Abstract
An indoor channel system was colonised with fluvial biofilms to study the chronic effects of high Fe and SO4 2− concentrations and acidic pH, the water chemistry in the surrounding streams of Aljustrel mining area (Alentejo, Portugal), and their contribution to community (in)tolerance to metal toxicity by short-term experiments with Cu and Zn. Biofilms were subjected to four different treatments during 8 weeks: high Fe and SO4 2− concentrations (1 mg Fe l−1 + 700 mg SO 2− 4 l−1) and acidic pH, high Fe and SO4 2− at alkaline pH; lower Fe and SO4 2− at acidic pH: and lower Fe and SO4 2− concentrations at alkaline pH as negative control. During chronic exposure, acidic pH affected growth negatively, based on low values of algal biomass and the autotrophic index, high values of the antioxidant enzyme activities and low diversity diatom communities, dominated by acidophilic species (Pinnularia aljustrelica) in acidic treatments, being the effects more marked with high Fe and SO4 2−. Co-tolerance to metals (Cu and Zn) was also shown in biofilms from the acidic treatments, contrasting with the higher sensitivity observed in the alkaline treatments. We can conclude that the Aljustrel mining area acidic environment limits algal growth and exerts a strong selection pressure on the community composition which is in turn, more tolerant to metal exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The streams surrounding the Aljustrel mining area (Alentejo Region, Southern Portugal: 37º52′37.90′′N, 8º10′54.64′′W) in the Iberian Pyrite Belt are characterized by acidic pH and high dissolved metals. They are affected by acidic mine drainage as a result of the sulphidic minerals (mainly pyrite) oxidation. This process, with many steps, involves iron oxidation in the minerals. The acidophilic chemolithotrophic microorganisms, mainly bacteria present in these environments (González-Toril et al. 2003) have the potential to accelerate the oxidation and dissolution reactions of the minerals, resulting in high sulphate contents, high dissolved metal concentrations, as arsenic (As), cadmium (Cd), copper (Cu), iron (Fe), manganese (Mn), lead (Pb), zinc (Zn) and H+ ions released as mineral acidity (Skousen et al. 1994; Nordstrom and Alpers1999). A number of studies have described the algal communities of streams affected by acid mine drainage, characterized by having lower diversity than pristine streams (for example, Bennett 1969; Hargreaves et al. 1976; Foster 1982; Verb and Vis 2000; Niyogi et al. 2002). In the Aljustrel streams, diatoms (Bacillariophyceae), part of the algae composing the biofilm, have low species diversity. Diatom communities are dominated by few diatom taxa typical of acidic waters with high metal contents (Luís et al. 2009, 2012).
Biofilm studies provide reliable information on biochemical and physiological changes, indicating an initial stress response of the organisms, that can be used as an early indicator of ecosystem damage (Sabater et al. 2007), providing also an ecotoxicological approach with higher ecological realism than just single species studies (Clements and Newman 2002; Guasch et al. 2010; Bonet et al. 2013). In addition, biofilms can also respond to chronic exposure to toxicants (Gold et al. 2003). Several studies have highlighted the biofilm sensitivity to both acidity and heavy metals (e.g. Guasch et al. 2002; Sabater et al. 2002; Ivorra et al. 2002; Pinto et al. 2003; Baker et al. 2004, Ferreira da Silva et al. 2009). Community structure in the streams surrounding the Aljustrel mining area has been attributed to chronic exposure to a mixture of metals (especially Fe) and acidic pH (Luís et al. 2012), but the specific influence of each factor has never been investigated.
Assessing contaminants impact on lotic systems is complicated due to the co-occurrence of several stress factors. Experimental investigations using artificial streams to examine the effect of acidification on periphyton communities have been conducted by Hendrey (1976) and Hall et al. (1980), and in natural lotic ecosystem with low pH, by Hamilton and Duthie (1984), and Mulholland et al. (1986). Therefore, the use of microcosms may be a pertinent alternative to field studies as this system allows a combination of pH/metals contamination to be simulated under controlled conditions (Clements and Newman 2002). In this study, we tried to mimic the extreme chemical environment described in the Aljustrel mining area: high contents of iron (upto 1 mg l−1), sulphates (700 mg l−1) and acidic pH (3.5–4.0).Artificial streams were used to grow biofilms under different chemical environments and compare them in terms of diversity and diatoms species composition, algal biomass, total biomass, photosynthetic parameters, antioxidant enzyme activities (AEAs) and antioxidant non-enzymatic mechanisms (glutathione (GSH) and phytochelatins (PCs)). Finally, differences in sensitivity to metals were also compared using short-term toxicity tests.
We hypothesize that the selection pressure exerted by the Aljustel mining area extreme chemical environment is mainly driven by acidity and the selected community will also be tolerant (co-tolerant) to metals. Low pH may effectively reduce toxicity at the cell surface via competition between metals and H+ ions (Riseng et al. 1991).
This study will contribute to understand the selection pressure exerted by acidity and/or Fe + SO4 2− and how these extreme environmental conditions influence metal toxicity in biofilms of AMD field waters aiding to define a strategy to improve the conditions of streams in the Aljustrel mining area.
Materials and methods
Experimental indoor channel system
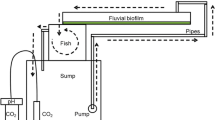
The experiments were carried out in an indoor channel system consisting of eight Perspex channels, each with 170 cm long and 9 cm wide, as described in Serra et al. (2009). Each treatment was composed by two channels named as replicates (R1 and R2). Dechlorinated tap water, previously filtered through an active carbon filter, was supplied from 8 L carboys located at the end of each channel and recirculated at a rate of 1 L min−1 through centrifuge pumps. Light was provided by LEDS (130 µE m−2 s−1) with a 12 h:12 h light : dark and the temperature was kept between 17 and 20 °C. Two water replacements per week were done, to maintain the good nutrients supply to the biofilms.
Periphyton communities’ colonisation and maturation
During 8 weeks (1 March to 27 April 2010), the channels were colonised with fluvial biofilms under different chemical conditions. A two-way ANOVA design was applied to test the specific effects of acidity and high dissolved salts (referred to as pH and Fe treatment factors, respectively) and to identify differences among treatments by post hoc comparisons. Four different treatments were applied: one with low addition of iron and sulphate (0.01 mg l−1 Fe; 300 mg l−1 SO4 2−) and alkaline pH (8.0), referred to as Non-Exposed treatment (Non-Exp); the second with the same iron and sulphate concentrations but with the addition of sulphuric acid to reduce pH (3.5 as in the field), Acid treatment; the third with high iron and sulphate (1 mg l−1 Fe; 700 mg l−1 SO4 2−) and alkaline pH, referred to as Fe treatment; and the fourth with the same iron and sulphate concentrations as the previous but with the addition of sulphuric acid to reduce pH at 3.5, referred as Acid + Fe treatment. Fluvial biofilms were obtained from an iron spring: Font SantVerdaguer (Guasch et al. 2012) and from Água Forte and Pero Bonito streams (Aljustrel, Portugal, Luís et al. 2012), the natural systems used to define the chemical conditions of Fe (iron spring), Acid + Fe (Água Forte) and Non-Exp (Pero Bonito) treatments. A mixture of the three biofilm inocula was added in each replicate.
Colonisation was carried out during 8 weeks (adding inocula in the channels, once a week, in one of the two water replacements) and biofilms were allowed to colonise the glass substratum (two glass types: 7.5 × 2 cm2 and 12 × 8.5 cm2), placed at the bottom of each channel. Water from the channels was completely replaced twice a week and macronutrients: 100 mg l−1 of calcium as Ca(NO3)2, 60 mg l−1 of magnesium as MgSO4·7H2O and 100 mg l−1 of sodium as NaCl and micronutrients: 0.03 mg l−1 of phosphate (nominal concentration) as KH2PO4 and 0.015 mg l−1 of silica as Na2SiO3 were added at each water renewal to avoid nutrient depletion and to simulate field conditions.
Endpoints sampling
During the whole experiment (8 weeks), the physicochemical parameters (temperature, pH, dissolved oxygen and conductivity) were measured using a multi-parametric probe (WTW METERS, Weilheim, Germany) before and after each water renewal (twice a week). In weeks 3, 6 and 8, 10 mL of water was taken to determine phosphate, anions and cations. For Fe, Cu and Zn analyses, 5 mL of water was taken in weeks 7 and 8. All samples were immediately filtered using 0.2 µm nylon membrane filters (Whatman) and just the samples for metal analysis were acidified with 1 % nitric acid (65 % suprapure, Merck). Samples were stored at 4 °C until analysis. In weeks 6 and 8, one glass of each replicate was sampled to analyse: algal biomass (Chlorophyll-a (Chl-a)), diatom composition, photosynthetic performance (through chl-a fluorescence parameters) and antioxidant enzymatic activities. The fluorescence measurements were carried out using undisturbed biofilm. Maximal quantum yield (ΦPSII) and effective quantum yield (Φ′PSII) parameters were used as photosynthesis capacity and photosynthesis efficiency indicators, respectively. Fo was used as an alternative non-destructive measure of algal biomass. Antioxidant enzymatic activities (catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione reductase (GR) were taken. As well as non-enzymatic activities to minimize the reactive oxygen species: total GSH, PCs, and total biomass (Ash-Free-Dry-Weight, AFDW). These last three were taken on week 8.
Metal (M) toxicity tests
After growth under the four different treatments, their effects in biofilm’s sensitivity to metals (Zn and Cu) were investigated. A 24 h acute toxicity test was performed. Biofilms of the four treatments were scraped and distributed in glass vials containing four increasing Cu and Zn concentrations: controls (no metal addition); concentration 1 (75 µg l−1 Cu and 500 µg l−1 Zn); concentration 2 (600 µg l−1 Cu and 4000 µg l−1 Zn) and concentration 3 (1200 µg l−1 Cu and 8000 µg l−1 Zn). Each glass vial contained: 11.5 mL of water from the four treatments previously tested during 8 weeks in the artificial streams, 2 ml of a scraped biofilm mixture (biofilm from six scrapped glasses, each 2 × 7.5 cm in 20 mL of treatment water) and 0.75 ml of the corresponding Cu and Zn solution. Samples were incubated during 24 h under the same conditions as in the colonisation, using a single-speed orbital mixer (KS260 Basic, IKA®) to maintain a constant agitation of 100 rpm. After 24 h of exposure, two replicates were sampled for AEA, PCs and GSH analyses to determine acute toxicity of Cu and Zn at concentration 1 (75 µg l−1 Cu and 500 Zn µg l−1) and Chl-a fluorescence measurements were made for all samples. AEA, PCs and GSH were focused on the lowest concentration because at higher concentrations enzymes may not respond anymore due to cell damage (Bonnineau et al. 2011). Water samples used to analyse dissolved metals (Fe, Cu, Zn) were filtered and preserved as described above.
Chemical analysis
Metal concentrations (Fe, Cu, Zn) in water were analysed by inductively coupled plasma mass spectrometry ICP-MS (7500c Agilent Technologies, Inc. Wilmington, DE). The accuracy of the analytical method was checked periodically using certified water reference (SPS-SW2 Batch 113, Oslo, Norway).
Phosphate concentration (PO4 3−) was analysed using the Murphy and Riley (1992) and dissolved major cations (Ca2+, K+, Mg2+, Na+) and anions (Cl−, SO4 2−, NO3 −) were analysed by ion-chromatography (761 Compact IC, METROHM Ltd., Herisau, Switzerland).
Biofilm analysis
Algal biomass
Chlorophyll-a (Chl-a) concentration, was used as a measure of algal biomass. Chl-a concentration was obtained after extraction with 90 % acetone and measured spectrophotometrically following Jeffrey and Humphrey (1975).
Total biofilm biomass
Total biofilm biomass was measured as AFDW. 15 cm2 of biofilm was scraped and then dried for 24 h at 105 °C in order to calculate dry matter (DW). Afterwards, samples were combusted for 4 h, in an oven at 450 °C (Hobersal MOD MF12-124, Spain) and then weighted again to calculate the mineral matter (AW). The AFDW was calculated subtracting the mineral matter from the total DW and after divided by the area of the glass slide. It was expressed in mg cm−2.
AFDW values were used with Chl-a values as a measure of the trophic status in streams, expressed as Autotrophic Index (AI). According to USEPA-RBP (Barbour et al. 1999), the formula to calculate the AI, is: AI = Chl-a (mg cm−2)/AFDW (mg cm−2).
Metals (Fe, Cu, Zn) quantification in biofilms
Biofilms were harvested by centrifugation at 4000×g for 10 min, washed in distilled H2O, and centrifuged again. In order to obtain the Fe, Cu and Zn bounded to the frustule, the method described by Purchase et al. (1997), with some modifications, was used; the cell pellet obtained was suspended in 2 ml of 0.1 M HNO3, sonicated in an ultrasonic bath for 5 min, centrifuged at 14.000×g for 10 min and collected into a microtube. The pellet was re-suspended in 1 ml of distilled H2O and transferred to Teflon bombs. Cells were digested overnight, at 115 °C, with 2 ml of an equivolume mixture of concentrated H2SO4 and concentrated HNO3. The cooled digested was filled up to 5 ml, using 1 M HNO3, and the concentration of Fe, Cu and Zn (equivalent to that taken up by cells) was determined by ICP-AES (inductively coupled plasma-atomic emission spectroscopy) model JobinYvonJy Plus.
Diatom treatment and identification
Biofilm samples were fist fixed with 4 % formaldehyde in order to preserve the sample for diatom study. Then after removing the formaldehyde, the samples were treated with HNO3 (65 %) and potassium dichromate (K2Cr2O7), at room temperature/24 h in order to eliminate the organic matter and to obtain clean diatom suspensions, followed by three centrifugations (1500 rpm) to rinse the samples. Permanent slides were mounted using the high refractive index (1.74) medium Naphrax® (Brunel Microscopes Ltd, UK). Light microscopy identifications were taken with a Leitz Biomed 20 EB microscope using a 100 × objective (A.N. 1.32); 400 valves were counted for each sample. Morphological terminology followed Krammer and Lange-Bertalot (1986, 1988, 1991a, b).
Chlorophyll-a fluorescence parameters
The chlorophyll fluorescence measurements were carried out by PhytoPAM (Pulse Amplitude Modulated) fluorometer (Heinz Walz GmbH) according to the procedure described in Corcoll et al. (2011) used to measure maximal quantum yield (ΦPSII), effective quantum yield (Φ′PSII) and Fo of dark-adapted cells (at 665 nm). The Fo of dark-adapted cells at 665 nm, is proportional to Chl-a concentration (Rysgaard et al. 2001); thus, Fo was used to follow the growth of the biofilm during the colonisation. All fluorescence parameters were calculated from the fluorescence signal recorded at 665 nm and given as relative units of fluorescence (Genty et al. 1989).
Antioxidant enzymes activities (AEA)
Three glass substrata (15 cm2 each) of biofilm were scrapped from each channel (considered as pseudo-replicates). Sampling, extraction and quantification of protein and AEA measurements were performed as described Bonnineau et al. (2011) and Bonet et al. (2013). For all AEA assays protein concentration used was 4 mg. For each AEA assay the optimal concentration of substrate or cofactor was optimised, thus the concentrations used here were 40 mM of H2O2 for CAT assay, 4 mM of H2O2 for the APX assay, 0.30 mM of NADPH for the GR assay and 0.075 mM of WST-1 SOD assay. AEA were calculated as specific activities (i.e. per microgram of proteins), thus catalase (CAT) is express in μmol H2O2 μg protein−1 min−1; APX express in μmol ascorbate μg protein−1 min−1; GR express in μmol NADPH μg protein−1 min−1, and SOD express in U μg protein−1.
Non-enzymatic activities
Phytochelatins, the fresh weight determination for each sample (two glass slides per replicate) was done and then samples were preserved at −80 °C prior to analysis. Peptide extraction was performed as described by Klapheck et al. (1994). Samples were sonicated for 20 s with 400 μl of 0.1 M HCl. The homogenates were centrifuged at 40000×g for 10 min, at 4 °C and the resulting supernatant was immediately used. The method for derivatisation of thiol compounds was performed as described earlier (Lima et al. 2006). Extracts (100 μl) were neutralised with 0.1 M NaOH, after the addition of 200 μl of 0.1 M Tris–HCl buffer (pH 8.0), 1 mM EDTA and 25 μl of 2 mM DTE. After incubation for 1 h at room temperature, 50 μl of 20 mMmBBr were added. Derivatisation was performed in the dark, for 40 min at a temperature of 35 °C. The reaction was stopped by the addition of 5 % (v/v) acetic acid up to a total volume of 1.5 ml. Samples were stored at 4 °C before HPLC–RP analysis.
The highly fluorescent derivatives were separated by RP–HPLC (Gilson liquid chromatograph, model 306), as described earlier (Lima et al. 2006). Samples were centrifuged at 40000×g, for 5 min, at 4 °C, filtered, and 20 μl aliquots were injected on a RP C18 column (250 × 4.6 mm2 i.d, 5 μl, Gilson). The column was equilibrated with previously degassed eluant A (0.01 % aqueous TFA (v/v)) and developed by a linear gradient of 0–20 % eluant B (90 % acetonitrile, in 0.01 % aqueous TFA) during the first 20 min, followed by an isocratic elution of 20 % eluant B for the next 30 min and finally a linear gradient of 20–90 % of eluant B during the last 10 min. The complete analysis was performed in 60 min. After each run, the column was washed by raising the concentration of eluant B to 100 % and re-equilibrated with eluant A. Thiols were resolved and eluted at a flow rate of 1 ml min−1 and detected by fluorescence (Jasco 821- FP Intelligent Spectrofluorometer) with excitation at 380 nm and emission at 480 nm (Lima et al. 2006). In order to overcome the problem of the decrease in derivatisation with chain length increase, adverted by other authors (Sneller et al. 2000), thiol identification was based on Cys and GSH standards (Sigma) and synthesised PCs standards (kindly offered by Dr. M.H. Zenk), with n ranging from 2 to 5.
Total Glutathione (GSH) was determined by HPLC as described above for PCs (Lima et al. 2006). GSH quantification was based on reduced GSH standards (Sigma).
Data treatment
In the acute exposure, data are presented in % of their relative controls to compare the response to chronic exposure. Potential differences between controls and treatments for physico-chemical variables (SO4 2−, PO4 3−, Fe in water, Fe in biofilm, conductivity and pH), Chl-a fluorescence parameters (F0, ΦPSII and Φ′PSII), AI, Chl-aenzymatic activities (APX, CAT and GR) and non-enzymatic protection mechanisms (GSH) were tested using two-way analysis of variance (ANOVA); effects were post hoc analysed by a Tukey’s test if significant differences were found. This analysis was performed using Sigma Plot 12.0 software. For all the analyses, statistical significance was set at p < 0.05. Primer 6 software was used to do PERMANOVA, Cluster and dbRDA analyses of diatom abundances.
Results and discussion
Chronic exposure
Experimental conditions and biofilm growth
The experimental setting allowed the reproduction of the chemical environment prevailing in acidic zones, such Aljustrel mining area: low pH with high dissolved salts (Fe and SO4 2−) with a characteristic biofilm in the Acid + Fe treatment. Treatment’s effect on pH was clearly achieved (Table 1). Fe + SO4 2− addition caused a marked increase in Fe concentration in water under acidic condition, but not under alkaline pH (Table 1). This result was expected since Fe solubility is very low at neutral/alkaline pH, and is supported by Fe contents in biofilms, much higher in all Fe treatments at alkaline pH due to retention and/or precipitation of Fe in biofilms (Table 1). On the other hand, sulphate concentration was affected by the two treatment factors, Fe and pH, since acidity was achieved by adding sulphuric acid. In addition, phosphate, while being equally added to all treatments, was also affected by the two treatment factors, leading to differences in P-availability that might influence biofilms growth. The influence of Fe on P-availability has already been described as one of the causes of algal growth reduction in Fe-rich environments due to an effective co-precipitation of Fe and P (e.g. Das et al. 2009; Guasch et al. 2012) in order to maintain the intracellular neutral conditions and diurnal shift of oxygen concentration in the biofilm layer (Das et al. 2009). Consequently, differences in algal growth and biofilm characteristics between treatments should be regarded as a result of the set of chemical alterations each treatment has created.
Biofilms characteristics
Fo showed the lowest values in Acid and Acid + Fe biofilms (Fig. 1), in accordance with Chl-a results (up to two orders of magnitude lower, Table 2).
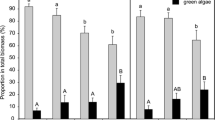
While Chl-a was much lower at acidic pH than at alkaline pH, total biomass (AFDW) was similar among biofilms exposed to different treatments, illustrating the shift from autotrophic to heterotrophic biofilms, dominated by bacteria, fungi and cyanobacteria (microscopic observations) and leading to marked differences in the Autotrophic Index (AI), Fig. 2. These results are in agreement with field investigations showing that acidophilic chemolithotrophic bacteria are the most adapted groups of microorganisms to these extreme conditions (González-Toril et al. 2003). Diatoms, while being also affected by the pH treatment factor, were present in all biofilms. From the initial three inocula mixture, each specific condition selected, during colonisation, the typical and dominant diatom species with ecological preferences closer to the physico-chemical characteristics of the water of the respective treatment. The inoculum from AMD affected streams (e.g. RibeiraÁgua Forte, Aljustrel, Portugal), containing a well-adapted community to chronic exposure of acidic conditions and metal contamination (mostly Fe) and dominated by few taxa. A similar community was found in the Acid and Acid + Fe biofilms (H’ = 0.50 and H’ = 0.99). In these biofilms, Pinnularia aljustrelica dominated with abundance always above 54 %, contrasting with diatom communities in Non-Exp and Fe biofilms dominated by Craticula halophila and Achnanthes thermalis (respectively), with higher diversity values (H’ = 2.28 and H’ = 1.50).
Cluster analysis and dbRDA show that pH was the main treatment factor driving the communities (Fig. 3). PERMANOVA reinforced pH as more significant than Fe (p = 0.001). This result is in concordance with other studies which concluded that the pH factor reduced diatom diversity (Mulholland et al. 1986; Planas 1996; Verb and Vis 2000) and caused a change in the assemblage structure through shifts from sensitive to tolerant taxa. In highly acidic environments, cells must overcome the high H+ concentration that may lead to rapid acidification of the cytosol (Gross and Robbins 2000), as well as develop strategies to protect themselves from adverse effects commonly associated with acidity, such as decreasing in nutrients, increasing in dissolved metal concentrations (Olaueson and Stokes 1989), and limited supply of CO2 for photosynthesis due to bicarbonate pool absence (Olaueson and Stokes 1989; Gross and Robbins 2000). The effective quantum yield, Ф’PSII, was affected by both treatment factors (Table 2) indicating that selection pressure had also affected the photosynthetic efficiency of the community. On the other hand, there were no significant effects in ФPSII. Probably it could also be linked with community adaptation. Adapted species to each treatment can have similar photosynthetic capacity.
a Dendrogram performed on a Bray-Curtis similarity matrix of diatom abundances in two sampling moments: 07/04/2012 and 21/04/2010; two replicates per sampling: 1, 2 from 07/04/2012; 3, 4 from 21/04/2010; b dbRDA: distance based redundancy analysis for the diatom resemblance matrix evidencing the samples disposition and the environmental variables which explain 78 % of biological variation. pH separates treatments in two groups: A acid and B alkaline: acidic pH is more relevant in the communities discrimination than Fe
Chronic exposure to acidic pH (pH treatment factor) caused also an increase in several AEAs (CAT, GR and APX) (Table 2) of these communities (Acid and Acid + Fe biofilms), which can be due to amelioration in oxidative stress to overcome the negative effects of acidic pH.
The effects of the Fe treatment factor were less clear and of lower magnitude than those caused by the pH factor. However, biofilm responses were more marked in Acid + Fe than in Acid biofilms. While Non-Exp and Fe biofilms had similar algal biomass (measured as Fo), it was higher in Acid than in Acid + Fe biofilms (Fig. 1), suggesting that elevated concentrations of Fe + SO4 2−constrained their ability to grow under acidic conditions. Differences in phosphate availability may partially explain this result. In agreement with these results, GR while being stimulated due to pH factor (acid treatment), it was less stimulated by the Fe factor, mainly at acidic treatments, leading to a significant interaction between treatment factors (Table 2). These results support the hypothesis of additive negative effects of elevated concentrations of Fe + SO4 2− and acidic pH reducing, in this case, the capacity of biofilms to cope with oxidative stress.
Influence of chronic exposure on metal toxicity
Acute exposure to metals may lead to transitory physiological effects if the community is not adapted because metal exposure is expected to cause oxidative stress, increased activity of the enzymes that may cope with this stress, photosynthesis inhibition and finally toxicity which may damage cell defense capacities. On the other hand, low pH may reduce toxicity due competition in acute exposure between metals and H+ ions at the cell surface (Riseng et al. 1991). Both aspects: co-tolerance and the prevailing chemical environment may explain the increase in tolerance found in our investigation. Biofilms from acidic treatments (Acid + Fe and Acid), were not inhibited after acute exposure to high Cu and Zn concentrations (Table 3), having values of Fo and ΦPSII similar to controls (80–150 %) whereas those from high pH treatments (Non-Exp and Fe) were clearly inhibited (Fig. 4), thus we can conclude that adaptation to acidity led to higher tolerance to acute metal exposure. Furthermore, communities of acidic treatments (Acid, Acid + Fe) did not increase enzyme activities after exposure to lower metal concentration, indicating that metals were not causing oxidative stress (Fig. 5). Other mechanisms, such as the production of PCs might help cells to cope with metal toxicity, reducing the reactive oxygen species (ROS) (Gaur and Rai 2001; Le Faucheur et al. 2005). PCs were just found in Acid treatment (Table 2), probably supported by higher contents of Cu and Zn (Table 4) together with low toxicity in biofilms in this treatment (Fig. 4). Metal toxicity was very clear but slightly different in Non-Exp biofilms compared to Fe biofilms. While effects of high metal concentrations on the photosynthetic capacity (ΦPSII) were slightly higher in Fe than in Non-Exp biofilms (Fig. 4), CAT, GR and GSH response to metal exposure was higher in Fe biofilms, indicating a better ability to cope with oxidative stress leading finally to lower toxicity (Fig. 5). CAT is mainly involved in the removal of H2O2 excess, converting it into water and molecular oxygen (Noctor and Foyer 1998). In agreement with our results, Li et al. (2006), showed the increase in AEA (e.g. CAT, GPX and SOD) levels in cultures of Pavlova viridis (Prymnesiophyceae) after Cu–Zn treatment indicating the protective role of these enzymes against Cu–Zn induction of oxidative stress. In addition, metal exposure caused a marked increase of total glutathione (GSH), a non-enzymatic antioxidant protective mechanisms in the same biofilms (Fig. 5). This increase indicates that metal exposure (Cu and Zn) induced the cells to activate non-enzymatic antioxidant protective mechanisms at high pH and high Fe + SO4 2− concentrations. Non-enzymatic antioxidants include the major cellular redox buffer glutathione. GSH is oxidised by ROS forming oxidised glutathione (GSSG). Dehydroascorbic acid DHA is reduced to ascorbate by the action of DHA reductase, using GSH as the reducing substrate in response to increased H2O2 levels (Foyer and Halliwell 1976). This reaction generates glutathione disulphide (GSSG), which is in turn re-reduced to GSH by nicotinamide adenine dinucleotide phosphate.
Metal (Cu and Zn) effects on chl-a fluorescence parameters after short-term exposure. Average Effective quantum yield (Φ′PSII), maximum quantum yield (ΦPSII) and minimum fluorescence (Fo) values (% of controls) for each treatment (n = 2), after 24 h of exposure to concentrations 2 (600 µg l−1 Cu and 4000 µg l−1 Zn; bars with lines) and 3 (1200 µg l−1 Cu and 8000 µg l−1 Zn; bars with no pattern), C2 and C3 respectively. Significant differences based on two-way ANOVA results are also indicated: A C3: Fe p < 0.01; B C2: pH p < 0.05, C3: pH p < 0.01; C C2, C3: pH p < 0.05. Different letters (a, b, c) indicate different treatments based on post hoc comparisons. Φ′PSII results after exposure to concentration 2 are not presented due to the lack of effects
Metal (Cu and Zn) effects on AEAs after short-term exposure. CAT, GR activities and GSH values (% of controls) for each treatment after 24 h of exposure to concentration 1 (75 µg l−1 Cu and 500 µg l−1 Zn). Significant differences based on two-way ANOVA results are also indicated: A pH p < 0.05, Fe*pH p < 0.05; B Fe p = 0.077; C Fe p < 0.05, Fe*pH p = 0.061. Different letters (a, b) indicate different treatments based on post hoc comparisons
(NADPH) as a reducing agent, a reaction catalysed by GR, thus having an important role in the non-enzymatic protective system against toxic metal damage (Sabatini et al. 2009). Organisms increase the activity of GSH biosynthetic enzymes (Vanacker et al. 2000) and GSH levels in the presence of abiotic stresses (Noctor et al. 2002). GR response was also higher in Fe than in Non-Exp biofilms, but the variability was very high and the differences were not statistically significant (Fig. 4).
Conclusions
During chronic exposure, acidic pH affects growth more negatively than high Fe and SO 2− 4 concentrations, based on low values of Fo and AI, high values of CAT, APX, GR and low diversity of diatom communities, dominated by acidic species in biofilms growing at acidic pH. This is the cost to maintain neutral the cytosolic pH, while the surrounding environment is acid. However, high Fe and SO 2− 4 concentrations were also constraining the ability to live under acidic conditions because the negative effects of acidity were aggravated under these conditions. In addition, when Cu and Zn were added (acute exposure), algal biomass (Fo) and the photosynthetic capacity of biofilms (ΦPSII) were not affected in acidic treatments (Acid andAcid + Fe biofilms) showing low metal toxicity in communities adapted to acidic pH, irrespective of the Fe and SO4 2− concentrations during growth. Metal toxicity was much higher to biofilms non-exposed and to those only exposed to high Fe and SO4 2− concentrations during growth. However, in contrast to the results obtained in biofilms growing at acidic pH, the Fe treatment factor influenced metal sensitivity of biofilms growing at alkaline pH. Antioxidant responses were more marked in Fe than in Non-Exp biofilms, indicating that the structural changes resulting from chronic exposure to elevated concentrations of Fe and SO4 2− were linked to a higher ability to respond to acute metal exposure leading to lower sensitivity. This interaction was not observed at acidic pH.
Through this lab experiment, it was possible to show that acidic pH was the parameter that affected most the diatom community and that high Fe and SO4 2− concentrations were also contributing to control algal growth. Acidic pH seems to ameliorate metals’ effects if the community has been pre-exposed and hence adapted to the prevailing extreme chemical environment. This may explain why acidic areas (as pyrite mines) with several high metal concentrations have algal growth with typical acidic diatoms as Pinnularia aljustrelica, found in high abundances. It is thus possible to extrapolate these findings to the complex pyrite mines, mainly under stable conditions since these systems are also exposed to drastic changes in water chemistry linked to high rainfall, for instance, with effects on diatom communities difficult to predict from this lab experiment.
References
Baker BJ, Lutz MA, Dawson SC, Bond PL, Banfield JF (2004) Metabolically active eukaryotic communities in extremely acidic mine drainage. Appl Environ Microbiol 70(10):6264–6271
Barbour MT, Gerritsen J, Snyder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish, 2nd edn. EPA 841-B-99-002. US Environmental Protection Agency, Office of Water, Washington, DC
Bennett HD (1969) Algae in relation to mine water. Castanea 34:306–328
Bonet B, Corcoll N, Acuňa V, Sigg L, Behra R, Guasch H (2013) Seasonal changes in antioxidant enzyme activities of freshwater biofilms in a metal polluted Mediterranean stream. Sci Tot Environ 444:60–72
Bonnineau C, Bonet B, Corcoll N, Guasch H (2011) Catalase in fluvial biofilms: a comparison between different extraction methods and example of application in a metal-polluted river. Ecotoxicol 20:293–303
Clements WH, Newman MC (2002) Community Ecotoxicology. John Wiley, Chichester
Corcoll N, Bonet B, Leira M, Guasch H (2011) Chl-a fluorescence parameters as biomarkers of metal toxicity in fluvial biofilms: an experimental study. Hydrobiologia 673:119–136
Das BK, Roy A, Koschorreck M, Mandal SM, Wendt-Potthoff K, Bhattacharya J (2009) Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res 43:883–894
Ferreira da Silva E, Almeida SFP, Nunes ML, Luís AT, Borg F, Hedlund M, Marques de Sá C, Patinha C, Teixeira P (2009) Heavy metal pollution downstream the abandoned Coval da Mó mine (Portugal) and associated effects on epilithic diatom communities. Sci Total Environ 407:5620–5636
Foster PL (1982) Species associations and metal contents of algae from rivers polluted by heavy metals. Freshwater Biol 12:17–39
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133(1):21–25
Gaur JP, Rai LC (2001) Heavy metal tolerance in algae. In: Rai LC et al (eds) Algal adaptation to environmental stresses. Springer, Berlin, pp 363–388
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990(1):87–92
Gold C, Feurtet-Mazel A, Coste M, Boudou A (2003) Impacts of Cd and Zn on the development of periphytic diatom communities in artificial streams located along a river pollution gradient. Arch Environ Contam Toxicol 44(2):189–197
González-Toril E, Llobet-Brossa E, Casamayor EO, Amann R, Amils R (2003) Microbial ecology of an extreme acidic environment, the Tinto River. Appl Environ Microbiol 69(8):4853–4865
Gross S, Robbins EI (2000) Acidophilic and acid-tolerant fungi and yeasts. Hydrobiologia 433:91–109
Guasch H, Paulsson M, Sabater S (2002) Effect of copper on algae communities from oligotrophic calcareous streams. J Phycol 38(2):241–248
Guasch H, Atli G, Bonet B, Corcoll N, Leira M, Serra A (2010) Discharge and the response of biofilms to metal exposure in Mediterranean rivers. Hydrobiologia 657:143–157
Guasch H, Acosta XG, Urrea G, Bañeras L (2012) Changes in the microbial communities along the environmental gradient created by a small Fe spring. Freshwater Sci 31(2):599–609
Hall RJ, Likens GE, Fiance SB, Hendrey GR (1980) Experimental acidification of a stream In the Hubbard Brook Experimental Forest, New Hampshire. Ecology 61:976–989
Hamilton PB, Duthie HC (1984) Periphyton colonization of rock surfaces in a boreal forest stream studied by scanning electron microscopy and track autoradiography. J Phycol 20(4):525–532
Hargreaves JW, Lloyd EJH, Whitton BA (1976) Chemistry and vegetation of highly acidic streams. Freshwater Biol 5:563–576
Hendrey GR (1976) Effects of pH on the growth of periphytic algae in artificial stream channels (IR 25/76.SNSF project). SNSF, Ås-NLH, Oslo, pp 50
Ivorra N, Barranguet C, Jonker M, Kraak MHS, Admiraal W (2002) Metal-induced tolerance in the freshwater microbenthic diatom Gomphonemaparvulum. Environ Pollut 116:147–157
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pfl 167:191–194
Klapheck S, Fliegner W, Zimmer I (1994) Hydroxymethyl-Phytochelatins [γ-glutamylcysteine n-serine] are metal-induced peptides of the Poaceae. Plant Physiol 104(4):1325–1332
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae, Naviculaceae, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae, Bacillariaceae, Epithemiaceae, Surirellacea, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 596
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae, Centrales, Fragilariaceae, Eunoticeae, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 577
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae, Achnanthaceae, Kristische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gesamtliteraturverzeichnis, Süßwasserflora von Mitteleuropa. Gustav Fischer Verlag, Stuttgart, p 437
Le Faucheur S, Bhera R, Sigg L (2005) Thiol metal contents in periphyton exposed to elevated copper and zinc concentrations: a field and microcosm study. Environ Sci Technol 39(20):8099–8107
Li M, Hu C, Zhu Q, Chen L, Kong Z, Liu Z (2006) Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 62(4):565–572
Lima A, Pereira S, Figueira E, Caldeira G, Caldeira H (2006) Cadmium detoxification in roots of Pisumsativum seedlings: relationship between toxicity levels, thiol pool alterations and growth. Environ Exp Bot 55:149–162
Luís AT, Novais MH, Van de Vijver B, Almeida SFP, Ferreira da Silva EA, Hoffmann L, Ector L (2012) Pinnularia aljustrelica sp. nov. (Bacillariophyceae), a new diatom species found in acidic waters in the Aljustrel mining area (Portugal) and further observations on the taxonomy, morphology and ecology of P. acidophila HOFMANN et KRAMMER and P. acoricola HUSTEDT. Fottea 12(1):27–40
Luís AT, Teixeira P, Almeida SFP, Ector L, Matos JX, Ferreira da Silva EA (2009) Impact of acid mine drainage (AMD) on water quality, stream sediments and periphytic diatom communities in the surrounding streams of Aljustrel mining area (Portugal). Water Air Soil Pollut 200:147–167
Mulholland PJ, Elwood JW, Palumbo AV, Stevenson RJ (1986) Effect of stream acidification on periphyton composition, chlorophyll, and productivity. Can J Fish Aquat Sci 43(10):1846–1858
Murphy J, Riley JP (1992) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Niyogi DK, Lewis WM, McKnight DM (2002) Effects of stress from mine drainage on diversity, biomass, and function of primary producers in mountain streams. Ecosystems 5:554–567
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant MolBiol 49:249–279
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53(372):1283–1304
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site. California. PNAS 96(7):3455–3462
Olaueson MM, Stokes PM (1989) Responses to the acidophilic alga Euglena mutabilis (Euglenophyceae) to carbon enrichment at pH 3. J Phycol 25(3):529–539
Pinto E, Sigaud-Kutner TCS, Leitão MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39(6):1008–1018
Planas D (1996) Acidification effects. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: Freshwater benthic ecosystems. Academic, San Diego, CA, USA, pp 497–530
Purchase D, Miles RJ, Young TWK (1997) Cadmium uptake and nitrogen fixing ability in heavy-metal-resistant laboratory and field strains of Rhizobium leguminosarum biovar trifolii. FEMS Microbiol Ecol 22(1):85–93
Riseng CM, Gensemer RW, Kilham SS (1991) The effects of pH, aluminium, and chelator manipulations on the growth of acidic and circumneutral species of Asterionella. Water Air Soil Pollut 60:249–261
Rysgaard S, Kühl M, Glud RN, Hansen JW (2001) Biomass, production and horizontal patchiness of sea ice algae in a high-Arctic fjord (Young Sound, NE Greenland). Mar Ecol Prog Ser 223:15–26
Sabater S, Navarro E, Guasch H (2002) Effects of copper on algal communities at different current velocities. J Appl Phycol 14:391–398
Sabater S, Guasch H, Ricart M, Romaní A, Vidal G, Klünder C, Schmitt-Jansen M (2007) Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal Bioanal Chem 387:1425–1434
Sabatini SE, Juarez AB, Eppis MR, Bianchi L, Luquet CM, Molina MCR (2009) Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicol Environ Saf 72(4):1200–1206
Serra A, Guasch H, Martí E, Geiszinger A (2009) Measuring in-stream retention of copper by means of constant-rate additions. Sci Total Environ 407(12):3847–3854
Skousen J, Sextone A, Garbutt K, Sencindiver J (1994) Acid mine drainage treatment with wetlands and anoxic limestone drains. In: Kent DM (ed) Applied wetland science and technology. Lewis, Boca Raton, pp 263–282
Sneller FE, van Heerwaarden LM, Koevoets PL, Vooijs R, Schat H, Verkleij JA (2000) Derivatization of phytochelatins from Silene vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman’s reagent and monobromobimane. J Agric Food Chem 48(9):4014–4019
Vanacker H, Carver TLW, Foyer CH (2000) Early H2O2 accumulation in mesophyll cells leads to induction of glutathione during the hyper-sensitive response in the barley-powdery mildew interaction. Plant Physiol 123(4):1289–1300
Verb RG, Vis ML (2000) Comparison of benthic diatom assemblages from streams draining abandoned and reclaimed coal mines and non-impacted sites. J N Am Benthol Soc 19:274–288
Acknowledgments
The authors are grateful to the Biology and Geosciences Departments of the University of Aveiro, Portugal, to the Fundação para a Ciência e Tecnologia, Portugal (grant number SFRH/BD/36137/2007) and to the University of Girona for their financial support by the project FLUVIALMULTISTRESS funded by the Spanish “Ministerio de Economía y competitividad” ref: CTM2009-14111-CO2-01. The authors also express their gratitude to the “ServeisTècnics de Recerca” of the University of Girona for offering their facilities and technical assistance during the metal analysis. We thank Ana Lima for doing the phytochelatins (PCs) and total gluthatione (GSH) analyses.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luís, A.T., Bonet, B., Corcoll, N. et al. Experimental evaluation of the contribution of acidic pH and Fe concentration to the structure, function and tolerance to metals (Cu and Zn) exposure in fluvial biofilms. Ecotoxicology 23, 1270–1282 (2014). https://doi.org/10.1007/s10646-014-1270-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1270-2