústria de zinco. Rev Bras Cienc Solo 22:631–640
Dias LE, Campello, EFC, Ribeiro Jr ES (2000) Uso de leiras na revegetação de um substrato remanescente da exploração de ouro em Paracatu-MG. In: IV Simpósio Nacional de Recuperação de Áreas Degradadas. Silvicultura Ambiental. Blumenau – SC, Cd-Rom
Dick RP, Breakwell DP, Turco RF (1996) Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In: Doran JW, Jones AJ (eds) Methods for assessing soil quality. Soil Sci Soc Am, Madison, pp 247–272
Dong Y, Zhu YG, Smith FA, Wang Y, Chen B (2008) Arbuscular mycorrhiza enhanced arsenic resistance of both white clover (Trifolium repens Linn.) and ryegrass (Lolium perenne L.) plants in an arsenic-contaminated soil. Environ Pollut 155:174–181
Edvantoro BB, Naidu R, Megharaj M, Singleton I (2003) Changes in microbial properties associated with long-term arsenic and DDT contaminated soils at disused cattle dipsites. Ecotoxicol Environ Safe 55:344–351
Feigl BJ, Sparling GP, Ross DJ, Cerri CC (1995) Soil microbial biomass in Amazonian soils: evaluation of methods and estimates of pool sizes. Soil Biol Biochem 27:1467–1472
Ferreira DF (2011) Sisvar a computer statistical analysis system. Cienc Agrotec 35:1039–1042
Geets J, Vangronsveld J, Diels L, Taghavi S, van der Lelie D (2008) Chapter 21 Microbial activities, monitoring and applications as part of a management strategy for heavy metal-contaminated soil and ground water. Dev Soil Sci 32:521–559
Ghosh AK, Bhattacharyya P, Pal R (2004) Effect of arsenic contamination on microbial biomass and its activities in arsenic contaminated soils of Gangetic West Bengal, India. Environ Int 30:491–499
Gratão PL, Prasad MNV, Cardoso PF, Lea PJ, Azevedo RA (2005) Phytoremediation: green technology for the clean up or toxic metals in the environment. Braz J Plant Physiol 17:53–64
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Bio 19:125–140
Huang JW, Poynton CY, Kochian LV, Elless MP (2004) Phytofiltration of arsenic from drinking water using arsenic-hyperaccumulating ferns. Environ Sci Technol 38:3412–3417
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil: measuremente and turnover. In: Paul E, Ladd JN (eds) Soil biochemistry. Marcel Dekker, New York, pp 425–471
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil–root interface. Plant Soil 321:5–33
Juma NG, Tabatabai MA (1977) Effects of trace elements on phosphatase activity in soils. Soil Sci Soc Am J 41:343–346
Kabata-Pendias A (2010) Trace elements in soil and plants, 4th edn. CRC, Boca Raton
Kaiser EA, Martens R, Heinemeyer O (1995) Temporal changes in soil microbial biomass carbon in an arable soil. Plant Soil 170:287–295
Lee JS, Lee SW, Chon HT, Kim K (2008) Evaluation of human exposure to arsenic due to rice ingestion in the vicinity of abandoned Myungbong Au–Ag mine site. Korea J Geochem Explor 96:231–235
Li YT, Rouland C, Benedetti M, Li FB, Pando A, Lavelle P, Dai J (2009) Microbial biomass, enzyme and mineralization activity in relation to soil organic C, N and P turnover influenced by acid metal stress. Soil Biol Biochem 41:969–977
Martin JP (1950) Use of acids rose-bengall and streptomicin in the plate method for estimating soil fungi. Soil Sci 134:215–232
Mastretta C, Barac T, Vangronsveld J, Newman L, Taghavi S, van der Lelie D (2006) Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. In: Harding SE, Tombs MP (eds) Biotechnology and genetic engineering reviews, vol 23. Lavoisier, Paris, pp 175–207
Mehlich, A. (1953) Determination of P, Ca, Mg, K, Na and NH4. North Carolina Soil Testing Laboratories, Raleigh, NC
Moreira FMS, Siqueira JO, Brussaard L (2006) Soil biodiversity in Amazonian and other Brazilian ecossystems. CABI, Wallingford
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bunemann EK, Oberson A, Frossard E (eds) Phosphorus in Action. Soil Biology vol. 26. Springer Verlag, Berlin, pp 215–241
Nautiyal CS (1999) An effect microbiological grouwth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 70:265–270
Oliveira A, Pampulha ME (2006) Effects of long-term heavy metal contamination on soil microbial characteristics. J Biosci Bioeng 102:157–161
Paivoke AEA, Simola LK (2001) Arsenate toxicity to Pisum sativum: mineral nutrients, chorophyll content, and phytase activity. Ecotox Environ Safe 49:111–121
Powlson DS, Hirsch PR, Brookes PC (2001) The role of soil microorganisms in soil organic matter conservation in the Tropics. Nutr Cycl Agroecosy 61:41–51
Raskin I, Ensley BD (2000) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York
Renella G, Landi L, Ascher J, Ceccherini MT, Pietramellara G, Mench M, Nannipieri P (2008) Long-term effects of aided phytostabilisation of trace elements on microbial biomass and activity, enzyme activities, and composition of microbial community in the Jales contaminated mine spoils. Environ Pollut 152:702–712
Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Lett 529:86–92
Sarathchandra U (1978) Nitrification activities and the changes in the populations of nitrifying bacteria in soil perfused at two different H-ion concentrations. Plant Soil 50:99–111
Sarkar A, Kazy SK, Sar P (2013) Characterization of arsenic resistant bacteria from arsenic rich groundwater of West Bengal, India. Ecotoxicology 22:363–376
Schinner F, Ohlinger R, Kandeler E, Margesin R (1996) Methods in soil biology. Springer, Heidelberg
Silva RR, Silva MLN, Cardoso EL, Moreira FMS, Curi N, Alovisi AMT (2010) Biomassa e atividade microbiana em solo sob diferentes sistemas de manejo na região fisiográfica campos das vertentes – MG. Rev Bras Cienc Solo 34:1585–1592
Smith JL, Paul EA (1990) The significance of soil microbial biomass estimations. In: Bollag JM, Stotzky G (eds) Soil biochemistry. Marcel Dekker Inc., New York, pp 357–396
Sparling GP (1992) Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust J Soil Res 30:195–207
Speir TW, Ross DJ (2002) Hydrolytic enzyme activities to assess soil degradation and recovery. In: Burns RG, Dick RP (eds) Enzymes in the environment: activity, ecology and applications. Marcel Dekker Inc., New York, pp 407–431
Speir TW, Kettles HA, Parshotam A, Searle PL, Vlaar LNC (1999) Simple kinetic approach to determine the toxicity of As(V) to soil biological properties. Soil Biol Biochem 31:705–713
Sylvester-Bradley R, Asakawa N, Torraca SLA, Magalhães FMM, Oliveira LA, Pereira RM (1982) Levantamento quantitativo de microrganismos solubilizadores de fosfatos na rizosfera de gramíneas e leguminosas forrageiras na Amazônia. Acta Amaz 12:15–22
Tabatabai MA (1994) Enzymes. In: Weaver RW, Angle JS, Bottomley PS (eds) Methods of soil analysis Part 2. Microbiological and Biochemical Properties. Soil Sci Soc Am, Madison, pp 775–833
Tangahu BV, Abdullah SRS, Basri H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. doi:10.1155/2011/939161
Ter braak CJF, Smilauer P (2002) CANOCO Reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca
USEPA (2007). Method 3051 A: microwave assisted acid digestion of sediments sludges, soils and oils: revision 1. Technical report. US Environmental Protection Agency, Washington, DC
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass. Soil Biol Biochem 19:703–707
Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M (2009) Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ Sci Pollut Res 16:765–794
Vásquez-Murrieta MS, Garduño-Migueles I, Franco-Hernández O, Govaerts B, Dendooven L (2006) C and N mineralization and microbial biomass in heavy-metal contaminated soil. Eur J Soil Biol 42:89–98
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wang S, Zhao X (2009) On the potential of biological treatment for arsenic contaminated soils and groundwater. J Environ Manag 90:2367–2376
Wang FE, Chen YX, Tian GM, Kumar S, He YF, Fu QL, Lin Q (2004) Microbial biomass carbon, nitrogen and phosphorus in the soil profiles of different vegetation covers established for soil rehabilitation in a red soil region of southeastern china. Nutr Cycl Agroecosys 68:181–189
Wang YP, Shi JY, Lin Q, Chen XC, Chen YX (2007) Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. J Environ Sci 19:848–853
Wang Q, He M, Wang Y (2011) Influence of combined pollution of antimony and arsenic on culturable soil microbial populations and enzyme activities. Ecotoxicology 20:9–19
Williamson JC, Johnson DB (1994) Conservation of mineral nitrogen in restored soils at opencast coal mine sites: II. The effects of inhibition of nitrification and organic amendments on nitrogen losses and soil microbial biomass. Eur J Soil Sci 45:319–326
Wollum AG (1982) Cultural Methods for soil Microorganisms. In: Page AL, Miller RH, Keeny (eds) Methods of soil analysis. Part 2: chemical and microbiological properties, 2nd edn. ASA, Madison, pp 781–814
Yang RY, Tang JJ, Chen X, Hu SJ (2007) Effects of coexisting plant species on soil microbes and soil enzymes in metal lead contaminated soils. Appl Soil Ecol 37:240–246
Acknowledgments
The authors would like to thank the National Council for Scientific and Technological Development [Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-No 304574/2010-4)], the Coordination of Improvement of Higher Education Personnel [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes)] and the Foundation for Research Support of the State of Minas Gerais [Fundação de Amparo à Pesquisa do estado de Minas Gerais (Fapemig) (Parceria CAPES-FAPEMIG- 2618/2012, Processo no 23038.008715/2012)] for their financial support and fellowships granted to the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Santos, J.V., de Melo Rangel, W., Azarias Guimarães, A. et al. Soil biological attributes in arsenic-contaminated gold mining sites after revegetation. Ecotoxicology 22, 1526–1537 (2013). https://doi.org/10.1007/s10646-013-1139-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1139-9