Abstract
Despite many estuaries having high levels of metal pollution, species are found to persist in these stressful environments. Populations of estuarine invertebrates exposed to toxic concentrations of such metals may be under selection. However, in species with a wide-dispersal potential, any short-term results of localized selection may be counteracted by external recruitment from populations not under selection. The barnacle Amphibalanus variegatus is found in nearshore coastal environments as well as sheltered embayments and estuaries, including metal-impacted estuaries, from New South Wales, Australia to Western Australia. The fertilised eggs of A. variegatus are brooded internally and released as larvae (nauplii), which remain in the water-column for ~14 days before settling. Hence the species has a considerable dispersal capacity. The purpose of this study was to examine whether populations of A. variegatus from metal-impacted sites, displayed a greater tolerance to a toxicant (copper) than reference populations. Adult barnacles where collected from twenty sites within two metal-impacted and fourteen sites within two reference estuaries. Within 24 h, adults were induced to spawn and the offspring were exposed to copper in a laboratory assay. Larvae collected from the metal-impacted estuaries demonstrated a greater tolerance to copper compared to those from reference sites. To determine if selection/localised in the metal impacted sites was occurring, the genetic structure of populations at three sites was examined using an AFLP methodology. No evidence of unique population identity and or selection (outlier loci) was detected suggesting that: (1) the tolerance displayed in the assay was derived from acclimation during development; and/or (2) that the populations are open preventing the fixation of any unique alleles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities are changing the nature of estuarine and coastal environments with many organisms now being exposed to novel agents and/or unnaturally high levels of trace elements (Medina et al. 2007; Sarkar et al. 2006; Bryan and Langston 1992). Consequently, the phenotype and genetic identity of populations residing in these locations may undergo substantial changes (Klerks and Weis 1987; Posthuma and Van Straalen 1993; Meyer and Giulio 2003). When a contaminant enters a system, an individual can respond in a number of ways: it may tolerate the stress, avoid it, or die (Lopes et al. 2004). The first response will have no effect on the genetic structure of the population, whereas the latter two may affect it by changing the frequency/identity of alleles within that population (Belfiore and Anderson 2001; Wirgin and Waldman 2004).
Exposure to contaminants can change the phenotype of an organism (Klerks and Weis 1987; Wang and Rainbow 2005), with these changes reflecting both its exposure history and its genetic background (Morgan et al. 2007). Following exposure, individuals can respond by activation of physiological/biochemical pathways which can ameliorate detrimental effects in subsequent exposures (e.g. Fritsch et al. 2011; Klerks and Lentz 1998). Although tolerance acquired through acclimatory responses is based on traits which are genetically-determined, in contrast to resistance acquired through selection, the induced effect is not generally transmitted across multiple generations and should disappear in remediated environments (Wirgin and Waldman 2004).
At a population level, genetically-determined resistance is acquired through the survival of tolerant genotypes and the demise of those that are sensitive (Klerks and Weis 1987). This can lead to a change in the distribution of a trait/s within an affected population (Belfiore and Anderson 2001). Traits conferring resistance may not be limited to a single gene, and may involve a suite of genes (Van Straalen et al. 2011). In addition, in an affected population, there may be a diverse range of genotypes that confer tolerance (Depledge 1994), and between populations the genes involved may not be conserved (Fisher and Oleksiak 2007), i.e. tolerance can evolve independently.
Although there are a number of studies that simultaneously examine both the phenotypic and genetic affects of contaminants in freshwater and terrestrial organisms (reviewed in Klerks and Weis 1987; Posthuma and Van Straalen 1993; Johnston 2011; Belfiore and Anderson 2001; Wirgin and Waldman 2004; Morgan et al. 2007; Grant 2002), there are few examples of this in marine organisms (examples see Klerks and Levinton 1989; Grant et al. 1989; Miliou et al. 2000; Untersee and Pechenik 2007). One notable exception to this is the detailed research on the estuarine fish Fundulus heteroclitus (reviewed in Wirgin and Waldman 2004).
The extent to which resistance is maintained within a local population, is a balance between the selection pressures acting on an individual versus the level of gene flow into the affected population (García-Ramos and Kirkpatrick 1997; Slatkin 1987). While natural selection acts as a driving force for local adaptation, the introduction of new alleles (gene flow) can act as an opposing force by homogenizing genotypes (Clarke et al. 2010; Lenormand 2002; Barton and Partridge 2000; Kawecki and Ebert 2004) preventing resistant genotypes from becoming fixed within a population. In freshwater systems there can be strong barriers to gene flow (e.g. ponds and lakes Coors et al. 2009) and flow is unidirectional (e.g. riverine systems, Groenendijk et al. 2002), which ensures that populations are closed or of a metapopulation structure (i.e. there are restrictions to gene flow). This can promote the fixation of alleles in response to a localized impact. In contrast, in the marine environment there are few barriers to gene flow, and it is commonly considered that many populations are panmictic in structure i.e. they are large and genetically/demographically ‘open’ (Caley et al. 1996; Cowen and Sponaugle 2009). Thus, in marine organisms, it is unclear what mechanisms drive local adaptation, and based on a paucity of studies, how commonly it occurs (see Sanford and Kelly 2011 and Sotka 2005 for review of local adaptation in marine invertebrates).
Marine species with a long larval duration have the potential to disperse across wide distances, thus demographic and genetic connectivity between widely separated populations is thought to be high (Sanford and Kelly 2011; Palumbi 1994). Untersee & Pechenik (2007) have suggested that larval duration may be an important determinant of the likelihood of localized adaptation, with adaptation less likely to occur in species with a long larval duration (i.e. a widespread dispersal capacity). To test this hypothesis, they examined the copper tolerance of two gastropod species (Crepidula fornicata and C. convexa), collected from metal-polluted and reference sites, which produce offspring with different larval longevities. For the species with the short-dispersing larvae, populations in polluted sites exhibited a heritable tolerance, whereas no evidence of this was exhibited by the species with the wide dispersing larvae. Rainbow et al. (1999) have posited a similar hypothesis, but in contrast to Untersee & Pechenik (2007), they found no evidence of physiological differences between populations of species which brooded their young (amphipod crustaceans) and a species with produced long-lived larvae (crabs) between metal-contaminated and reference sites.
The barnacle Amphibalanus variegatus is native to Australia, and found in nearshore coastal environments as well as estuaries and sheltered embayments from Western Australia to Northern NSW, including industrialized/metal-impacted estuaries along the NSW coast (Dafforn et al. 2009; Gall et al. 2012). A. variegatus produce larvae which remain in the water-column for up to 14 days (Egan and Anderson 1986) before settlement and hence have a widespread dispersal capacity. The purpose of this study was therefore to determine whether populations of the widely-dispersing barnacle A. variegatus residing within metal-impacted estuaries have a greater tolerance to a toxicant (copper) compared to those from reference populations. Secondary to this, was to determine whether pollution is having an impact at the population genetic level, which would indicate localised selection and/or adaptation. The specific aims of this study were:
-
1)
examine whether populations from metal-impacted estuaries display a greater tolerance to a toxicant compared to reference populations.
-
2)
using an Amplified fragment length polymorphism (AFLP) methodology, examine whether there are differences in population structure which correspond with level of estuarine pollution.
Methods
Study sites
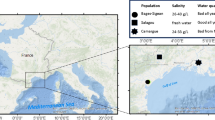
Populations of A. variegatus were collected from four estuaries along the east coast of NSW, two metal-impacted (‘industrialised’, Port Kembla & Botany Bay), and two reference (The Clyde & Wagonga Inlet, see Fig. 1). In each estuary, adult barnacles were collected from 5 to 10 sites. The designated a priori estuarine categories (industrialised versus reference) were verified by the measurement of copper levels in the tissue of oysters and the benthic sediments, which were deployed/collected in a larger, parallel study, throughout the duration of this experiment (see Dafforn et al. 2012, also summarized in Fig. 2).
Map of study sites located along the NSW coastline, SE Australia. a Botany Bay and b Port Kembla are industrialised estuaries; c The Clyde and d Wagonga Inlet are reference estuaries. Circles represent the sites within each estuary at which A. variegatus were collected from. Levels of copper in the benthic sediments and tissue of oysters also measured at each of these sites (some exceptions, white and grey-filled circles represent sites where no copper measurement taken from the sediment and oyster tissue respectively, see Dafforn et al. (2012)). Stars represent the sites also used in the molecular study
Copper content measured in the tissue of oysters and sediment (left axis, based on dry weight values) and mean EC50 24 h for Amphibalanus variegatus nauplii from each estuary (right axis). Sediment and oyster tissue values from Dafforn et al. (2012). NB: mean copper values presented are based on measurements taken from each of the sites at which A. variegatus were collected from. Error bars represent standard error
Port Kembla (‘industrialised’) is a small artificial-estuary which functions as a commercial port. The shoreline and area adjacent to the port has supported heavy industry for over 80 years including the processing and production of steel, copper and zinc products (He and Morrison 2001). During 1990, monitoring studies found substantial improvements in the levels of metals and polycyclic hydrocarbons in fish, sediments and dissolved in the water column, however in particular levels of lead, copper and arsenic were still found to be severely elevated above the current high trigger sediment quality guideline values (reviewed in He and Morrison, 2001). A recent study by Gall et al. (2012) determined that the levels of copper and lead in the tissue of oysters are still elevated above ecologically-significant levels of contamination (Scanes and Roach 1999) and a negative relationship was observed between the density A. variegatus and copper and lead concentrations (also in Dafforn et al. 2009). Dafforn et al. (2012) in a parallel study, reported loadings of copper between 64–1,737 and 76–956 mg/kg in the benthic sediments and transplanted oyster tissue, respectively across the sites used in this study. Botany Bay (‘industrialised’) is a commercial port, with several sources of contaminants from both industrial and residential sources. The two main tributaries into Botany Bay, Georges and Cooks River, are documented to have elevated levels of copper and other metal contaminants in the sediments (Hayes et al. 1998; Spooner et al. 2003), particularly in upstream areas with pollutants radiating as far as the mouth of the estuary (see Birch 1996). Correspondingly, Dafforn et al. (2012) found that benthic sediments in the upper estuary had the highest copper loadings, but also found levels of copper measured in the tissue of oysters to be elevated at a number of sites in the outer harbour, with the highest copper reading recorded at a site near the mouth of the estuary. Loadings of copper across the sites used in this study, range from 18–66 to 52–262 mg/kg in the benthic sediments and oyster tissue respectively (Dafforn et al. 2012).
The Clyde and Wagonga Inlet (reference estuaries) contain no commercial ports or any significant industrial presence, current or historical. Over 95 % of the catchment running into the Clyde is National Park and state forest (DLWC 2000) and both are part of the Batemans Bay Marine Reserve, i.e. the level of commercial and urban development is restricted.
Larval collection
Amphibalanus variegatus adults were collected from each site (Fig. 1) by deploying settlement panels (60 × 60 cm grey PVC sheets) for 3 months between November 2009 and March 2010 and allowing barnacles to recruit to the surface. Panels were deployed by attaching them to a rope which was anchored to the seafloor at 5 m depth and held upright in the water-column by a float. Individual panels were retrieved from ten sites in Botany Bay (Fig. 1a), ten sites in Port Kembla (Fig. 1b), six sites in The Clyde (Fig. 1c), and from eight sites in Wagonga Inlet (Fig. 1d).
Upon retrieval, the panels were ‘cleaned’ by gently scrubbing with a soft brush in FSW and all other invertebrates removed. Panels were only used if A. variegatus was found to be occupying >15 % cover of the panel (~150 individuals). To induce spawning, the panels were left overnight in the dark maintained at 20 °C and exposed to a bright light the following morning. This induced the release of nauplii, which were attracted to point sources of light enabling collection with a pipette.
24 h larval toxicity test
All test solutions were derived from a stock solution of 1,000 mg/l of copper which was prepared by dissolving 2.5114 g of reagent grade CuSO4·5H20 in 1,000 ml of filtered Milli-Q© water and kept at 4 °C to prevent reduction of copper ions in the solution. A 1,000 μg/l Cu solution was prepared each day from this stock solution and diluted with UV sterilized, filtered seawater (0.2 μm) to obtain the treatment concentrations.
The tolerance sensitivity of A. variegatus nauplii was investigated by measuring the swimming activity of nauplii during a 24 h toxicity assay, which encompassed the moult from Nauplii 1 to Nauplii 2. Within 2 h of spawning, nauplii from each site were exposed to five nominal concentrations of copper (50, 100, 150, 200, 250 μg/l Cu) and a seawater control. Containers were presoaked in appropriate solutions overnight to ensure the concentrations of the test solutions were maintained. For each test concentration, twenty nauplii were exposed to 10 ml of each test solution. Exposures were carried out in 6-well Corning© polystyrene culture plates, with each plate containing a single replicate of each test solution. For each site, each treatment was replicated six times in order to obtain a more robust measure of within site variation (i.e. 120 larvae × 5 treatments and control, for each site). After 24 h, treatments were censused by counting the number of nauplii which had become immobilized under a light microscope. Nauplii were deemed ‘immobilized’ if they lay on the bottom surface of the well for 15 s without swimming (as in Qiu et al. 2005).
Molecular methods
Amplified fragment length polymorphism fingerprinting was performed on individuals collected from one site within Port Kembla (15 individuals, 285 mg/kg and 330 mg/kg copper recorded in the benthic sediment and oyster tissue respectively), Botany Bay (13 individuals, 63.5 and 243 mg/kg copper recorded in the benthic sediment and oyster tissue respectively) and Wagonga inlet (15 individuals, 28 and 35 mg/kg copper recorded in the benthic sediment and oyster tissue respectively). The choice of site tested within each estuary was random. Adults were harvested from the panels following larval collection and stored in 95 % ethanol. DNA was extracted using a standard proteinase K digestion and phenol–chloroform extraction procedure (Hillis et al. 1996). The AFLP protocol was adapted from, and similar to, the original protocol of Vos et al. (1995) as follows: for each digest reaction, 1 μl of DNA stock solution (50–200 ng) was mixed with 20 μl PCR H2O, 2.50 μl NEBbuffer 4 (New England BioLabs (NEB), Ipswich, USA), 1 μl MseI (10 U) and 0.50 μl EcoRI (10 U). Mixtures were incubated at 37 °C for 2 h and then at 70 °C for 15 min to denature the restriction enzymes. Five micro liter of digest products were combined with a ligation reaction mixture (9.75 μl PCR H2O, 2.00 μl NEB T4 DNA Ligase reaction buffer (10×), 0.25 μl T4 DNA Ligase ((50 U–rxn)), 1 μl pre-prepared adapters Eco (5 pMol) and 2.00 μl pre-prepared adapters Mse (5 pMol)) and incubated at 37 °C for 3 h.
One μl of ligation product was then combined with a pre-selective PCR reaction mix consisting of 12.25 μl PCR H2O, 2.50 μl dNTP mix (0.25 mM), 0.25 μl NEB Taq (1.25 U), 2 μl Thermopol buffer (10×), 1 μl forward primer (0.5 μM) and 1 μl reverse primer (0.5 μM) (see Table 1 for primer sequences). PCR conditions were 20 cycles of 94 °C for 30 s, 56 °C for 60 s, and 72 °C for 60 s. For the selective amplification, a 1:10 dilution of the pre-selective PCR products was made and 1 μl of each added to a selective amplification mix consisting of 12.25 μl PCR H2O, 2.50 μl dNTP mix (0.25 mM), 0.25 μl NEB Taq (1.25 U), 2 μl Thermopol buffer (10×), 1 μl selective fluorescent labelled (6-FAM, VIC, NED, PET) forward (E01) primer (0.5 μM) and 1 μl selective reverse (M02) primer (0.5 μM). PCR conditions in the first cycle was an initial denaturation at 94 °C for 2 min, followed by 94 °C for 30 s, 65 °C for 30 s, 72 °C for 60 s with the annealing temperature reduced by 0.7 °C for 13 cycles, then 18 cycles of 94 °C for 30 s, 56 °C for 30 s and 72 °C for 2 min, followed by a final extension step of 72 °C for 30 min.
Initially, 32 different primer pairs were tested, from which 8 primer pairs (see Table 1) were selected, based on their ability to discriminate between samples and their consistency. Reactions using selective forward (E01) primers with different fluorescent labels were subsequently pooled, 1:1:1:2 (6-FAM:VIC:NED:PET) and analysed using an Applied Biosystems 3730 DNA Analyzer with GeneScan-500 (LIZ) (Applied Biosystems, Foster City, USA) as the size standard. Fragment analysis was performed using SequentiX GelQuest software version 2.6.5 (SequentiX, Klein Raden, Germany) with bands being scored in a binary fashion as present (1) or absent (0). Bands were assigned to bins based upon three base pair (bp) size intervals. All fragments below 100 bp were excluded from the analysis. The quality and quantity of the digest, pre-amplified and amplified products was checked by running a diluted sample of each product on a 1.5 % agarose gel.
Data analysis
The median effects concentration (EC50) for each site was calculated using the trimmed Spearman-Karber method (US EPA Trimmed Spearman-Karber Analysis Program, Ver 1.5, Environmental Monitoring Systems Laboratory, Cincinnati, OH, USA). A nested ANOVA was used to test for the effects of copper with category (industrialised vs reference) as a main factor, and estuary as a random, nested factor. Data was log-transformed to meet the assumptions of ANOVA.
For the population genetic analyses, total genetic diversity was partitioned between each estuarine category using an analysis of molecular variance (AMOVA) using GenAlEx version 6.41 (Peakall and Smouse 2006) with P-values generated based on 9,999 permutations. A nested AMOVA was performed, with Port Kembla and Botany Bay nested within the ‘industrialised’ category, and Wagonga Inlet within the ‘reference’ category (termed as ‘regions’ in the AMOVA software package). Pairwise comparisons between all estuaries were also performed in order to elucidate individual variance components. The percentage of polymorphic loci was determined using AFLPsurv version 1.0. To visualize the data, a PCO plot was generated using the binary dataset and based on a Euclidean distance matrix.
To determine whether there was evidence of loci under directional selection within the populations from the industrialized estuaries, the genetic data was analysed using Bayescan version 2.1 (Foll and Gaggiotti 2008), which scans the data for marker loci which show excess differentiation (outliers) from neutral evolution expectations. The software generates and compares two alternative models; one model includes the effects of selection, the other excludes it. Default values as suggested by Foll and Gaggiotti (2008) were used, monomorphic loci were removed and a low threshold of log(BF) > 0.5 was used in accordance with Fischer et al. 2011 (i.e. a log(BF) > 0.5 would be considered as substantial evidence for selection, log(BF) > 1, strong and log(BF) > 2, decisive). Analysis was made at the global level (i.e. industrialised versus reference populations) and pairwise comparisons also performed to check for population specific outliers.
Results
Variation in 24 h EC50 among estuaries
The 24 h EC50 for barnacle nauplii exposed to copper varied among industrialised and reference estuaries. Nauplii from the industrialised estuaries showed a significantly greater tolerance to copper than those from the reference estuaries (F1,2 = 194.62, P = 0.001). There was no significant difference in the 24 h EC50 values between estuaries within each category (F2,29 = 0.1474 P = 0.864).The average 24 h EC50 for nauplii from the industrialised and reference estuaries was 111.01 μg/l Cu (SE = 3.01) and 81.29 μg/l Cu (SE = 3.28) respectively (see Fig. 2 for mean estuary 24 h EC50 values).
Population genetics and evidence of selection
The eight primer combinations used produced 392 loci among the 43 samples, 78.83 % of which were polymorphic. The percentage of loci which were polymorphic for Botany Bay, Port Kembla and Wagonga inlet is 61.0, 58.7 and 54.8 % respectively. No differentiation was found among estuarine categories (PhiPR = 0.008 P = 0.816) and/or among the 3 populations (PhiPT = 0.003, P = 0.296, see Table 2). Examination of the data using a PCO plot revealed, in line with the AMOVA, no evidence of any structure (Fig. 3). No evidence for the selection of loci (outliers) was detected at the global level and/or between populations even at the lowest threshold PO value (logPO > 0.5), thus all of the loci amplified can be considered neutral (see Table 3).
Discussion
In-line with the general response of a range of aquatic organisms (reviewed in Johnston 2011), larvae of populations of A. variegatus from the impacted estuaries displayed a greater tolerance to copper than those from the reference estuaries. This response was observed for populations collected from twenty sites within the two industrialised estuaries and fourteen sites within the two reference estuaries. To our knowledge this represents the largest assessment of metal-tolerance of wild-collected organisms ever conducted in a marine system. However, A. variegatus brood their fertilised eggs for a period and offspring may have acclimated to copper and/or other contaminants during development. Based on results of the laboratory assay alone, we cannot distinguish whether the bioassay response is due to acclimation, or reflects a greater resistance. The lack of associated changes in population genetic structure and absence of outlier loci within the industrialised populations would indicate the former. There was, however, evidence of high gene flow between the three estuaries, so it is possible that selection has occurred but is not fixed within the population (i.e. local adaptation). With examination of the genome using a technique which provides greater resolution, the presence of loci under selection may have become evident.
Although the population genetic results indicate that no selection for resistant individuals has occurred, evidence from other work suggests that copper and/or other toxicants may be operating as selective agents within the impacted estuaries. Over the past 80 years, estuarine-wide levels of copper in Port Kembla and Botany Bay, based on water, biotic and sediment measures, have been shown to be high, or in the case of Port Kembla, grossly elevated and ecologically-detrimental (He and Morrison 2001; Birch et al. 1996; Birch 1996; Teutsch 1992; Evenden 1992; Hayes et al. 1998; Moran 1984). Over the past decade, conditions within Port Kembla have undergone significant improvement, but currently levels are still elevated above ANZECC guidelines and are predicted to be of ecological significance (Gall et al. 2012; Dafforn et al. 2009; Dafforn et al. 2012). Recently, in two independent studies, lower rates of recruitment were documented for A. variegatus in Port Kembla, although small numbers were still recorded suggesting tolerant individuals were being selected for and surviving (Dafforn et al. 2009; Gall et al. 2012). In another study, levels of copper found within recreational estuaries, were shown to be highly toxic to A. variegatus adults, although only a portion of individuals within the assemblage were affected (50 % mortality recorded, Piola and Johnston 2008). In the barnacle A. amphitrite, differences in tolerance to the anti-fouling agent copper pyrithione, were found among families suggesting there may be substantial genetic variability in barnacle tolerance to a toxicant (Romano et al. 2010).
Although a greater tolerance to copper was seen in the bioassay, this effect may not be due to previous exposure to copper alone. In the real-world, polluted locations are rarely found to be composed of a single pollutant, but rather are characterised by a suite a pollutants (Klerks and Moreau 2001). The impacted locations selected for this study conform to this pattern, and although copper was found to be elevated, high levels of other metals and contaminants have also been documented (see Dafforn et al. 2012). Exposure to other contaminants has been shown to increase tolerance to other metals, which is likely to due to similarities in the pathways involved in processing the contaminant (Wang and Rainbow 2005). For example Münzinger and Monicelli (1992) found that Daphnia magna strains which had been acclimated to chromium over seven generations displayed a greater tolerance to copper and nickel compared to individuals with no previous chromium exposure (see also Wang and Rainbow 2005; Klerks 1999).
No evidence of differences in population structure was seen and no outlier loci detected, indicating selection had not occurred. However, whilst the AFLP methodology provides a genome-wide census, which makes it a powerful tool in detecting differences in loci which might be implicated with selection (Wang et al. 2012), it is possible the loci/genes associated with tolerance were not amplified as the AFLP methodology only provides information on a limited portion of the genome. In a study by Williams and Oleksiak (2008), where breeding experiments have determined that there is considerable adaptation to a strong pollutant in the estuarine fish F. heteroclitus, examination of the genome using the AFLP methodology, detected only a small number of loci (1–6 %) under selection. In our study, by increasing the number of loci, using a methodology with greater genetic resolution (e.g. 454 sequencing, Stapley et al. 2010) and/or targeting specific regions of the genome, the effects of selection may have become evident. However, if a strong localised structuring force was in effect (i.e. selected loci fixed within the population/local adaptation), we would expect the molecular approach used to have the power to detect this (Campbell and Bernatchez 2004).
The population genetic results indicate, in-line with predictions based on larval duration, that A. variegatus is a widely-dispersing species. Populations separated by as much as 300 km were found to show a high degree of genetic similarity (PhiPT = 0.003). Thus, as postulated by Untersee and Pechenik (2007), it is possible that although selection may be operating at a local scale, for species whose populations experience high and frequent inputs of external recruitment, the effect may be quickly lost. For example Groenendijk et al. 2002 found that metal tolerance in the F1 generation of the midge Chironomus riparius was quickly lost when adapted and non-adapted individuals were crossbred.
Implications
Risk assessments often rely heavily on results of tests performed on laboratory-reared animals, which have been acclimated to laboratory conditions, often for many generations. As already discussed by other authors, the development of tolerance by natural populations, has implications for how toxicity data should be extrapolated to actual risks faced by biota. Tests, which do not incorporate the influence of acclimation and/or genetic variability arising due to previous exposure, could lead to overestimations of long-term ecological risks. Furthermore, tests performed on field-collected populations whose exposure history has not been considered, could lead to the ecological assessments which underestimate potential impacts. Although, in this instance, the differences seen between populations, were not extreme, in other studies, differences of up to 8-fold have been documented (Johnston 2011).
In this study, a greater tolerance to the toxicant copper was observed across a large number of sites spread across two impacted and two reference estuaries in one of the most spatially-extensive aquatic studies to date. In a recent review Johnston (2011) examined the literature on tolerance in aquatic organisms and found that most studies tested organisms from only 2 to 4 sites, thus limiting our ability to generalise with regards to how common or widespread toxicant tolerance is in aquatic systems. The spatially-extensive toxicant response we observed would suggest that, for at least the studied species, tolerance may not be a rare phenomenon. Thus, where induced tolerance may previously been considered a complicating, but uncommon ‘nuisance’ (Millward and Klerks 2002; Chapman 1985), perhaps it may be more extensive and prevalent than previously thought.
References
Barton N, Partridge L (2000) Limits to natural selection. Bioessays 22(12):1075–1084
Belfiore NM, Anderson SL (2001) Effects of contaminants on genetic patterns in aquatic organisms: a review. Mutat Res 489(2–3):97–122
Birch GF (1996) Sediment-bound metallic contaminants in Sydney’s estuaries and adjacent offshore, Australia. Estuar Coast Shelf Sci 42(1):31–44
Birch GF, Evenden D, Teutsch ME (1996) Dominance of point source in heavy metal distributions in sediments of a major Sydney estuary (Australia). Environ Geol 28(4):169–174
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76(2):89–131
Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA (1996) Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500
Campbell D, Bernatchez L (2004) Generic scan using AFLP markers as a means to assess the role of directional selection in the divergence of sympatric whitefish ecotypes. Mol Biol Evol 21(5):945–956
Chapman GA (1985) Acclimation as a factor influencing metal criteria. In: Bahner RC, Hansen DJ (eds) Aquatic toxicology and hazard assessment: eighth symposium. American Society for Testing Metals, Philadelphia, 1985, pp 119–136
Clarke LM, Munch SB, Thorrold SR, Conover DO (2010) High connectivity among locally adapted populations of a marine fish (Menidia menidia). Ecology 91(12):3526–3537
Coors A, Vanoverbeke J, De Bie T, De Meester L (2009) Land use, genetic diversity and toxicant tolerance in natural populations of Daphnia magna. Aquat Toxicol 95(1):71–79
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. In: Annual Review of Marine Science, vol 1. Annual Reviews, Palo Alto, pp 443–466
Dafforn KA, Glasby TM, Johnston EL (2009) Links between estuarine condition and spatial distributions of marine invaders. Divers Distrib 15(5):807–821
Dafforn KA, Simpson SL, Kelaher BP, Clark GF, Komyakova V, Wong CKC, Johnston EL (2012) The challenge of choosing environmental indicators of anthropogenic impacts in estuaries. Environ Pollut 163:207–217
Depledge MH (1994) Genotypic toxicity: implications for individuals and populations. Environ Health Perspect 102:101–104
DLWC (2000) Estuaries of New South Wales. Sydney, Australia: Department of Land and Water Conservation
Egan EA, Anderson DT (1986) Larval development of Balanus amphitrite Darwin and Balanus variegatus Darwin (Cirripedia, Balanidae) from New South Wales, Australia. Crustaceana 51(2):188–207
Evenden D: Heavy metal concentrations in the sediments of the Georges River, N. S. W. unpublished honours thesis (1992)
Fischer MC, Foll M, Excoffier L, Heckel G (2011) Enhanced AFLP genome scans detect local adaptation in high-altitude populations of a small rodent (Microtus arvalis). Mol Ecol 20(7):1450–1462
Fisher M, Oleksiak M (2007) Convergence and divergence in gene expression among natural populations exposed to pollution. BMC Genomics 8(1):108
Foll M, Gaggiotti O (2008) A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: a bayesian perspective. Genetics 180(2):977–993
Fritsch C, Coeurdassier M, Gimbert F, Crini N, Scheifler R, Vaufleury A (2011) Investigations of responses to metal pollution in land snail populations (Cantareus aspersus and Cepaea nemoralis) from a smelter-impacted area. Ecotoxicology 20(4):739–759
Gall ML, Poore AGB, Johnston EL (2012) A biomonitor reflects an ecologically-significant fraction of metals in an industrialised harbour. J Environ Monitor 14(3):830–838
García-Ramos G, Kirkpatrick M (1997) Genetic models of adaptation and gene flow in peripheral populations. Evolution 51(1):21–28
Grant A (2002) Pollution-tolerant species and communities: intriguing toys or invaluable monitoring tools? Hum Ecol Risk Assess 8(5):955–970
Grant A, Hateley JG, Jones NV (1989) Mapping the ecological impact of heavy metals on the estuarine polychaete Nereis diversicolor using inherited metal tolerance. Mar Pollut Bull 20(5):235–238
Groenendijk D, Lucker SMG, Plans M, Kraak MHS, Admiraal W (2002) Dynamics of metal adaptation in riverine chironomids. Environ Pollut 117(1):101–109
Hayes WJ, Anderson IJ, Gaffoor MZ, Hurtado J (1998) Trace metals in oysters and sediments of Botany Bay, Sydney. Sci Total Environ 212(1):39–47
He ZJ, Morrison RJ (2001) Changes in the marine environment of Port Kembla Harbour, NSW, Australia, 1975–1995: a review. Mar Pollut Bull 42(3):193–201
Hillis DM, Mable BK, Larson A, Davis SK, Zimmer EA (1996) Nucleic acids IV: sequencing and cloning. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics, 2nd edn. Sinauer Asssociates Inc., Massachusetts
Johnston EL (2011) Tolerance to contaminants: evidence from chronically exposed populations of aquatic organisms. In: Romeo M, Rainbow PS, Amiard-Triquet C (eds) Tolerance to environmental contaminants. CRC Press, Boca Raton, pp 25–46
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7(12):1225–1241
Klerks P (1999) Acclimation to contaminants by the grass shrimp Palaemonetes pugio: individual contaminants vs. mixtures. Ecotoxicology 8(4):277–286
Klerks P, Lentz S (1998) Resistance to lead and zinc in the western mosquitofish Gambusia affinis inhabiting contaminated Bayou Trepagnier. Ecotoxicology 7(1):11–17
Klerks PL, Levinton JS (1989) Rapid evolution of metal resistance in a benthic oligochaete inhabiting a metal-polluted site. Biol Bull 176(2):135–141
Klerks PL, Moreau CJ (2001) Heritability of resistance to individual contaminants and to contaminant mixtures in the sheepshead minnow (Cyprinodon variegatus). Environ Toxicol Chem 20(8):1746–1751
Klerks PL, Weis JS (1987) Genetic adaptation to heavy metals in aquatic organisms: a review. Environ Pollut 45(3):173–205
Lenormand T (2002) Gene flow and the limits to natural selection. Trends in ecology & evolution (Personal edition) 17(4):183–189
Lopes I, Baird DJ, Ribeiro R (2004) Genetic determination of tolerance to lethal and sublethal copper concentrations in field populations of Daphnia longispina. Arch Environ Contam Toxicol 46:43–51
Medina MH, Correa JA, Barata C (2007) Micro-evolution due to pollution: possible consequences for ecosystem responses to toxic stress. Chemosphere 67(11):2105–2114
Meyer JN, Giulio RTD (2003) Heritable adaptation and fitness costs in killifish (Fundulus heteroclitus) inhabiting a polluted estuary. Ecol Appl 13(2):490–503
Miliou H, Verriopoulos G, Maroulis D, Bouloukos D, Moraitou-apostolopoulou M (2000) Influence of life-history adaptations on the fidelity of laboratory bioassays for the impact of heavy metals (Co2+ and Cr6+) on tolerance and population dynamics of Tisbe holothuriae. Mar Pollut Bull 40(4):352–359
Millward RN, Klerks PL (2002) Contaminant-adaptation and community tolerance in ecological risk assessment: introduction. Hum Ecol Risk Assess 8(5):921–932
Moran PJ (1984) Water-quality control and its effect on the concentration of heavy-metals in Port-Kembla Harbor, NSW. Mar Pollut Bull 15(8):294–297
Morgan AJ, Kille P, Stürzenbaum SR (2007) Microevolution and ecotoxicology of metals in invertebrates. Environ Sci Technol 41(4):1085–1096
Münzinger A, Monicelli F (1992) Heavy metal co-tolerance in a chromium tolerant strain of Daphnia magna. Aquat Toxicol 23(3–4):203–216
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Syst 25:547–572
Peakall ROD, Smouse PE (2006) Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6(1):288–295
Piola RF, Johnston EL (2008) Pollution reduces native diversity and increases invader dominance in marine hard-substrate communities. Divers Distrib 14(2):329–342
Posthuma L, Van Straalen NM (1993) Heavy-metal adaptation in terrestrial invertebrates: a review of occurrence, genetics, physiology and ecological consequences. Comp Biochem Physiol C 106(1):11–38
Qiu J-W, Thiyagarajan V, Cheung S, Qian P-Y (2005) Toxic effects of copper on larval development of the barnacle Balanus amphitrite. Mar Pollut Bull 51(8–12):688–693
Rainbow PS, Amiard-Triquet C, Amiard JC, Smith BD, Best SL, Nassiri Y, Langston WJ (1999) Trace metal uptake rates in crustaceans (amphipods and crabs) from coastal sites in NW Europe differentially enriched with trace metals. Mar Ecol Prog Ser 183:189–203
Romano JA, Rittschof D, McClellan-Green PD, Holm ER (2010) Variation in toxicity of copper pyrithione among populations and families of the barnacle, Balanus amphitrite. Biofouling 26(3):341–347
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Annu rev marine sci 3(1):509–535
Sarkar A, Ray D, Shrivastava A, Sarker S (2006) Molecular biomarkers: their significance and application in marine pollution monitoring. Ecotoxicology 15(4):333–340
Scanes PR, Roach AC (1999) Determining natural ‘background’ concentrations of trace metals in oysters from New South Wales, Australia. Environ Pollut 105(3):437–446
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236(4803):787–792
Sotka EE (2005) Local adaptation in host use among marine invertebrates. Ecol Lett 8(4):448–459
Spooner DR, Maher W, Otway N (2003) Trace metal concentrations in sediments and oysters of Botany Bay, NSW, Australia. Arch Environ Contam Toxicol 45(1):92–101
Stapley J, Reger J, Feulner PGD, Smadja C, Galindo J, Ekblom R, Bennison C, Ball AD, Beckerman AP, Slate J (2010) Adaptation genomics: the next generation. Trends Ecol Evol 25(12):705–712
Teutsch ME: The distribution of heavy metals in Botany Bay and the lower Georges River, N.S.W. unpublished honours thesis, University of Sydney (1992)
Untersee S, Pechenik JA (2007) Local adaptation and maternal effects in two species of marine gastropod (genus Crepidula) that differ in dispersal potential. Mar Ecol Prog Ser 347:79–85
Van Straalen NM, Janssens TS, Roelofs D (2011) Micro-evolution of toxicant tolerance: from single genes to the genome’s tangled bank. Ecotoxicology 20(3):574–579
Vos P, Hogers R, Bleeker M, Reijans M, Lee Tvd, Hornes M, Friters A, Pot J, Paleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23(21):4407–4414
Wang W-X, Rainbow PS (2005) Influence of metal exposure history on trace metal uptake and accumulation by marine invertebrates. Ecotoxicol Environ Saf 61(2):145–159
Wang T, Chen G, Zan Q, Wang C, Su Y-j (2012) AFLP genome scan to detect genetic structure and candidate loci under selection for local adaptation of the invasive weed Mikania micrantha. PLoS One 7(7):e41310
Williams LM, Oleksiak MF (2008) Signatures of selection in natural populations adapted to chronic pollution. BMC Evol Biol 8(1):282
Wirgin I, Waldman JR (2004) Resistance to contaminants in North American fish populations. Mutat Res 552(1–2):73–100
Acknowledgments
We would like to thank members of the Subtidal Ecology and Ecotoxicology laboratory and volunteers for their assistance. This Project was supported by an Australian Research Council Grant awarded to Johnston EL and Doyle C.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gall, M.L., Holmes, S.P., Dafforn, K.A. et al. Differential tolerance to copper, but no evidence of population-level genetic differences in a widely-dispersing native barnacle. Ecotoxicology 22, 929–937 (2013). https://doi.org/10.1007/s10646-013-1063-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1063-z