Abstract
Synthetic pyrethroids are classified as moderately toxic to mammals and birds; nevertheless, they are highly toxic to non-target aquatic organisms such as fish and zooplankters. Chemical pollutants produce different effects in exposed organisms, ranging from biochemical to population responses. Cladocerans can modify the energy content of their offspring according to the surrounding medium as a way to improve their odds in case they have to cope with stressful conditions at birth. In this study, the effect of a synthetic pesticide on two levels of response in a Daphnia species different from those traditionally used as test organisms was evaluated. With this aim, Daphnia schoedleri neonates (<24 h) were exposed for 21 days to three sublethal concentrations of α-cypermethrin, 0.54, 5.4, and 54 ng L−1, which correspond to 48-h EC1/100, EC1/10, and EC1, respectively. Effects were measured through a life table analysis for fecundity and survivorship. For effects on progeny, protein, carbohydrates, and lipids were determined and then transformed to caloric content. Biomarkers (BM) were expected to be the most sensitive evaluated response; nevertheless, population parameters such as survivorship and net reproductive rate (R0) were more sensitive since they presented significant differences with respect to controls at the lowest tested concentration. Neonates’ caloric content varied during the reproductive period assessed and was negatively correlated to fecundity: as more neonates were born, less energy was provided by the adult females. Macromolecules concentration and caloric content values in cypermethrin-exposed adults were not different from those recorded in the control at the end of exposure time. The results herein presented suggest that stressed daphnids allocate more energy reserves to their offspring, although this strategy can vary depending on the number of reproductive events during the lifecycle, and on the toxicant’s concentration. Sub-individual approaches to assess toxicant effects should be accompanied by demographic studies, which support population effect predictions inferred from BMs assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The core idea for designing pesticides is to kill noxious organisms, for which they are intentionally released into the environment. Nevertheless, non-target organisms could also get in contact with such chemicals that can cause their death or affect them negatively. In addition, severe environmental problems can appear in contaminated media that induce reduction in abundance, species richness or distribution (Hanazato 2001).
Pesticides can also affect aquatic invertebrate’s populations dynamics by altering their age structure (Liess et al. 2006), growth and development (Forbes and Cold 2005), and mortality rates (Schroer et al. 2004). Moreover, the effects on fecundity will have repercussions at the population level (Goedkoop et al. 2010).
Pyrethroids are synthetic derivatives from pyrethrins—active substances naturally produced by certain chrysanthemum species—that possess neurotoxic effects and affect the central nervous system of insects, which are the main target of such pesticides (Hu et al. 2010; Pettit et al. 2010). Nevertheless, pyrethroids can reach water bodies and affect non-target organisms because of their usage in both urban and agricultural areas for pest control; their posterior runoff could eventually affect the aquatic biota (Weston et al. 2009; Shen et al. 2012). Although pyrethroids are considered as slight to moderate toxicants to mammals, it has been observed that aquatic organisms, including fish and aquatic invertebrates, are much more sensitive (Friberg-Jensen et al. 2003).
The major environmental concern about cypermethrin (CYP) is related to its high toxicity to aquatic organisms, since several studies have confirmed adverse effects on different taxa at very low concentrations, including lethal and sublethal responses. Reported EC50 values include CYP concentrations as low as 0.19 μg L−1 (72 h) for the benthic copepod Acartia tonsa (Medina et al. 2002), or 0.5 μg L−1 for rainbow trout (Bradbury and Coats 1989); sublethal effects may occur at much lower concentrations (Moore and Waring 2001). CYP is specifically toxic to crustaceans and, for this reason, it is used for the treatment of parasitic copepods infestations in salmonids culture (Gowland et al. 2002); after treatments, therapeutic bath media are released to the surrounding water, and this leads to direct exposure of this pesticide to the natural aquatic biota. It should be noted that the metabolism of this synthetic pyrethroid involves the glutathione S-transferase (GST) enzyme, producing oxidative stress in crustaceans (Gowland et al. 2002).
Cypermethrin is highly toxic to Daphnia magna and Ceriodaphnia dubia, by decreasing their reproductive output and growth as well as survival when these cladocerans were exposed to concentrations as low as a few nanograms per liter (Shen et al. 2012). Furthermore, carbohydrates content was reduced related directly to CYP concentration (Christensen et al. 2005). Although carbohydrates play an important role in energy budget, the major energy reserve in cladocerans is represented by lipids, which are firstly mobilized for reproduction or involved in energy-demanding processes, like detoxification (Sancho et al. 1996).
How cladocerans accumulate energy depends on intrinsic (e.g., age, physiological condition) and extrinsic (environmental conditions) factors that will modify the energy allocation and the way in which these resources can be stored by organisms as lipids, protein, or carbohydrates (Tessier and Goulden 1982; Peters 1987), and how they are destined to maintenance, growth, and reproduction (Kooijman and Bedaux 1996). The way in which these organisms obtain and how they use the energy, under both ideal and stressful environmental conditions, have been thoroughly studied by several authors, being Kooijman the most recognized and the author of the dynamic energy budget (DEB) theory as a useful explanation of this process (Nisbet et al. 2012). Energy obtained through food is allocated to two main large physiological processes: somatic growth-structure maintenance and sexual maturity-reproductive maintenance-gametes production; chemical pollutants affect negatively growth and reproduction of organisms and under stress conditions both processes compete for energy. DEB theory is the result of several studies carried out more than 20 years ago by Kooijman and coworkers, summarized in Kooijman (2001) and Kooijman et al. (2009).
The present study was aimed at evaluating to what extent can energy reserves be allocated to Daphnia schoedleri offspring when they are exposed to α-CYP and figuring out if there is any correlation between the energy resources allocated to neonates and the responses at supra-individual level. D. schoedleri is a zooplanktonic cladoceran not traditionally used as test organism in aquatic ecotoxicology, hence the obtained information will be relevant for the knowledge of toxic responses in a different freshwater microcrustacean species.
Materials and methods
The D. schoedleri strain was obtained from the cladoceran collection of the Experimental Hydrobiology Laboratory (Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional); this strain has been maintained active for more than 15 years. Test specimens were obtained from controlled cultures of known age, where ten parthenogenetic females were grown in 500-mL containers with 400-mL of reconstituted hard water (206 mg L−1 CaCl2·2H2O, 247 mg L−1 MgSO4·7H2O, 193 mg L−1 NaHCO3, and 8 mg L−1 KCl) (US EPA 2002). Specimens were maintained at 25 ± 1 °C, with a 16:8 h (light:dark) photoperiod and fed on Pseudokirchneriella subcapitata at 6 × 105 cells mL−1.
Acute toxicity tests were carried out to determine the 48 h-EC50 value for α-CYP (PESTANAL®, analytical standard 99 % purity. supplied by Sigma-Aldrich), according to standardized guidelines (US EPA 2002). Dilution water was reconstituted hard water, and the endpoint was immobilization measured at 24 and 48 h. EC50 values were used afterwards as reference to determine CYP sublethal concentrations for the sub-chronic toxicity exposure bioassays; the corresponding toxicity units (TU) of tested concentrations were also determined.
Effect of α-cypermethrin on D. schoedleri reproductive fitness
Based on previously determined EC1’s for this study, neonates were exposed to EC1 and fractions of this value (1/10 and 1/100), resulting the following concentrations: 0.54, 5.4, and 54 ng L−1 of CYP, equal to 0.0009, 0.009, and 0.09 TU; experiments were conducted until cladocerans reached 21-days. Additionally, two controls in reconstituted hard water were carried out, one with no toxicant and another with dimethylsulfoxide (DMSO) at 50 μL L−1, since this was the solvent concentration used as CYP carrier in all the treatments. DMSO is one of the solvents recommended for toxicity bioassays with Daphnia at concentrations not exceeding 100 μL L−1 (OECD 1998). Five replicates of each treatment were performed using ten specimens per replicate. All assays were conducted in 250-mL containers with 200-mL test solution under the same feeding regimen as described previously; test solution was completely renewed every other day. Once reproduction began, progeny was daily separated and recorded in the different treatments and controls during the 21-days exposure period; no complete life-cycle studies were carried out for controls, because after 21 days mortality was recorded for all the α-CYP concentrations. For the biochemical analysis, produced neonates were euthanized by fast freezing and kept at −20 °C until their use. At the end of experiments, adult females were euthanized in the same manner and kept under the same conditions.
Life table analysis
With adults’ survival and fecundity data recorded during 21 days of CYP exposure and through a partial life table analysis, the demographic parameters, survivorship (l x ) and age related fecundity (m x ), were determined. In addition, the intrinsic rate of population growth (r) (estimated by iteration with Euler’s equation), net reproductive rate (R 0), life expectancy at birth (e x ), generation time (G), and the average lifespan were also calculated for survivorship and fecundity values recorded during 21 days in each cohort, according to Krebs (1985) and Martínez-Jerónimo and Martínez-Jerónimo (2007). Even though these demographic parameters are supported by data collected only during 21 days, these are valid for this period and enable comparison with values determined for the control series.
Caloric content determination
Once reproduction began, the progeny produced daily in all the replicates of each treatment was pooled, and the total number of neonates was divided in three equal parts for the posterior analysis of total protein, lipids, and carbohydrates. Thus, these three neonates’ replicates (with at least 40 individuals) and five adults’ replicates (with at least 3 daphnids) were homogenized in 1-mL methanol:water solution (1:1). Lipids were extracted twice with equal volumes of chloroform. Thereafter, the organic phase was heated to dryness; 250 μL of water, 250 μL H2SO4 and 1 mL of vanillin reactive were added as described by Zöllner and Kirsch (1962). Lipids in the samples were quantified by comparing absorbance (532 nm) data with the standard curve of cholesterol.
Total protein and total carbohydrates were determined in the aqueous phase resultant from lipid extraction. 300 μL of the Bradford’s reactive (Bradford 1976) were added to the same volume of the daphnids homogenate, and protein content was obtained by interpolation in the standard curve of bovine serum albumin (absorbance at 595 nm).
The Dubois’ method was used for carbohydrates quantification, using dextrose as standard (Arzate-Cárdenas and Martínez-Jerónimo 2012). 50 μL of saturated phenol solution (about 80 %) were added to 250 μL of the homogenate and the final reaction was performed at 80 °C after sulfuric acid addition (500 μL). Absorbance was read at 490 nm and interpolated in dextrose standard curve.
Finally, the caloric content was calculated as described in Mann and Gallager (1985) and results are expressed as millicalorie individual−1.
Statistical analysis
α-Cypermethrin toxic effective concentrations (ECX) were calculated through Probit analysis (Stephan 1977). Results from energy content in offspring were compared through bifactorial ANOVA (factors: maternal age and CYP concentration), and Dunnett’s multiple comparisons test (to establish differences with respect to the control group at each time). On the other hand, caloric content in adult females as well as the population parameters herein determined were compared through one-way ANOVA and Dunnett’s post hoc test using Statistica 7 software. Survivorship curves were compared with the control group through the Mantel-Cox log rank and Grehan–Breslow–Wilcoxon tests (GraphPad Prism 6 software). Pearson’s correlation coefficients were determined for fecundity (mx) versus caloric content and macromolecules concentration in produced progeny. Significant differences were established when P ≤ 0.05.
Results
Cypermethrin values for the 48-h EC50 and EC1 for D. schoedleri neonates were calculated through Probit analysis (Stephan 1977). The calculated EC50 was in average 600 ng L−1 with a 95 % confidence interval (CI) from 520 to 680 ng L−1, whereas the EC1 value was 54 ng L−1 (CI = 42–65 ng L−1); EC1 was used as criterion to choose the sublethal concentrations here studied.
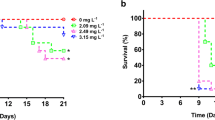
Figure 1 shows the survivorship curves observed for CYP-exposed D. schoedleri. Survival decreased as CYP concentrations increased, reaching mortalities up to 96 % with the highest CYP concentration. At the end of experiments (21-days), survivorship in 5.4 and 0.54 ng L−1 treatments was intermediate between that observed in controls (around 85 %) and that determined for the most affected group (4 % of survival), with values of 32 and 52 %, respectively. Survival curves were compared by Mantel-Cox statistical test; with respect to control group, significant reduced survival was determined for D. schoedleri exposed to all tested CYP concentrations (P < 0.01 for 0.54 and 5.4 ng L−1, and P < 0.001 for 54 ng L−1).
The main demographic parameters obtained with the life table analysis are shown in Fig. 2. As a general trend, all population parameters were significantly reduced by CYP exposure (P < 0.05). The average life span (ALS) was statistically reduced at the two highest CYP concentrations (Fig. 2a). Life expectancy at birth (e x ) decreased with increasing toxicant concentrations, revealing a significant reduction in the two highest concentrations (Fig. 2b). Because experiments were capped at 21 days, values for ALS and e x determined for controls are close to this maximum period of observation, but these demographic parameters could be somewhat different, taking into account that the normal lifespan of this cladoceran is larger than 21 days; nevertheless, they are valid for comparison among results here obtained for CYP-exposed individuals. The net reproductive rate (R 0) (the average offspring produced by an average female during her life cycle, in this case during the observed period of time) was significantly reduced in all treatments exposed to CYP; reduced fecundity in DMSO-exposed organisms was not significant with respect to control without solvent (Fig. 2c). Generation time (G) (the average time between the birth of parents and the birth of their offspring) was significantly decreased with the two highest CYP concentrations (P < 0.05) (Fig. 2d), but this could be an artifact because the Life Table analysis was partial. Finally, the intrinsic rate of population increase (r) was significantly reduced only at 54 ng L−1 (P < 0.001), showing no differences between the other treatments and the control.
Results of Life Table Analysis for Daphnia schoedleri exposed to different concentrations of α-cypermethrin; average values (n = 5) and standard error bars are shown. Data were compared through one-way ANOVA and Dunnet’s post hoc test. Significant differences were established by *(P < 0.05), and ***(P < 0.001)
Figure 3 shows the adult daphnids macromolecules and caloric content. As observed, no significant differences were established between the different treatments and the control group. No datum is available for the EC1 (54 ng L−1) CYP group since the resultant survivorship at day 21 was 4 %; thereafter, the amount of cladocerans’ biomass for the quantification of macromolecules was insufficient.
Figure 4 shows fecundity values (m x) for D. schoedleri in the different exposure conditions; graphs here included show the variation in fecundity along the observed period. Trends are similar for the two lower CYP values, with respect to control, but for the highest concentration (equivalent to the EC1), lower fecundity and a clear reduction in reproduction were observed at the end of the exposure time.
Regarding macromolecules content determined in the progeny produced with the different treatments shown in Fig. 5, no clear, distinguishable pattern was recognized, probably due to changes during the reproductive period, likely related with the clutch size. Although there were some offspring groups that significantly varied from the control (P < 0.05), the group that differed the most was that exposed to the highest CYP concentration tested; in such a group, the macromolecules and energy contents were frequently higher than in the controls. Comparatively with the CYP-exposed groups, variation in the controls lied within a narrower range.
The peaks observed in neonates’ caloric content correspond to low reproductive outcome of CYP-exposed daphnids, whereas the reduction in energy content, observed with the highest CYP concentration, was related to high reproductive rate at that maternal age. Two-way ANOVA demonstrated significant differences elicited for both the age of the mothers and the CYP concentration regarding all macromolecules and the caloric content as well; although no pattern could be clearly established for the daily variations recorded, CYP concentration accounted for most of the observed effects. The Dunnett’s comparison with respect to the control group evidenced that for most of the daily determinations, macromolecule concentrations and caloric content were higher for neonates released from mothers exposed to the highest CYP concentration (equivalent to the EC1), being the difference highly significant (P < 0.001) with respect to the control.
Pearson’s correlation coefficients demonstrated that macromolecules content as well as caloric content in neonates were negatively correlated with fecundity (m x ), as observed in Table 1. In consequence, as m x values increased, protein, lipids, carbohydrates, and caloric content per neonate decreased.
Table 2 shows the lowest tested concentration at which significant effects were observed (LOEC), the non-observed effect concentration (NOEC), and the maximum allowable toxic concentration (MATC) for CYP-exposed D. schoedleri; these values were established after post hoc comparison were carried out, and significant difference with respect to the control were determined. MATC was calculated as the geometric mean of LOEC and NOEC. Table 2 also shows the acute to sub-chronic ratio (calculated as the ratio of the α-CYP 48-h EC50 divided by the LOEC) and the corresponding TU needed to produce the effect on that endpoint. The values of NOEC and MATC for l x and R 0 are expressed as intervals, because the lowest tested concentration caused significant differences (P < 0.05), with respect to the control group. Comparison of these data revealed that the less sensitive responses were r and the caloric content in both neonates and adults, by presenting the highest NOEC, MATC, and LOEC values. On the other hand, the most susceptible responses were l x and R 0, which presented NOEC values under the CYP concentrations tested, since even the lowest assayed (0.54 ng L−1) caused significant reduction on these demographic parameters.
Discussion
Notwithstanding all the here tested CYP concentrations could be considered safe, because they were established from the EC1 and below (including this as the highest tested value), most of the assessed endpoints evidenced toxic effects indeed. Results of the acute toxicity tests with D. schoedleri exposed to CYP cannot easily be compared to other reported values (EC50) because all experiments in this study were conducted at a higher temperature (25 °C), since this condition prevails in subtropical environments. It has been described that pyrethroids toxicity can vary depending on the temperature at which exposures occur (Ratushnyak et al. 2005). Nevertheless, lethal effects on D. magna have been observed at concentrations as low as 0.0006 μg L−1 (1.44127 × 10−11 M) (Kim et al. 2008), and also at relatively higher concentrations such as 4.81 mg L−1 (1.15542 × 10−5 M) (Sheng et al. 2004). The present study revealed that survivorship was significantly affected at all pyrethroid tested concentrations since the beginning of the test (Fig. 1). These findings can be considered of high relevance because reduction of survival was documented at concentrations that occur in contaminated water bodies (Marino and Ronco 2005).
Pyrethroids are known to affect the nervous system by altering the activity of ATPases (Begum 2009) and sodium gates in the nervous system (among others) in insects (Breckenridge et al. 2009); Friberg-Jensen et al. (2010) documented that impairment of thoracic appendices movement with the consequent reduction in food intake is likely to occur in cladocerans. Reduced food consumption leads to less energy income that could be followed by fecundity decline, and by a rise in mortality, even if food supply is not limited. For other pesticides, diminution in filtration and ingestion rate has been reported, besides the subsequent reduction in energy reserves in organisms exposed at concentrations as low as 1/1,000 of the respective EC50 (Sancho et al. 2009; Villarroel et al. 2009). Assuming this as a possible effect produced in CYP-exposed cladocerans, failure in obtaining energy (through food intake) and its consequences in daphnids’ metabolism (increase of energy demand) could partially explain the decrement in l x observed in the present study.
It has been described that adult females cannot allocate as much energy to maintenance and reproduction to the same extent when detoxification processes occur that consume most of the available resources (Arzate-Cárdenas and Martínez-Jerónimo 2012). Kim et al. (2008) reported that fecundity and net reproductive rate are susceptible to be diminished in stressed cladocerans, as occurred with Daphnia magna exposed to CYP at 0.002, 0.02, and 0.2 ng L−1 for 21-days.
In the present study, D. schoedleri exhibited decreased reproduction at CYP concentrations as low as 0.54 ng L−1, which also affected their survival. In this case, a trade-off was expected to be observed, in which fecundity could be relegated in order to allocate more energetic resources (available through food ingestion and assimilation) to maintenance (survivorship). Nevertheless, obtained results permit to assume that D. schoedleri would have allocated its energy resources to both processes (reproduction and maintenance) instead of generating a trade-off between them, which led to the reduction in both demographic responses (fecundity and survival).
As seen in Fig. 3, D. schoedleri generation time was decreased with the two highest CYP concentrations herein tested. Nevertheless, this parameter was expected to increase because of delayed age at first reproduction and lengthening of the interbreeding time, effects that have been documented in organisms exposed to environmentally concerning toxicants (Martínez-Jerónimo and Martínez-Jerónimo 2007). Contrarily, these daphnids could have shortened their intermolt and interbreeding times and decreased their clutch size as a strategy to guarantee their persistence, by reducing fecundity but increasing frequency, which could explain why the generation time in stressed D. schoedleri (by CYP exposure) was shorter than in controls.
The intrinsic rate of population increase seems not to be a good indicator of toxic effects in this study, as it was not significantly modified in CYP-exposed D. schoedleri. Kim et al. (2008) observed similar results for CYP and D. magna, where increased mortality and decreased fecundity were documented, but the life table analysis did not reflect effects on the intrinsic rate of population increase; this demographic parameter has been also referred by Villarroel et al. (2009) as a nonsensitive population parameter in comparison to BMs. In C. dubia exposed to CYP, 8-day growth was a more sensitive endpoint than survival and reproduction (Shen et al. 2012). Nevertheless, the sensitivity of r as a demographic endpoint should not be discarded as it represents the difference between the instantaneous rates of birth and death in the population, two important population parameters susceptible to be modified under environmental stress conditions. It could be expected that under different stress-causing conditions, r could reflect better negative effects at the population level.
Regarding effects on energy resources, Arzate-Cárdenas and Martínez-Jerónimo (2012) reported a decreasing pattern of available energy in chronically chromium-exposed D. schoedleri, which was concentration dependent. Furthermore, it has been reported that CYP is able to affect appendices and oral apparatus movements in D. magna at low concentrations, which leads to lower feeding capacity (lower food and energy intake) (Friberg-Jensen et al. 2010); nevertheless, after 21 days no significant differences were observed in CYP-exposed adult daphnids with respect to the control in the present study.
According to McCauley et al. (1990) and Bradley et al. (1991) models, energy resources in cladocerans should be allocated first to maintenance, and once this demand is satisfied, energy can be reallocated to reproduction. Under stressful conditions, a trade-off between these two responses can be seen. Thus, offspring would be provided with enough energy resources to cope with the stressful conditions in which they would be released. This is a strategy followed by some organisms in order to warrant population persistence. Daphnids can follow both of these models depending on the environmental conditions, and their reproduction is susceptible to be modified by age, during their life time (Glazier and Calow 1992), which could explain the variation in the energy content in D. schoedleri offspring seen in Fig. 5. In addition, Arzate-Cárdenas and Martínez-Jerónimo (2012) reported that toxicant exposure can induce adult females to improve their offspring odds in stress conditions by increasing their energy reserves, generating a trade-off from quantity to quality (fewer neonates with higher energy reserves).
It is a generalized notion that as clutch size grows in number, the energy content per individual is less in comparison to clutches constituted by fewer neonates. In this case, there are minimums and maximums in the energy content of D. schoedleri offspring, which are related to the maximum and minimum fecundity (m x ) values, respectively (Figs. 4, 5).
Life table analyses are useful to assess the effect of different toxic pollutants on cladocerans but exposure time should be extended to include most of the life cycle. On the other hand, caloric content (result of protein, lipids, and carbohydrates in tissues) has been selected as a general physiological status BM, because it is susceptible to be modified by several environmental factors, including toxicant exposure (Sancho et al. 2009). BMs are referred as more sensitive endpoints than population parameters (Villarroel et al. 2009), but inference of effects at population level is required.
The use of BMs is intended to shorten the exposure period and provide information that could be relevant at higher levels of biological organization, from individuals to populations. Sub-individual responses offer the possibility to infer long-term effects by evaluating short-term exposure endpoints. However, studies that report correlations between BMs and demographic parameters do not take into consideration how sub-individual responses can be modified by the organisms’ age. For example, De Coen and Janssen (1997) described that cellular energy allocation (CEA) of D. magna neonates and demographic parameters such as the intrinsic rate of population increase, r, showed a good correlation. Furthermore, CEA has been applied in organisms different from daphnids and has shown that energy budgets are good predictors for long-term effects at higher biological levels (Smolders et al. 2004). Nonetheless, the results herein presented showed that caloric content cannot be always considered as the most sensitive BM, since there was no modification in adult daphnids energy reserves, and the energy that was allocated to neonates was significantly increased only at the highest CYP concentration assayed (54 ng L−1). Some demographic parameters could be more sensitive than BMs such as the caloric content, but this may not be generalized for all toxicants since toxic effects will depend on their physicochemical properties, besides the organisms’ physiological condition, which can be modified during the life cycle, as shown in the results herein presented.
Conclusions
CYP produced significant toxic effects in D. schoedleri even at concentrations low enough to be be considered safe for aquatic biota; the main effect was observed on survival, fecundity, and average lifespan. As a general trend, all demographic parameters were significantly reduced by CYP exposure (P < 0.05); these results evidence the high sensitivity of this cladoceran to this insecticide, and warn about possible toxic effects in natural environments reached by CYP remnants. Although there are reports that confirm energy content decrease in Daphnia exposed to different sorts of toxicants, and that neonates susceptibility is not altered by maternal age, our results point out that generalizations should be carefully established because of natural variability on how energy is allocated by cladocerans. In particular, reproduction was affected by CYP concentration, and the produced neonates showed negative correlation with macromolecules concentration and caloric content; this means that neonates in large clutch sizes had lower caloric content. The endpoints here assessed at both studied levels showed different sensitivity, but they can be regarded as complementary. Although BMs offer the possibility to shorten exposure time and rapidly assess effects, their results should be accompanied by demographic information obtained from (sub) chronic bioassays that support the former results, as the toxic effect can be under- or over-estimated, depending on the selected endpoint.
References
Arzate-Cárdenas MA, Martínez-Jerónimo F (2012) Energy resource reallocation in Daphnia schoedleri (Anomopoda: Daphniidae) reproduction induced by exposure to hexavalent chromium. Chemosphere 87:326–332
Begum G (2009) Enzymes as biomarkers of cypermethrin toxicity: response of Clarias batrachus tissues ATPase and glycogen phosphorylase as a function of exposure and recovery at sublethal level. Toxicol Mech Methods 19:29–39
Bradbury SP, Coats JR (1989) Comparative toxicology of the pyrethroid insecticides. Rev Environ Contam Toxicol 108:133–177
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bradley MC, Perrin N, Calow P (1991) Energy allocation in the cladoceran Daphnia magna Straus, under starvation and refeeding. Oecologia 86:814–818
Breckenridge CB, Holden L, Sturgess N, Weiner M, Sheets L, Sargent D, Soderlund DM, Choi JS, Symington S, Clark JM, Burr S, Ray D (2009) Evidence for a separate mechanism of toxicity for the type I and the type II pyrethroid insecticides. Neurotoxicology 30(Suppl 1):17–31
Christensen BT, Lauridsen TL, Ravn HW, Bayley M (2005) A comparison of feeding efficiency and swimming ability of Daphnia magna exposed to cypermethrin. Aquat Toxicol 73:210–220
De Coen WM, Janssen CR (1997) The use of biomarkers in Daphnia magna toxicity testing: IV. Cellular energy allocation: a new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J Aquat Ecosyst Stress Recov 6:43–55
Forbes VE, Cold A (2005) Effects of the pyrethroid esfenvalerate on life-cycle traits and population dynamics of Chironomus riparius—importance of exposure scenario. Environ Toxicol Chem 24:78–86
Friberg-Jensen U, Wendt-Rasch L, Woin P, Christoffersen K (2003) Effects of the pyrethroid insecticide, cypermethrin, on a freshwater community studied under field conditions. I. Direct and indirect effects on abundance measures of organisms at different trophic levels. Aquat Toxicol 63:357–371
Friberg-Jensen U, Nachman G, Christoffersen KS (2010) Early signs of lethal effects in Daphnia magna (Branchiopoda, Cladocera) exposed to the insecticide cypermethrin and the fungicide azoxystrobin. Environ Toxicol Chem 29:2371–2378
Glazier DS, Calow P (1992) Energy allocation rules in Daphnia magna: clonal and age differences in the effects of food limitation. Oecologia 90:540–549
Goedkoop W, Spann N, Akerblom N (2010) Sublethal and sex-specific cypermethrin effects in toxicity tests with the midge Chironomus riparius Meigen. Ecotoxicology 19:1201–1208
Gowland BT, Moffat CF, Stagg RM, Houlihan DF, Davies IM (2002) Cypermethrin induces glutathione S-transferase activity in the shore crab, Carcinus maenas. Mar Environ Res 54:69–177
Hanazato T (2001) Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Pollut 112:1–10
Hu Z, Du Y, Nomura Y, Dong K (2010) A sodium channel mutation identified in Aedes aegypti selectively reduces cockroach sodium channel sensitivity to type I, but not type II pyrethroids. Insect Biochem Mol Biol 41:9–13
Kim Y, Jung J, Oh S, Choi K (2008) Aquatic toxicity of cartap and cypermethrin to different life stages of Daphnia magna and Oryzias latipes. J Environ Sci Health B 43:56
Kooijman SALM (2001) Quantitative aspects of metabolic organization: a discussion of concepts. Philos Trans R Soc Lond B Biol Sci 356:331–349
Kooijman SALM, Bedaux JM (1996) Analysis of toxicity tests on Daphnia survival and reproduction. Water Res 30:1711–1723
Kooijman SALM, Baas J, Bontje D, Broerse M, van Gestel CAM, Jager T (2009) Ecotoxicological applications of dynamic energy budget theory. In: Devillers J (ed) Ecotoxicology modeling. Emerging topics in ecotoxicology: principles, approaches and perspectives. Springer, Berlin, pp 23–259
Krebs CJ (1985) Ecology: the experimental analysis of distribution and abundance, 3rd edn. Harper and Row, New York, pp 145–160
Liess M, Pieters BJ, Duquesne S (2006) Long-term signal of population disturbance after pulse exposure to an insecticide: rapid recovery of abundance, persistent alteration of structure. Environ Toxicol Chem 25:1326–1331
Mann R, Gallager SM (1985) Physiological and biochemical energetics of larvae of Tereo navalis L and Bankia gouldi (Bartsch) (Bivalvia: Teredinidae). J Exp Mar Biol Ecol 85:211–218
Marino D, Ronco A (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826
Martínez-Jerónimo F, Martínez-Jerónimo L (2007) Chronic effect of NaCl salinity on a freshwater strain of Daphnia magna Straus (Crustacea: Cladocera): a demographic study. Ecotoxicol Environ Saf 67:411–416
McCauley E, Murdoch WW, Nisbet RM, Gurney WSC (1990) The physiological ecology of Daphnia: development of a model of growth and reproduction. Ecology 71:703–715
Medina M, Barata C, Telfer T, Baird DJ (2002) Age- and sex-related variation in sensitivity to the pyrethroid cypermethrin in the marine copepod Acartia tonsa Dana. Arch Environ Contam Toxicol 42:17–22
Moore A, Waring CP (2001) The effects of a synthetic pyrethroid pesticide on some aspects of reproduction in Atlantic salmon (Salmo salar L.). Aquat Toxicol 52:1–12
Nisbet RM, Jusup M, Klanjscek T, Pecquerie L (2012) Integrating dynamic energy budget (DEB) theory with traditional bioenergetic models. J Exp Biol 215:892–902
OECD (1998) Guidelines for testing of chemicals. Guidelines 211: Daphnia magna reproduction test. Paris, France
Peters RH (1987) Metabolism in Daphnia. In: Peters RH, DeBernardi R (eds) Memorie dell’Istituto Italiano di Idrobiologia, Daphnia, vol 45. Istituto Italiano di Idrobiologia, Verbania, Pallanza
Pettit WJ, Whelan PI, McDonnell J, Jacups SP (2010) Efficacy of alpha-cypermethrin and lambda-cyhalothrin applications to prevent Aedes breeding in tires. J Am Mosq Control Assoc 26:387–397
Ratushnyak A, Andreeva MG, Trushin MV (2005) Effects of type II pyrethroids on Daphnia magna: dose and temperature dependences. Riv Biol 98:349–357
Sancho E, Ferrando MD, Andreu E (1996) Physiological stress responses of Anguilla anguilla to fenitrothion. J Environ Sci Health B 31:87–98
Sancho E, Villarroel MJ, Andreu E, Ferrando MD (2009) Disturbances in energy metabolism of Daphnia magna after exposure to tebuconazole. Chemosphere 74:1171–1178
Schroer AF, Belgers JD, Brock TC, Matser AM, Maund SJ, Van den Brink PJ (2004) Comparison of laboratory single species and field population-level effects of the pyrethroid insecticide lambda-cyhalothrin on freshwater invertebrates. Arch Environ Contam Toxicol 46:324–335
Shen MF, Kumar A, Ding SY, Grocke S (2012) Comparative study on the toxicity of pyrethroids, α-cypermethrin and deltamethrin to Ceriodaphnia dubia. Ecotoxicol Environ Saf 78:9–13
Sheng XM, Xiong L, Wu ZB, Tang HF, Liu T, Wang Y (2004) Toxicity of cypermethrin to Daphnia magna HB. J Environ Sci 16:770–771
Smolders R, Bervoets L, De Coen W, Blust R (2004) Cellular energy allocation in zebra mussels exposed along a pollution gradient: linking cellular effects to higher levels of biological organization. Environ Pollut 129:99–112
Stephan CE (1977) Methods for calculating an LC50. In: Mayer FI, Hamelink JL (eds) Aquatic toxicology and hazard evaluation. ASTM STP 534. American Society for Testing and Materials, Philadelphia, pp 65–84
Tessier AJ, Goulden CE (1982) Estimating food limitation in cladoceran populations. Limnol Oceanogr 27:707–717
U.S. Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th edn. EPA-821-R-02-012
Villarroel MJ, Sancho E, Andreu-Moliner E, Ferrando MD (2009) Biochemical stress response in tetradifon exposed Daphnia magna and its relationship to individual growth and reproduction. Sci Total Environ 407:5537–5542
Weston DP, Holmes RW, Lydy MJ (2009) Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut 157:287–294
Zöllner N, Kirsch K (1962) Microdetermination of lipids by the sulphophosphovanillin reaction. Z Ges Exp Med 135:545–561
Acknowledgments
F. Martínez-Jerónimo and R. Ortiz-Butrón are thankful for financial support provided by the Sistema de Estímulo al Desempeño de los Investigadores (EDI) and Comisión de Operación y Fomento de Actividades Académicas (COFAA) of the Instituto Politécnico Nacional. M. Arzate-Cárdenas is grateful for support from CONACYT (fellow No. 205561). Finally, thanks to three anonymous reviewers whose detailed analysis and critical comments helped to significantly improve this paper.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
Experiments of this study comply with the current laws of Mexico regarding ethic aspects on the use of biological material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Jerónimo, F., Arzate-Cárdenas, M. & Ortiz-Butrón, R. Linking sub-individual and population level toxicity effects in Daphnia schoedleri (Cladocera: Anomopoda) exposed to sublethal concentrations of the pesticide α-cypermethrin. Ecotoxicology 22, 985–995 (2013). https://doi.org/10.1007/s10646-013-1077-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1077-6