Abstract
The use of herbicides has increased over the last decades. Glyphosate is the most widely used herbicide commercialized in more than 750 formulations. While information about glyphosate’s toxicity on different non-target aquatic organisms has been vastly documented, we know little about the transgenerational effects in aquatic biota. This study determined the cross-generation effects produced by the glyphosate-based herbicide Faena® on the American cladoceran Daphnia exilis. Measured endpoints were survival, reproductive responses, metabolic biomarkers, and the size of neonates. D. exilis was exposed to glyphosate concentrations of 2.09, 2.49, and 3.15 (mg L−1) (as content in Faena®) during 21 days starting from neonates, at 25°C, 16:8 photoperiod, fed with 8 × 105 cells mL−1 of Pseudokirchneriella subcapitata. The LC50 was 4.22 mg L−1. Survival, accumulated progeny, and the number of clutches in the parental generation (P1) were significantly higher than those observed in the first generation (F1). Exposure to the herbicide completely inhibited reproduction in the F1. The size of the neonates varied among treatments and broods in P1; nevertheless, neonate size (body and total lengths, as well as body width) was significantly affected in F1. Toxic effects on the survival and reproduction of D. exilis were significantly increased in the F1 exposed to Faena®. Results warn about the augmented effect on progeny where parents were exposed to this herbicide. Multigenerational adverse effects could be expected in freshwater zooplankton exposed to Faena®. The frequently claimed low toxicity of glyphosate must be revised to control the indiscriminate use of this herbicide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture is one of the most important ways to produce foodstuffs for human consumption and animal raising. Every year, agrochemicals are extensively used around the world, most of them without restriction in their commercialization and application in farmlands; consequently, residual concentrations are being accumulated in the environment (Gill et al. 2018). Pesticides are a group of natural and synthetic substances used to eradicate agricultural pests, as well as human and livestock disease vectors (Mnif et al. 2011). Residues of pesticides come into aquatic ecosystems through agricultural drainage, urban sewage, and surface runoff (Banaee et al. 2020), and also can be moved to water via atmospheric process (Battaglin et al. 2014). Herbicides are used to control weeds in crops of economic importance and lawns. Glyphosate (N-(phosphonomethyl) glycine) is the herbicide most used worldwide (Myers et al. 2016; Singh et al. 2020)); it is marketed under different commercial denominations, being Roundup® the most popular brand (de Brito Rodríguez et al., 2017). At present, glyphosate-based herbicides (GBH) are produced by many companies (Woodburn 2000), with more than 750 commercial formulations, accounting for a global consumption ranging from 0.6 to 1.2 × 106 tons every year (de Brito et al. 2017). In Mexico, Faena® is a commercial formula used in agriculture, aquaculture, and urban green areas (gardens and lawns).
Glyphosate is a broad-spectrum, non-selective contact herbicide, which persistence was formerly stated to be low in soil and water (half-life of 60 and 35 days, respectively) (Leyva-Morales et al. 2014; Kang et al. 2017). It is now recognized that glyphosate and its metabolites persistence in soil and water are more prolonged than previously documented (Myers et al. 2016). Updated information indicates that glyphosate half-life is variable in water, soil, and air, and depends on environmental factors. For example, in seawater, this ranges from 47 days (in low light) to 267 days (in darkness). However, this time can increases to 315 days in the dark at a higher temperature (Singh et al. 2020). Its success in agriculture is due to its mode of action on target organisms. Glyphosate affects the growth of weeds through inhibition of the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), an enzyme necessary for the synthesis of aromatic amino acids required for structural proteins and also useful for metabolism and defense mechanisms (Duke and Powles 2008; Battaglin et al. 2009).
Different studies suggest that surfactants and other adjuvants in commercial formulations are more toxic than glyphosate (Battaglin et al. 2009; Myers et al. 2016). Polyoxyethylene tallow amine (POEA) is a common surfactant included in glyphosate-based herbicides; it is assumed to be the main responsible for the toxicity of the commercial products in some aquatic organisms (Perkins et al. 2000; Thompson et al. 2004). Tsui and Chu (2003) reported the acute toxicity of POEA to be 1.15 mg L−1 in C. dubia. Other studies indicate that POEA’s acute toxicity in aquatic organisms ranges from 0.097 to 2 mg L−1 (Giesy et al. 2000; Brausch et al. 2007). Therefore, adjuvants’ contribution to toxicity in GBH cannot be excluded, despite the type and concentrations of these and other supposed inert substances that are unknown for most of the commercial formulations in many countries. The whole toxicity assessment provides an essential measure of the impact that commercial formulations produce in organisms, notwithstanding the co-formulants added to the active substance are unknown. In the natural environment, aquatic biota will be exposed to all the chemical compounds included in the formulation.
The extensive use of this pesticide has grown exponentially since its commercial launch in 1974 (Woodburn 2000). The introduction of genetically modified crops resistant to glyphosate (corn, cotton, soybean, and canola) has contributed to the increase in the production and sale of glyphosate-based commercial formulations (Battaglin et al. 2014; Myers et al. 2016). Currently accumulated evidence of the environmental impacts of this herbicide through toxic effects to the biota and to the human (Gill et al. 2018) has conducted to the prohibition of glyphosate in many countries around the world, including recently in Mexico.
Regarding the concentration of glyphosate in the aquatic environment, in Southern México Ruiz-Toledo et al. (2014) reported average values of 0.35 μg L−1 and 5.69 μg L−1 in dry and rainy seasons, respectively. However, higher values, ranging from 100 to 700 μg L−1 have been measured in watercourses in the Argentine pampa (Paravani et al., 2016). Glyphosate has also been determined in groundwater in concentrations up to 2.6 μg L−1, not only in agriculture areas but also in urban areas where glyphosate has been intensively used to control weeds in lawns, roads, streams, and drains (Sanchis et al., 2012).
Based on its acute and dermal toxicity, glyphosate is classified into the class IV “slightly toxic” herbicide (U.S. Environmental Protection Agency 2002). Notwithstanding, the negative impacts of glyphosate on freshwater systems have been extensively documented. Acute and chronic effects on non-target organisms, like phytoplankton in one generation bioassays (Tsui and Chu 2003; Qiu et al. 2013), fish (Kreutz et al. 2011; Rondón-Barragán et al. 2012; Singh et al. 2020), and crustaceans (Domínguez-Cortinas et al. 2008; Reno et al. 2018; Rodríguez-Miguel et al. 2018), have been reported, but no multigenerational or transgenerational effects in aquatic biota have been reported.
Recently, the impact of xenobiotics across generations has been evaluated to determine whether the toxic effects in an organism may persist in its subsequent generations. Inference of toxic impacts based on the effects observed in the parental generation is not enough to forecast damages in wild populations in natural environments (Hammers-Wirtz and Ratte 2000), nor to discard the impact on future generations in aquatic biota.
Multigenerational exposure is a novel approach to determine the long-term impact of pollutants in more than one generation in a population because the changes in the parental generation can modify the behavior and health of individuals in the next generations (Yu et al. 2013; Prud’homme et al. 2017). Skinner (2008) defines multigenerational effects of environmental factors (including chemical pollutants), as the exposure that produces effects in multiple generations (in the exposed pregnant mothers and their embryos). In contrast, transgenerational effects are observed in consecutive generations, not involving direct progeny exposure. Transgenerational toxic effects involve alterations in the epigenome mainly related to DNA methylation, which could be associated with some changes in genes, producing hereditary consequences in future generations. In contrast, multigenerational exposure does not imply epigenetic process but damages in the progeny during gestation that has consequences in the development of the filial generation. Both processes are environmentally important regarding chemical pollution effects, but impacts in the structure and function in aquatic communities are different.
An important factor that could determine the recovery and persistence of a population exposed to a toxic pollutant for a long time is the organisms’ capability to modify their tolerance through generations. Under these conditions, the sensitivity of the organisms can be modified in consecutive generations. Resistance could be expected when the individuals can express inducible mechanisms that make them more tolerant to the chemical stressor through improved physiological defense and detoxification mechanisms. Nevertheless, increased sensitivity could also be expected when some essential physiological processes are affected, and the progeny of these individuals displays impaired responses to the same pollutants. Cross generation effects have been observed in aquatic biota (Corrales et al. 2014; Campos et al. 2016), and in terrestrial invertebrates (Li et al. 2019), when they were exposed to endocrine disruptors (Benzo[a]pyrene), and to synergistic additives for pesticides (piperonyl butoxide).
Relevant information about the cross-generation toxicity of metals (Yu et al. 2013; Colombo et al. 2014), pharmaceutical drugs (Prud’homme et al. 2017), hydrocarbons (Corrales et al. 2014; Prud’homme et al. 2017), and vertebrate-type estrogens (Brennan et al. 2006) has been reported in different test models. This information is pertinent to know the potential damage in populations that survive under stressful conditions for a long time in natural environments, as happens in zooplankters thriving in polluted freshwater bodies. At this respect both, the study of multigenerational and transgenerational effects are important to assess the long-term effects of chemical stressors in aquatic ecotoxicology. The mechanisms involved in the routes of direct exposure to the progeny in pregnant females, as well as the induction to epigenetic transgenerational phenotypesare also important in this sense.
In ecotoxicology, the reproduction test with Daphnia magna is extensively used. A procedure using this cladoceran (based on the OECD guideline 211, OECD 2004) has been proposed to assess effects in two generations (Barata et al. 2017). Nevertheless, the study of impacts on other cladocerans is needed to know the effect of chemical stressors in succeeding generations. Daphnia exilis is a freshwater cladoceran distributed in temperate to subtropical latitudes in North America; it has been proposed as a test model in ecotoxicology due to its taxonomic similarities with D. magna (Hairston et al. 1999; Martínez-Jerónimo et al. 2008). Although D. magna and D. exilis are included in the same taxonomic complex (Ctenodaphnia subgenus), their sensitivity to the same stressor differs substantially, as demonstrated for the reference toxicant, hexavalent chromium, indicating a higher sensitivity for D. exilis (Martínez-Jerónimo et al. 2008). The use of alternative species with different geographical distribution can contribute to having a complete scenario to infer the effects of pollutants on the aquatic biota.
Reproduction in D. exilis is essentially by parthenogenesis, and the progeny is morphologically and genetically identical to the parents. In cladocerans, differences in the body size of neonates can happen in the clutches produced and released during the life cycle. Nevertheless, there are external factors that determine the shape and body size in Daphnia. According to Martínez-Jerónimo (2012), the temperature is a determining factor that conditions the increase of the body size in D. magna. Other abiotic factors which variation might increase or decrease the boost of body size are photoperiod, population density, quantity and quality of food, water composition, and biotic factors (Fontoura and Agostinho 1996; Martínez-Jerónimo 2012), but chemical stress could be an additional influence that can impact the size and the content of macromolecules in daphnids.
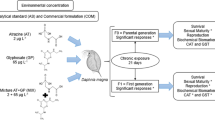
Because during chronic and subchronic exposure of daphnids to sublethal concentration of toxicants the progeny of adult females could also be indirectly exposed and negatively affected, the objective of this study was to determine whether the herbicide Faena®, as one of the most popular commercial formulations of glyphosate, can produce multigenerational toxic effects in a non-target species, the American cladoceran D. exilis. To test this hypothesis, the progeny produced in the F1 was raised in the same concentrations that the individuals were exposed in the parental generation. As endpoints, survival, reproduction, and biochemical effects in the parental and filial generation were assessed. The aim was to document the toxic multigenerational impacts of this commercial herbicide in this zooplankter to warn about its indiscriminate usage.
Materials and methods
Test organisms
Experiments were conducted using a clonal strain of D. exilis fed the green microalga Pseudokirchneriella subcapitata. Both strains were obtained from the plankton collection of the Laboratorio de Hidrobiología Experimental, Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, Mexico City, Mexico. Cultures of 10 D. exilis females of the same age were maintained in 500-mL glass jars with 350 mL of moderately hard reconstituted water (MHRW) (96 mg L−1 NaHCO3, 60 mg L−1 CaSO4•2H2O, 60 mg L−1 MgSO4, 4 mg L−1 KCl, in 1000 mL deionized water), in environmental chambers at 25 + 1°C and a 16:8 (light/darkness) photoperiod, fed with P. subcapitata.
Pseudokirchneriella subcapitata was axenically cultured in Bold’s basal medium (250 mg L−1 NaNO3, 25 mg L−1 CaCl2•2H2O, 75 mg L−1 MgSO4•7H2O, 75 mg L−1 K2HPO4, 175 mg L−1 KH2PO4, 25 mg L−1 NaCl, 4.98 mg L−1 FeSO4•7H2O, 0.001 mL L−1 H2SO4, 11.42 mg L−1 H3BO3, 50 mg L−1 EDTA, 31 mg L−1 KOH, 8.82 mg L−1 ZnSO4•7H2O, 1.44 mg L−1 MnCl2•4H2O, 0.71 mg L−1 MoO3, 1.57 mg L−1 CuSO4•5H2O, 0.49 mg L−1 Co (NO3)2•6H2O, in 1000 mL deionized water), at 25 + 1°C with continuous illumination for 10 days.
Glyphosate
Glyphosate was used as the commercial formulation Faena® (CAS:38641-94-0) (Monsanto Comercial, S. de R. L. de C. V., Zapopan, Jal., Mexico). This product contains the potassium salt of N-(phosphonomethyl) glycine (35.6% w/w equivalent to 363 g L−1 of acid equivalent), and different adjuvants of unknown composition and concentration, in an aqueous base (64.4% w/w in total). In all experiments, a stock solution containing the equivalent concentration of 500 mg L−1 glyphosate was prepared.
Acute toxicity test
The acute toxicity of Faena® on D. exilis was determined to select the subchronic concentrations used in the generational experiments. The median lethal concentration (LC50) was established according to the guideline of the U.S. Environmental Protection Agency (2002), testing the following nominal concentrations considering the glyphosate content: 2, 3, 4, 5, 6, and 7 mg L−1 with three replicates for each one; these values were selected after several range-finding tests. MHRW was used as dilution water and as a negative control. Test organisms were incubated at 25 + 1°C, 16:08-h photoperiod (light: darkness) in an environmental chamber. After 48 h, immobilized and death individuals were recorded. Five different complete bioassays were used to determine the average LC50.
Multigenerational effects
Subchronic 21-day experiments were performed to assess the generational effects of Faena® on D. exilis, in the parental (P1) and in the filial 1 generation (F1).
In the parental generation (P1), neonates (age < 24 h) of D. exilis were exposed to corresponding sublethal concentrations of glyphosate (mg L−1) equivalent to the previously determined LC0.1 (2.09 mg L−1), LC1 (2.49 mg L−1), and LC10 (3.15 mg L−1). One neonate was placed in 150-mL jars containing 100-mL test volume. Dilution water and negative control were MHRW. Pseudokirchneriella subcapitata (8 ×105 cells mL−1) was supplied as food. Each treatment had ten replicates. Test volume was completely renewed each 48 h. Bioassays were incubated in an environmental chamber at 25 ± 1°C and 16:08-h (light: darkness) photoperiod. Survival, accumulated progeny, age at first reproduction, and the number of clutches were daily recorded for 21 days.
To determine the cross-generation effects of Faena®, the F1 generation was prepared, starting with neonates obtained in the third brood from the control and all the glyphosate concentrations in the parental groups (P1). Ten neonates in each case were randomly chosen and individually placed in 100-mL test volume, in 150-mL glass beakers, in the same treatment in which the parents were grown. Maintenance, feeding, and incubation conditions were the same as for the P1 organisms. Survival, accumulated progeny, age at first reproduction, and the number of clutches were daily recorded for 21 days.
As abortions were observed, the number of aborted eggs and embryos per female were also recorded in P1 and F1.
Twenty neonates were randomly selected from six clutches from the control and the different concentrations of glyphosate, and the body sizes were measured using an Olympus SZ61 stereomicroscope and CellSens® software v. 1.0. Total length (TL, the distance from the anterior edge of the head to the extreme of the caudal spine), body length (BL, the distance from the anterior head edge to the base of the insertion of the caudal spine), and the body width (BW, the maximum distance between the dorsal and the ventral margin of the carapace) were determined.
Macromolecules analyses
The neonates of each brood in each treatment and generation were collected in 1.5-mL Eppendorf tubes with 1 mL of 100 mM potassium-phosphate buffer (pH 7.2) and immediately stored at −20°C for macromolecule analyses.
The concentration of proteins, lipids, and carbohydrates in D. exilis exposed to Faena® was determined only in the parental generation (P1) because herbicide exposure inhibited reproduction in the F1 completely. For each concentration of herbicide, ten organisms were homogenized in 1 mL of 100 mM potassium phosphate buffer at pH 7.2 for 2 min.
Protein content was determined according to Bradford (1976) in 100-μL samples, using bovine serum albumin as a standard. The absorbance of samples and calibration curve was read at 595 nm.
Total carbohydrates were determined according to Dubois et al. (1951) in 200-μL samples. A standard solution of glucose (1%) was used as a standard. Absorbance was read at 490 nm.
Lipids were quantified according to Zöllner and Kirsch (1962) in 200-μL samples. Cholesterol was used to prepare the standard solution. Absorbance was read at 525 nm.
Statistical analysis
The median lethal concentration (LC50) and 95% confidence limits were calculated by the Probit method, using RA software (Risk Hazard Assessment Tools, v. 1.0). Survival curves were analyzed using the Kaplan-Meier’s method. One-way analysis of variance (ANOVA) was used to determine significant differences in reproductive parameters (P < 0.05). LSD’s pairwise comparisons test was used to establish differences among treatments and control in accumulated progeny, the number of clutches, and macromolecules content in P1 and F1. Differences in body size and age to first reproduction between the glyphosate treatments and the control were analyzed using Dunnett’s test. Sigma Plot ver. 11.0 and Statistica v. 10.0 software were used for all the analyses.
Results
Acute toxicity of Faena® on D. exilis
The Probit determined median lethal concentration was 4.22 mg L−1 of glyphosate (95% limits: 3.48-5.43 mg L−1).
Parental and cross-generation effect of Faena® on D. exilis survival in chronic exposure
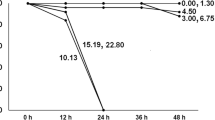
Survival curves of D. exilis exposed to sub-lethal concentrations of glyphosate during 21 days in the P1 and F1 generations are shown, respectively, in Fig. 1a and b. Survival in control conditions of both generations was 100%. In the P1, a significant reduction in survival was determined at glyphosate concentrations of 2.09 (LC0.1) and 2.49 (LC1) mg L−1; nonetheless, survivors were recorded at the end of the test for all concentrations. The percentage of survival was decreased in F1 adults exposed to all the Faena® concentrations (Fig. 1b); all exposed cases were significantly different from the control, with no survivors at the end of the test. At the 2.49 and 3.15 mg L−1 of glyphosate concentrations, a 100% mortality was documented at the 13th day, and 17 days for the lowest glyphosate concentration (2.09 mg L−1).
Survival of D. exilis exposed to three sub-lethal concentrations of glyphosate (contained in Faena®): 2.09, (LC0.1), 2.49 (LC1), and 3.15 mg L−1 (LC10) during 21 days. (a) P1, parental progeny. (b) F1, filial generation. Asterisks indicate differences with respect to the control, according to the Kaplan-Meier method (*P < 0.01, **P < 0.001), (n=10)
Reproductive effects of chronic exposure to Faena® in the parental and filial generations of D. exilis
The accumulated progeny recorded in P1 and F1 generations of D. exilis exposed to Faena® for 21 days is shown in Fig. 2a. In the parental generation, accumulated progeny decreased significantly in all the glyphosate treatments, in a roughly effect-concentration pattern. In the filial generation, reproduction was inhibited completely at the 2.49 and 3.15 mg L−1 concentrations and reduced to a minimum (95% inhibition) in the lowest glyphosate concentration (LSD’s test, P < 0.05). Accumulated progeny in P1 was higher than in F1. In P1 some females produced eggs, but they were released from the brood chamber before they completed their development and are reported as abortions. Aborted eggs and embryos were documented in all the glyphosate concentrations in the P1, with the highest number for the 3.15 mg L−1 glyphosate concentration (Fig. 2a). Abortions are a toxic effect of Faena® that negatively affected reproduction in the parental generation, but herbicide exposure produced comparatively more adverse effects on reproduction in the F1.
Effect of glyphosate in the parental (P1) and filial (F1) generations of D. exilis after 21 days of exposure to three sub-lethal concentrations of glyphosate (Faena®): 2.09, (LC0.1), 2.49 (LC1), and 3.15 mg L−1 (LC10). (a) Accumulated progeny and, for P1, number of abortions. (b) Number of clutches. Average values ± standard error limits. For P1 different uppercase letters, and for F1 different lowercase letters denote significant differences (post hoc LSD test, P < 0.05), (n=10)
The number of clutches per female in both generations is shown in Fig. 2b. In the parental generation, the number of reproductive events in the treatments was significantly reduced compared with the control (P < 0.05), following a dose-response trend. In the filial generation, a drastic reduction in fecundity was documented; this is associated with the inhibition in reproduction observed at all glyphosate concentrations, which were significantly lower than the values observed in the corresponding treatments in P1 (LSD’s test, P < 0.05).
The average age to first reproduction observed in adults in both generations is shown in Table 1. In P1, a significant reduction was observed at the two highest glyphosate concentrations. In F1, most females did not reach sexual maturity, and a substantial delay in the starting of reproduction was documented at the lowest herbicide concentration. Infertility in F1 and abortions in P1 were the toxic effects on reproduction produced by exposure of D. exilis to the herbicide.
Effects on macromolecules content in the parental and filial generations of D. exilis chronically exposed to Faena®
The concentrations of proteins, carbohydrates, and lipids in D. exilis exposed to Faena® are shown in Fig. 3a–c. Macromolecules content was determined only in the parental generation because the herbicide inhibited reproduction in the filial generation. In the P1 generation, proteins and lipids decreased at all glyphosate concentrations compared with the control, whereas carbohydrates content was not affected by the herbicide.
Biomarkers in neonates of the parental (P1) and filial (F1) generations of D. exilis exposed to glyphosate during 21 days: (a) proteins, (b) carbohydrates, and (c) lipids. Average values ± standard error. Differences among treatments in P1 are indicated with different uppercase letters. Inhibition of reproduction by all treatments in F1 did not allow performing these determinations, thus, only the results for the control are shown (LSD’s test comparisons; P < 0.05), (n=10)
Effects on the neonate and adult sizes in the parental and filial generations of D. exilis chronically exposed to Faena®
Figure 4 shows the size of neonates of D. exilis exposed to glyphosate in the P1 (Fig. 4a, c, and e) and the F1 generations (Fig. 4b, d and f ), measured as the total length (TL), body length (BL), and body width (BW). In F1, the inhibition of reproduction was almost 100% at the 2.09, 2.49, and 3.15 mg L−1 concentrations of glyphosate; for this reason, no measures were available. Although no clear trends were identified, significant smaller individuals were observed at the highest glyphosate concentrations (P < 0.05, Dunnett’s test).
Total length, body length, and body width of neonates measured in P1 (a, c, and e, respectively) and F1 (b, d, and f, respectively), in six consecutive clutches of D. exilis exposed to glyphosate (Faena®). Differences are denoted with * (P < 0.05, Dunnett’s test). Average values ± standard error, (n=10)
The size of neonates varied depending on the number of the clutch, and this was also related to the age of mothers. The BW was the most variable parameter among clutches, especially at 2.09 and 2.49 mg L−1 (P<0.05), whereas BL and TL depicted significant differences only in the control. In F1, the reproduction was highly inhibited; as a result, it was not possible to have a complete sample (n = 20 neonates) to register the body size for each clutch. However, the results in controls of F1 indicated that the size of the progeny was similar to that of P1.
Table 2 shows the size of adult females in P1 and F1 measured at the end of the experiments. There were significant differences in the size of adults exposed to glyphosate, being smaller in F1 than in P1. In controls, adult females in F1 were significantly larger in TL and BL, compared with P1. At the highest concentration (3.15 mg L−1), data are not available because the morphology of females was severely altered by Faena®, and no measurements could be made.
Discussion
The 48-h LC50 obtained for D. exilis exposed to Faena® was 4.22 mg L−1. In comparison with other cladocerans, it is similar to that reported for Simocephalus mixtus (5.27 mg L−1, Rodríguez-Miguel et al. 2018), and D. magna (4.1 mg L−1, Domínguez-Cortinas et al. 2008) for the same formulation known as Faena®. Comparison with other results published for GBH is difficult because formulations can vary from region to region and even at different times, being the precise composition and concentration of the inert and adjuvants ingredients, as well as the chemical form of the active ingredient in the commercial formulation, unknown, because this is classified as confidential business information (Mesnage et al. 2019). For this reason, even for the same commercial brand, differences in composition and toxicity could be expected. Nevertheless, limited comparison can be made to know the range of toxic effects that these formulations can produce in non-target species for practical purposes. With this in mind, for the Roundup® formulation tested by Alberdi et al. (1996) and Tsui and Chu (2003), the reported acute toxicity is 5.39 mg L−1, 61.72 mg L−1, and 66.18 mg L−1, respectively, for the freshwater cladocerans Ceriodaphnia dubia, D. magna, and Daphnia spinulata. Regarding other GBH, Reno et al. (2015) determined LC50 values of 14.49 and 0.31 mg L−1 for C. dubia exposed to Eskoba® and Sulfonato Touchdown®, respectively. For D. magna, LC50 values were 29.48 and 1.62 mg L−1, respectively, for the same products; these results evidenced that acute toxicity depends on the commercial formulation, and could be mainly related to the different adjuvants and inert ingredients. For Simocephalus vetulus exposed to the glyphosate formulation Eskoba® (48% w/v active ingredient content), Reno et al. (2014) reported an EC50 value of 21 mg L−1, indicating a lower sensitivity of this cladoceran than D. exilis. The data above confirms that the toxicity of this herbicide depends on the commercial formulation but also that the toxic effects is species-specific in cladocerans
The determined LC50 in C. dubia for the active ingredient, isopropylamine salt of glyphosate, is 415 mg L−1, whereas for the glyphosate acid it is 147 mg L−1 (Tsui and Chu 2003), evidencing toxicity differences in the active ingredients and confirming that the adjuvants in the commercial formulations contribute significantly to the toxicity of this herbicide. This situation has been confirmed in different studies. Despite glyphosate toxicity as the active ingredient could be comparatively reduced, chemical differences in the active ingredients, as well as differences in the co-formulants, make difficult the comparison of toxicity results. As opposite, Pochron et al. (2020) reported contrary effects in soil animals (Eisenia fetida), in which significant higher toxicity was observed in organisms exposed to glyphosate than the effect observed in two Roundup formulations; in this case, the microbial activity could be responsible for the observed results. Surfactants included in the GBH contribute to increased toxicity. Changes in the surfactant have been made in some of the new formulations aimed to reduce the herbicide toxicity to non-target species (Mesnage et al. 2019). Nevertheless, the formula and concentration of adjuvants and inert ingredients in the GBH are unknown in most cases.
Here, we observed that GBH Faena® at sublethal concentrations caused significant toxic effects on D. exilis, mainly in the F1 generation. In the parental generation, the herbicide formulation produced physiological effects that affected survival and reproduction in progenitors. When progeny were exposed to the same concentrations at which the parents were exposed, increased effects were observed. This outcome provoked that, in the F1, fecundity was almost entirely reduced. These increased toxic responses in the filial generation demonstrate transgenerational effect where accumulated toxic responses in individuals under chemical stress could contribute to the extinction of populations.
The survival in P1 decreased to 50% with the 2.49 mg L−1 (glyphosate) treatment, whereas in F1, survival was significantly reduced in all treatments. Campos et al. (2016) reported that during toxicity bioassays, survival and reproduction could be modified by the health status of the organisms before the test started. In the present study, this residual effect can be discarded because the health of test organisms was assured as neonates from the third clutch were obtained from a healthy standardized culture; for all experiments with the P1, organisms were tested and had similar sizes and locomotion (OECD 2004). By doing this, we ensured that the exposure to Faena® caused the observed effects in both in the parental and the filial generations.
All the concentrations of the herbicide Faena® inhibited reproduction in both generations. The F1 generation demonstrates a virtually 100% decrease in reproduction across all treatments. Rodríguez-Miguel et al. (2018) reported similar effects in Simocephalus mixtus exposed to Faena® at concentrations ranging from 2.19 to 4.06 mg L−1; they reported more than 50% abatement in reproduction for most treatments, and 1 to 2 days delay in becoming sexually mature the individuals. An increased number of abortions were also observed at the highest Faena® concentrations. Similarly, Cuhra et al. (2013) reported that in the Roundup® formulation they assessed, 1.35 mg L−1 produced 100% eggs abortion in D. magna, confirming adverse effects on reproduction through the interruption of egg development and the expulsion of the brood chamber. Gill et al. (2018) reported that Roundup® at 0.45 mg L−1 reduces the fecundity and increases the abortion rate in D. magna and that at 1.35 mg L−1 Roundup® produced 100% abortions; these findings are coincident whit the observed in the present study for Faena®.
Other possible metabolic pathways that can incorporate glyphosate to cause a toxic effect on non-target organisms (Metazoa) is through the inhibition of cytochrome p450, an enzymatic complex necessary for the biotransformation of xenobiotics (Samsel and Seneff 2013). Possible bioaccumulation of glyphosate in cladocerans might lead to the alteration of biochemical processes related to vital functions like growth, reproduction, and survival, thus affecting animals as observed in our results. Nevertheless, changes in the gut microbiome could also happen, modifying the nutrition and food assimilation process, interfering in this way with the energy balance in the individuals.
Abortions in cladocerans can be elicited by factors including temperature, toxic metabolites, and food quality (Chen and Folt 1996; Marques et al. 2004; Ismail et al. 2010). Our study shows that the herbicide Faena® can also induce abortion as a toxic effect on reproduction, impairing fecundity.
The content of macromolecules in P1 and F1 controls was lipids>proteins>carbohydrates, in a proportion that can be considered normal. The results obtained in P1 are similar to those reported by Ventura (2006), who concludes that proteins and lipids are the most abundant macromolecules in cladocerans and copepods. Additionally, Ventura (2006) described that organisms with accelerated growth and high reproductive rates have to incorporate and assimilate quality nutrients that enable their short life spans; thus, proteins and lipids are important macromolecules in the metabolism of daphnids. Although the content of macromolecules varies according to the age of the organism (Smirnov 2014), the content of structural molecules remains considerably constant (Ventura and Catalan 2005). For this reason, a change in the content of these macromolecules, even though they can be potentially obtained from a proper diet like the one provided in the present study, can be understood as toxic effects related to chemical stress.
Our results demonstrated that the content of proteins was the most affected, followed by lipids and carbohydrates. Papchenkova et al. (2009) demonstrated that the commercial formulation of the GBH Roundup® they assessed increased the proteolytic enzymatic activity while decreasing the amylolytic enzymatic activity. This could explain why in the P1 the protein content diminished, whereas the carbohydrate content had slight increases at all the concentrations of Faena®.
Lipids are the most studied molecules in cladocerans because they are involved in growth and reproduction. Cladocerans possess the fat body, a specialized organ that synthesizes and stores lipids. Before reproduction starts, this organ participates in the growth and maturation of the individual. Once cladocerans reach sexual maturity, the fat body facilitates the transference of lipids from the mother to the oocytes, which develop inside the brood pouch (Tessier et al. 1983). When a decrease in the lipid content occurs, the fat body might be severely affected, resulting in a low reproductive rate as observed in F1, where reproduction was null at some Faena® concentrations.
In this study, the photoperiod, concentration of food, and culture medium were constant factors in both P1 and F1 bioassays. Therefore, effects on the size of D. exilis can be related to the chemical stress elicited by glyphosate and other ingredients in the formulation of Faena®.
Green (1954) reported that the size of neonates is variable during the life of females and that the size of the offspring can vary from one clutch to another, especially in polyembryonic organisms such as Simocephalus and Daphnia genera. Nevertheless, the commercial formulation Faena® produced modifications in size in some clutches without a consistent pattern, but frequently the neonates were smaller at the higher herbicide concentrations. Similar effects are reported by Gill et al. (2018), indicating that glyphosate concentrations as low as 0.05 mg L−1 produced a reduction in the size of juveniles. Cuhra et al. (2013) reported that the body size in adults of D. magna exposed to Faena® (1.35 and 4.05 md L−1) was significantly reduced. The mechanisms to produce affectations in the size of cladocerans are not established but could be related to food assimilation that eventually will provide essential nutrients used for the increase in biomass.
The size of adult females in P1 and F1 indicates that F1 females were smaller than P1 females. The body size is an essential factor in the reproductive activity because, in addition to the normal physiology and an adequate lipids content, the capacity of the brood chamber is determined by the size of the female (Smirnov 2014). Also, smaller organisms can take more time to reach sexual maturity, and the result could be the production of smaller neonates (Campos et al. 2016).
In the present study, we documented that exposure to Faena® produced increased toxic effects on the filial generation, remarkably in the adults’ survival, reproduction, and macromolecules content. Mechanisms related to the increased sensitivity in the filial generation were not established. Still, vital physiological processes were impaired in the progeny so that, when they were exposed to similar Faena® concentrations as those to which the parents were exposed, toxic effects increased severely. Our findings contribute with pieces of evidence of toxic effects at sublethal glyphosate concentrations and support the argumentation about the banning of this herbicide in many countries around the world.
Conclusions
The study in two generations in the freshwater cladoceran D. exilis demonstrated cross-generation effects induced by the herbicide Faena®. The different responses in both generations (P1 and F1) suggest that even low concentrations of glyphosate in this GBH (LC1: 2.09 mg L−1) produce a toxic effect on survival and reproduction. Also, the herbicide provoked abortion in this cladoceran. Our study also showed that the GBH Faena® affected biomolecules content in D. exilis, mainly proteins and lipids. These results warn about the transgenerational, adverse effects that this herbicide can produce in aquatic biota.
Data Availability
All the raw data and datasheets are available for examination. The biological material used in this study is part of the live collections of Cladocerans, Microalgae, and Cyanobacteria of the Laboratorio de Hidrobiología Experimental, ENCB-IPN, México, and can be reviewed under request.
References
Alberdi JL, Sáenz ME, Di Marzio WD, Tortorelli MC (1996) Comparative acute toxicity of two herbicides, paraquat and glyphosate, to Daphnia magna and D. spinulata. Bull Environ Contam Toxicol 57:229–235. https://doi.org/10.1007/s001289900180
Banaee M, Akhlaghi M, Soltanian S, Sureda A, Gholamhosseini A, Rakhshaninejad M (2020) Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish Pontastacus leptodactylus. Ecotoxicology. 29:1500–1515. https://doi.org/10.1007/s10646-020-02233-0
Barata C, Campos B, Rivetti C, LeBlanc GA, Eytcheson S, McKnight S, Tobor-Kaplon M, de Vries BS, Choi S, Choi J, Sarapultseva EI, Coutellec MA, Coke M, Pandard P, Chaumot A, Quéau H, Delorme N, Geffard P, Martínez-Jerónimo F, Watanabe H, Tatarazako N, Lopes I, Pestana JLT, Soares AMVM, Pereira CM, De Schamphelaere K (2017) Validation of a two-generational reproduction test in Daphnia magna: an interlaboratory exercise. Sci Total Environ 579:1073–1083. https://doi.org/10.1016/j.scitotenv.2016.11.066
Battaglin WA, Rice KC, Focazio MJ, Salmons S, Barry RX (2009) The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005–2006. Environ Monit Assess 155:281–307. https://doi.org/10.1007/s10661-008-0435-y
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U. S. soils, surface water, groundwater and precipitation. J Am Water Resour As 50(2):275–290. https://doi.org/10.1111/jawr.12159
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brausch JM, Beall B, Smith PN (2007) Acute and sub-lethal toxicity of three POEA surfactant formulations to Daphnia magna. Bull Environ Contam Toxicol 78(6):510–514. https://doi.org/10.1007/s00128-007-9091-0
Brennan SJ, Brougham CA, Roche JJ, Fogarty AM (2006) Multigenerational effects of four selected environmental oestrogens on Daphnia magna. Chemosphere 64(1):49–55. https://doi.org/10.1016/j.chemosphere.2005.11.046
Campos B, Jordao R, Rivetti C, Lemos MFL, Soares AMVM, Barata C (2016) Two-generational effects of contaminants in Daphnia magna: effects of offspring quality. Environ Toxicol Chem 35(6):1470–1477. https://doi.org/10.1002/etc.3290
Chen CY, Folt CL (1996) Consequences of fall warming for zooplankton overwintering success. Limnol Oceanogr 41(5):1077–1086. https://doi.org/10.4319/lo.1996.41.5.1077
Colombo V, Pettigrove VJ, Golding LA, Hoffmann AA (2014) Transgenerational effects of parental nutritional status on offspring development time, survival, fecundity, and sensitivity to zinc in glyphosate (Roundup®) and Cosmoflux® 411F índice oxidative stress in red-bellied pacu (Piaractus brachypomus) midges. Ecotoxicol Environ Saf 110:1–7. https://doi.org/10.1016/j.ecoenv.2014.07.037
Corrales J, Thornton C, White M, Willet KL (2014) Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat Toxicol 148:16–26. https://doi.org/10.1016/j.aquatox.2013.12.028
Cuhra M, Traavik T, Bohn T (2013) Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in Daphnia magna. Ecotoxicology 22:251–262. https://doi.org/10.1007/s10646-012-1021-1
de Brito RL, de Oliveira R, Abe FR, Brito LB, Moura DS, Valadares MC, Grisolia CK, de Oliveira DP, de Oliveira GAR (2017) Ecotoxicological assessment of glyphosate-based herbicides: effects on different organisms. Environ Toxicol Chem 36(7):1755–1763. https://doi.org/10.1002/etc.3580
Domínguez-Cortinas G, Mejía-Saavedra J, Santos-Medrano GE, Rico-Martínez R (2008) Analysis of the toxicity of glyphosate and Faena® using the freshwater invertebrates Daphnia magna and Lecane quadridentata. Toxicol Environ Chem 90(2):377–384. https://doi.org/10.1080/02772240701529038
Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167. https://doi.org/10.1038/168167a0
Duke SO, Powles SB (2008) Glyphosate: once-in-a-century. Pest Manag Sci 64(4):319–325. https://doi.org/10.1002/ps.1518
Fontoura NF, Agostinho AA (1996) Growth with seasonally varying temperatures: an expansion of the von Bertalanffy growth model. J Fish Biol 48(4):569–584. https://doi.org/10.1111/j.1095-8649.1996.tb01453.x
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup® herbicide. Rev Environ Contam Toxicol 167:35–120. https://doi.org/10.1007/978-1-4612-1156-3_2
Gill JPK, Sethi N, Mohan A, Datta S, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16:401–426. https://doi.org/10.1007/s10311-017-0689-0
Green J (1954) Size reproduction in Daphnia magna (Crustacea: Cladocera). Proc Zool Soc London 124(3):535–545. https://doi.org/10.1111/j.1469-7998.1954.tb07796.x
Hairston NG, Perry LJ Jr, Bohonak AJ, Fellows MQ, Kearns CM (1999) Population biology of a failed invasion: paleolimnology of Daphnia exilis in upstate New York. Limnol Oceanogr 44(3):477–486. https://doi.org/10.4319/lo.1999.44.3.0477
Hammers-Wirtz M, Ratte HT (2000) Offspring fitness in Daphnia: Is the Daphnia reproduction test appropriate for extrapolating effects on the population level? Environ Toxicol Chem 19:1856–1866. https://doi.org/10.1002/etc.5620190720
Ismail HN, Qin JG, Seuront L, Adams M (2010) Impacts of male and food density on female performance in the brackish cladoceran Daphniopsis australis. Hydrobiologia 652(1):277–288. https://doi.org/10.1007/s10750-010-0359-8
Kang L, Wu JQ, Jiang LL, Shen LZ, Li JY, He ZH, Wei P, Lv Z, He MF (2017) Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere 171:40–48. https://doi.org/10.1016/j.chemosphere.2016.12.032
Kreutz LC, Barcellos LJG, Valle E, Silva T, Anziliero D, dos Santos ED, Pivato M, Zanatta R (2011) Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immun 30(1):51–57. https://doi.org/10.1016/j.fsi.2010.09.012
Leyva-Morales JB, García de la Parra LM, Bastidas-Bastidas PJ, Astorga-Rodríguez JE, Bejarano-Trujillo J, Cruz-Hernández A, Martínez-Rodríguez IE, Betancourt-Lozano M (2014) Pesticide use in a technified agricultural valley in Northwest Mexico. Rev Int Contam Ambient 30(3):247–261
Li Z, Ai F, Zhang J, Yu Z, Yin D (2019) Using Caenorhabditis elegans for studying trans and multigenerational effects of toxicants. J Vis Exp 149:1–18. https://doi.org/10.3791/59367
Marques CR, Abrantes N, Goncalves F (2004) Life history traits of standard and autochthonous cladocerans: II. Acute and chronic effects of acetylsalicylic acid metabolites. Environ Toxicol 19:527–540. https://doi.org/10.1002/tox.20060
Martínez-Jerónimo F (2012) Description of the individual growth of Daphnia magna (Crustacea: Cladocera) through the von Bertalanffy growth equation. Effect of photoperiod and temperature Lymnology 13:65–71. https://doi.org/10.1007/s10201-011-0356-2
Martínez-Jerónimo F, Rodríguez-Estrada J, Martínez-Jerónimo L (2008) Daphnia exilis Herrick, 1985 (Crustacea: Cladocera). Una especie zooplanctónica potencialmente utilizable como organismo de prueba en bioensayos de toxicidad aguda en ambientes tropicales y subtropicales. Rev Int Contam Ambient 24(4):153–159
Mesnage R, Benbrook C, Antoniou MN (2019) Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem Toxicol 128:137–145. https://doi.org/10.1016/j.fct.2019.03.053
Mnif W, Hassine A, Bouaziz IH, Bartegi A, Thomas O, Roig B (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8(6):2265–2303. https://doi.org/10.3390/ijerph8062265
Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, Vom Saal FS, Welshons WV, Benbrook CM (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15:19. https://doi.org/10.1186/s12940-016-0117-0
OECD (2004) Test No. 202: Daphnia sp., acute immobilization test. OECD Guidelines for the testing of chemicals, section 2. OECD publishing, Paris. 12p. https://doi.org/10.1787/9789264069947-en
Papchenkova GA, Golovanova IL, Ushakova NV (2009) Reproductive parameters, size, and hydrolases activity in successive generations of Daphnia magna Straus under effect of “Roundup” herbicide. Inland Water Biol 3:105–110. https://doi.org/10.1134/S1995082909030158
Paravani EV, Sasal M, Sione SM, Gabioud EA, Oszust JD, Wilson MG, Demonte L, Repetti MR. 2016. Determinación de la concentración de glifosato en agua mediante la técnica de inmunoabsorción ligada a enzimas (ELISA). Revista Internacional de Contaminación Ambiental, 32(4):399–406.
Perkins PJ, Boermans HJ, Stephenson GR (2000) Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay-Xenopus. Envrion Toxicol Chem 19(4):940–945. https://doi.org/10.1897/1551-5028(2000)019<0940:TOGATU>2.3.CO;2CO;2
Pochron S, Simon L, Mirza A, Littleton A, Sahebzada F, Yudell M (2020) Glyphosate but not Roundup® harms earthworms (Eisenia fetida). Chemosphere 241:125017. https://doi.org/10.1016/j.chemosphere.2019.125017
Prud’homme SM, Chaumont A, Cassar E, David JP, Reynaud S (2017) Impact of micropollutants on the life-history traits of the mosquito Aedes aegypti: On the relevance of transgenerational studies. Environ Pollut 220(Pt A):242–254. https://doi.org/10.1016/j.envpol.2016.09.056
Qiu H, Geng J, Ren H, Xia X, Wang X, Yu Y (2013) Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J Hazard Mater 248-249:172–176. https://doi.org/10.1016/j.jhazmat.2012.12.033
Reno U, Gutierrez MF, Regaldo L, Gagneten AM (2014) The impact of Eskoba®, a glyphosate ormulation, on the freshwater plankton community. Water Environ Res 86:2294–2300. https://doi.org/10.2175/106143014X13896437493580
Reno U, Gutierrez MF, Longo M, Vidal E, Regaldo L, Negro A, Mariani M, Zalazar C, Gagneten AM (2015) Microcrustaceans: biological models to evaluate a remediation process of glyphosate-based formulations. Water Air Soil Pollut 226:349. https://doi.org/10.1007/s11270-015-2616-y
Reno U, Doyle SR, Momo FR, Regaldo L, Gagneten AM (2018) Effects of glyphosate formulations on the population dynamics of two freshwater cladoceran species. Ecotoxicology 27:784–793. https://doi.org/10.1007/s10646-017-1891-3
Rodríguez-Miguel AV, Martínez-Jerónimo LG, Martínez-Jerónimo FF (2018) La exposición al herbicida glifosato (FAENA®) produce efectos a nivel poblacional en el cladócero Simocephalus mixtus. In: Galar-Martínez M, Ramírez-Romero P, Gasca-Pérez E, Gómez-Olivan LM, Zavala-Aguirre JL, Arzate-Cárdenas MA, Rico-Martínez R (eds) Contribuciones al conocimiento de la ecotoxicología y química ambiental en México Volumen 2. Instituto Politécnico Nacional, CDMX, pp 331–353
Rondón-Barragán IS, Marin-Mendez GA, Chacón-Novoa RA, Naranjo-Suarez L, Pardo-Hernández D, Eslava-Mocha PR (2012) Glyphosate (Roundup®) and Cosmoflux® 411F induce oxidative stress in red-bellied pacu (Piaractus brachypomus). Orinoquia 16(2):162–176
Ruiz-Toledo J, Castro R, Rivero-Pérez N, Bello-Mendoza R, Sánchez D (2014) Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of Southern Mexico. Bull Environ Contam Toxicol 93:289–293. https://doi.org/10.1007/s00128-014-1328-0
Samsel A, Seneff S (2013) Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: pathways to modern diseases. Entropy 15(4):1416–1463. https://doi.org/10.3390/e15041416
Sanchís J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, Garrido T, Farré M (2012) Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 402:2335–2345. https://doi.org/10.1007/s00216-011-5541-y
Singh S, Kumar V, Datta S, Wani AB, Dhanjal DS, Romero R, Singh J (2020) Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity and degradation: a review. Environ Chem Lett 18:663–702. https://doi.org/10.1007/s10311-020-00969-z
Skinner M (2008) What is an epigenetic transgenerational phenotype? F3 or F2. Review. Reprod Toxicol 25:2–6. https://doi.org/10.1016/j.reprotox.2007.09.001
Smirnov NN (2014) Physiology of the Cladocera. Elsevier, San Diego
Tessier AJ, Henry LL, Goulden CE, Durand MW (1983) Starvation in Daphnia: energy reserves and reproductive allocation. Limnol Oceanogr 28(4):667–676. https://doi.org/10.4319/lo.1983.28.4.0667
Thompson DG, Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR (2004) Chemical and biomonitoring to assess potential acute effects of Vision® herbicide on native amphibian larvae in forest wetlands. Environ Toxicol Chem 23(4):843–849. https://doi.org/10.1897/02-280
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197. https://doi.org/10.1016/S0045-6535(03)00306-0
U.S. Environmental Protection Agency (2002) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. EPA-821-R-02-012. fifth ed. Office of Research and Development, Cincinnati, OH United States Environmental Protection Agency
Ventura M (2006) Linking biochemical and elemental composition in freshwater and marine crustacean zooplankton. Mar Ecol Prog 327:233–246. https://doi.org/10.3354/meps327233
Ventura M, Catalan J (2005) Reproduction as one of the main causes of temporal variability in the elemental composition of zooplankton. Limnol Oceanogr 50:2043–2056. https://doi.org/10.4319/lo.2005.50.6.2043
Woodburn AT (2000) Glyphosate: production, pricing and use worldwide. Pest Manag Sci 56(4):309–312. https://doi.org/10.1002/(SICI)15264998(200004)56:4<309::AIDPS143>3.0.CO;2-C
Yu ZY, Chen XX, Zhang J, Wang R, Yin DQ (2013) Transgenerational effects of heavy metals on L3 larva of Caenorhabditis elegans with greater behavior and growth inhibitions in the progeny. Ecotoxicol Environ Saf 88:178–184. https://doi.org/10.1016/j.ecoenv.2012.11.012
Zöllner N, Kirsch K (1962) Microdetermination of lipids by the sulfo-phospho-vanillin reaction. Z die Gesamte Exp Med Einschl Exp Chir 135:545–561
Acknowledgements
Alma V. Rodriguez-Miguel thanks to Consejo Nacional de Ciencia y Tecnología (CONACyT), to the Beca de Estímulo Institucional de Formación de Investigadores of the Instituto Politécnico Nacional (BEIFI-IPN), and to the Escuela Nacional de Ciencias Biológicas for the support given for this study. Fernando Martínez-Jerónimo acknowledges the Secretaría de Investigación y Posgrado-IPN for the support granted.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Alma Rodríguez-Miguel, Miriam Hernández-Zamora, Laura Martínez-Jerónimo and Fernando Martínez-Jerónimo. The first draft of the manuscript was written by Alma Rodríguez-Miguel and Miriam Hernández-Zamora, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript written by Fernando Martínez-Jerónimo.
Corresponding author
Ethics declarations
Ethical approval
The animals used in experiments were ethically managed, with advising of the Institutional Ethical Committee. For the analysis, test individuals were ethically euthanized.
Consent to participate
All the coauthors accepted to participate in this research
Consent to publish
All the participants in this research agreed to be included in this manuscript as coauthors
Competing interests
The authors declare no competing financial interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rodríguez-Miguel, A., Hernández-Zamora, M., Martínez-Jerónimo, L. et al. Exposure to sublethal concentrations of the glyphosate-based herbicide Faena® increases sensitivity in the progeny of the American cladoceran Daphnia exilis (Herrick, 1895). Environ Sci Pollut Res 28, 38094–38105 (2021). https://doi.org/10.1007/s11356-021-13259-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13259-0