Abstract
Endosulfan is an environmentally persistent pesticide and has been shown to be genotoxic, neurotoxic and carcinogenic to surrounding organisms. Earthworms are widely used in environmental metabolomic studies to assess soil ecotoxicity. Previous nuclear magnetic resonance (NMR)-based metabolomic studies have analyzed earthworm tissue extracts after exposure to endosulfan and identified some key metabolic indicators that can be used as biomarkers of stress. However, some metabolites may have been masked due to overlap with other metabolites in the tissue extract. Therefore, in this study, the coelomic fluid (CF) and the tissue extract of the earthworm, Eisenia fetida, were both investigated using 1H NMR-based metabolomics to analyze their metabolic profile in response to endosulfan exposure at three sub-lethal (below LC50) concentrations. Principal component analysis determined the earthworm CF and earthworm tissue extract to both have significant separation between the exposed and control at the two highest sub-lethal endosulfan exposures (1.0 and 2.0 μg cm−2). Alanine, glycine, malate, α-ketoglutarate, succinate, betaine, myo-inositol, lactate and spermidine in the earthworm CF and alanine, glutamine, fumarate, glutamate, maltose, melibiose, ATP and lactate in earthworm tissue extract were all detected as having significant fluctuations after endosulfan exposure. An increase in ATP production was detected by the increase activity in the citric acid cycle and by anaerobic metabolism. A significant decrease in the polyamine, spermidine after endosulfan exposure describes an apoptotic mode of protection which correlates to a previous endosulfan exposure study where DNA damage has been reported. This study highlights that earthworm CF is a complementary biological medium to tissue extracts and can be helpful to better understand the toxic mode of action of contaminants at sub-lethal levels in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endosulfan is an organochlorine pesticide that emerged in the 1960s as one of the leading chemicals used against a wide spectrum of agriculturally important insect pests (Ayres and Ayres 1999). It is currently produced in at least six countries around the world with India being one of the largest producers. Endosulfan has been ubiquitously detected in air, soil and water, and at long distances from the direct source of application (Weber et al. 2010). In recent years, endosulfan has been recognized as a persistent toxic substance by many environmental agencies such as the United Nations Environment Programme, World Health Organization and Environmental Justice Foundation (Liu et al. 2009). Endosulfan has been shown to have genotoxic, neurotoxic and carcinogenic properties to mammals, birds, fish and bees (Liu et al. 2009; Siddique et al. 2003). Endosulfan has a half-life in water of around 180 days but in soil, it has much higher persistence with a half-life close to 60–900 days (Ayres and Ayres 1999). Due to its persistence in the environment, it is important to understand its impact on organisms in soil ecosystems at sub-lethal levels.
Past studies have demonstrated that the total contaminant concentrations in soil do not necessarily relate to the bioavailable fraction or to soil toxicity (Barthe and Pelletier 2007). Several ecotoxicological tests are frequently utilized that focuses on the endpoint of toxicity such as the organism’s mortality, reproduction rates and overall growth (Kroeger 2006). However, these results do not allow the understanding of the toxic mode of action (MOA) of the contaminant after sub-lethal exposure at a molecular level and how it affects the organism at a function level (Moore 2002). Environmental metabolomics is an emerging field of research that examines the changes in the metabolic profile of native living organisms in their environment to potential environmental stressors (Simpson and McKelvie 2009). Metabolomics offers the advantage of understanding the dynamic state of the organism (cell, tissue or biofluid) which can mechanistically relate to the organism’s phenotype (Bundy et al. 2009). The endogenous metabolites identified after exposure by metabolomics can uncover unforeseen relationships to further understand the organism’s biochemical response to the contaminant and is finding an increasing number of applications in ecotoxicology (Ekman et al. 2009).
Earthworms play a major role in the decomposition activity of the soil environment and contribute to the maintenance of the soil physical structure (Paoletti 1999). Their feeding and burrowing activities in the soil increase soil fertility, water infiltration and soil aeration (Sanchez-Hernandez 2006). Earthworms represent close to 60–80 % of the soil total biomass, are ubiquitous in a vast range of soils (Saint-Denis et al. 1999) and their absence or well-being in the soil is directly connected to microbial community health and biodiversity (Scheu et al. 2002). Due to their importance in the soil, earthworms are common biological indicators in ecotoxicological studies to assess the health of the soil environment. Metabolomic studies using earthworms have been frequently used to detect subtle biomarkers of exposure to environmental contaminants such as polyaromatic hydrocarbons (PAHS) (Brown et al. 2009, 2010) and metal contaminants (Bundy et al. 2004, 2007, 2008). Earthworm metabolomic studies have shown potential to be important monitoring tools to detect stress in the soil system (Brown et al. 2010; McKelvie et al. 2010). Nuclear magnetic resonance (NMR) spectroscopy has been commonly used in earthworm metabolomics to identify key metabolites as it is non-destructive and a rapid technique for high-throughput of samples (Brown et al. 2008, 2009). Previous metabolomics studies have analyzed the exposure of the earthworm, Eisenia fetida to endosulfan using one-dimensional (1D) and two-dimensional (2D) NMR spectroscopy (McKelvie et al. 2009; Yuk et al. 2010, 2011) on their aqueous tissue extract. From their results, leucine, phenylalanine, tryptophan, lysine, glutamate, valine, glycine, isoleucine, methionine, glutamine, alanine, maltose, glucose, melibiose, malate, fumarate and ATP were detected as significant in two sub-lethal concentrations (1.0 and 2.0 μg cm−2) using the earthworm tissue extract and a neurotoxic MOA was postulated (Yuk et al. 2011). However, the analysis of the earthworm tissue extract may mask the metabolic change of endogenous metabolites because of its chemical heterogeneity (Bundy et al. 2001). In addition, certain metabolites can be higher in concentration in the sample, dominate the spectrum to increase signal overlap and decrease sensitivity to other metabolites in the same NMR region. Sugars are a major component in earthworm tissue extracts and tend to exhibit complex NMR profiles which in turn mask metabolites at lower concentration over a large spectral region in both 1D and 2D NMR (Yuk et al. 2010). Past earthworm metabolomic studies (Lankadurai et al. 2011b; Rochfort et al. 2009), have experimented using a three solvent extraction system (chloroform, methanol and water) to separate the polar and non-polar metabolites for NMR analysis. However, in both studies, the polar fraction still exhibited significant overlap in the sugar region. Therefore, from both an analytical and ecotoxicity perspective, it may be advantageous to investigate another biological medium especially one where the concentration of sugars is reduced which in turn can help to detect obscured metabolites.
The earthworm coelomic fluid (CF) plays an important role in homeostasis and in immune defenses against external stimuli (Kurek et al. 2006). CF has many haemolytic, proteolytic and cytotoxic enzymes that are active against foreign cells and peptides (Bundy et al. 2001). Studies have shown alterations in the components of the CF to be early indicators of immunotoxicity or biomarkers (Kauschke et al. 2007; Hendawi et al. 2004). A past study analyzed the exposure of an environmental contaminant, 3-fluoro-4 nitrophenol, to the earthworm CF using 1D NMR and detected acetate and malonate to be significantly decreased in the exposed earthworms (Bundy et al. 2001). Another study analyzed the earthworm CF on their exposure to 3-trifluoromethylaniline using NMR spectroscopy and identified lactate as a potential biomarker of acute toxic stress compared to the control earthworms (Lenz et al. 2005). Past metabolomic studies have shown promise using either the earthworm tissue extracts or CF, however, to our knowledge, both have not been compared simultaneously to assess their ability to discriminate metabolites of exposure to a contaminant. In this study, the CF from one set of earthworms and the tissue extract from another set of earthworms (both with control and endosulfan-exposed treatments) will be analyzed using NMR. Readers should note that the tissue extract set will also contain the CF but as discussed previously, are masked due to the higher abundant metabolites such as carbohydrates. As such, the isolation of the CF can potentially reduce this background and permit a more targeted and in-depth analysis of the CF itself. The comparison of the CF to the remaining tissue after extraction was not investigated based on the principle that earthworm metabolomic studies do not remove the CF prior to analysis. Instead, this study compares the isolated CF to the whole tissue analysis to investigate what additional information the CF can provide when compared to the commonly employed technique in the field after contaminant exposure. Contacts tests are used to examine the specific changes in both the metabolic profile of the CF and tissue extracts after endosulfan exposure. Contacts tests are widely used for understanding chemical risk or screening before studying more complex matrices such as soil (Bundy et al. 2009). The results from this study will further our understanding of the MOA by an environmentally relevant contaminant such as endosulfan and provide further insight on the potential of utilizing the earthworm CF for environmental metabolomic studies.
Experimental methods
Earthworm contact test preparation and exposure
Eisenia fetida earthworms were purchased from The Worm Factory (Perth, ON, Canada) and were maintained according to Brown et al. (2008). Mature earthworms with a visible clitellum were depurated in groups of five in the dark for 96 h on Whatman 4 Qualitative filter paper with a diameter of 9 cm (Fisher Scientific, Waltham, MA, USA) in 500 mL jars to remove any residues from their intestinal tracts (Brown et al. 2008). To ensure there were no significant differences in the weights of the earthworms before exposure for the control and endosulfan treatment groups, an analysis of variance (ANOVA) was conducted. The earthworms had a mean weight of 0.60 ± 0.13 g and from the ANOVA analysis (ANOVA, F 7,71 = 0.892, p = 0.517), the p value was higher than the α = 0.05 level and therefore, no significant difference was seen between the controls (0.58 ± 0.08 g) and any of the endosulfan concentrations groups (0.61 ± 0.14 g) before exposure. Earthworms were then transferred to individual 120 mL amber glass jars containing pre-treated Whatman GF/A 4.25 cm diameter glass filter paper (Fisher Scientific). The half-lethal concentration (LC50) value for endosulfan is reported to be 5.7 μg cm−2 (Heimbach 1988). To ensure the exposure concentrations chosen for endosulfan were sub-lethal, endosulfan (99 % purity; Sigma Aldrich, St. Louis, MO, USA) was applied to the filter paper at three sub-lethal concentrations: 1/9th (0.5 μg cm−2), 1/6th (1.0 μg cm−2) and 1/3rd (2.0 μg cm−2) of the literature LC50 using 1 mL of acetone (HPLC grade; Caldeon, Georgetown, ON, Canada) as the carrier solvent. One milliliter of acetone was applied to control treatments. In all cases, the acetone was allowed to evaporate and 1 mL of distilled water was added prior to the addition of earthworms. All earthworms survived the exposure tests. Earthworms were kept in the dark for 48 h, as recommended by the OECD LC50 contact test guideline (OECD 1984). The experiment was kept at 24 °C which is an optimal temperature for E. fetida (Presley et al. 1996). Earthworms used for the CF extraction, were separated from the earthworms used for the tissue extraction (detailed procedures are outlined below). There were 10 replicates for control and 10 for each of the three concentrations of the contaminant for the exposed specimen.
Earthworm CF extraction and preparation for NMR
Each earthworm after exposure (control or exposed) were placed in individual 25 mL glass vials with 365 μL of 0.2 M monobasic sodium phosphate buffer solution (NaH2PO4·2H2O; 99.3 %; Fisher Scientific) containing 0.1 % (w/v) sodium azide (99.5 % purity; Sigma Aldrich) as a preservative. Buffer solution was made with D2O (99.9 % purity, Cambridge Isotope Laboratories Inc., Andover, MA, USA) and adjusted to a pD of 7.4 using NaOD (30 % w/w in 99.5 % D2O, Cambridge Isotope Laboratories Inc.). The buffer solution for all NMR samples also contained 10 mg/L of 2,2-dimethyl-2-silapentane-5-sulfonate sodium salt (DSS; 97 %, Sigma Aldrich) as an internal standard. Earthworm CF was extracted by electrical extrusion using a 9 V battery and using short exposure (<1 s) repeated 10 times (Bundy et al. 2002; Hendawi et al. 2004). The earthworm was then removed and the extracted fluid was placed in a 1.5 mL centrifuge tube and centrifuged for 20 min at 15,000 rpm (~17,000×g) using an International Equipment Company 21000 Centrifuge (Fisher Scientific, Canada) to remove any biosolids or coelomocytes. The supernatant was then transferred into a 5 mm High Throughputplus NMR tubes (Norell Inc., Landisville, NJ, USA). All samples were frozen immediately after preparation and each sample was thawed prior to NMR analysis.
Earthworm tissue extraction and preparation for NMR
After exposure, earthworms were immediately flash frozen in liquid nitrogen and lyophilized (Brown et al. 2008). The lyophilized earthworms were homogenized in a 1.5 mL centrifuge tube using a 5 mm wide stainless steel spatula. Samples were then extracted using 1 mL of a 0.2 M monobasic sodium phosphate buffer solution (NaH2PO4·2H2O; 99.3 %; Fisher Scientific) containing 0.1 % (w/v) sodium azide (99.5 % purity; Sigma Aldrich) as a preservative (Brown et al. 2008). Buffer solution was made with D2O (99.9 % purity, Cambridge Isotope Laboratories Inc.) and adjusted to a pD of 7.4 using NaOD (30 % w/w in 99.5 % D2O, Cambridge Isotope Laboratories Inc.). The buffer solution for all NMR samples also contained 10 mg/L of DSS (97 %, Sigma Aldrich) as an internal standard. Samples were vortexed for 30 s using a VX 100 vortexer (Labnet, Edison, NJ, USA) and then sonicated for 15 min using a FS60 sonicator (Fisher Scientific) to aid with the extraction. Samples were then centrifuged at 14,000 rpm (~15,000×g) using an International Equipment Company 21000 Centrifuge (Fisher Scientific) for 20 min and the supernatant was transferred into a new 1.5 mL centrifuge tube. The centrifuge process was then repeated two more times to ensure all additional particulates were removed and then samples were transferred into a 5 mm High Throughputplus NMR tubes (Norell Inc.). All samples were frozen immediately after preparation and each sample was thawed prior to NMR analysis.
NMR spectroscopy
All NMR spectra were acquired using a Bruker Avance 500-MHz spectrometer with a 1H−19F−15N−13C 5 mm broadband quadruple inverse probe fitted with an actively shielded Z gradient (Bruker BioSpin, Rheinstetten, Germany). The 1H 90° pulse was calibrated for each sample in the study. 1H NMR experiments were performed using presaturation utilizing relaxation gradients and echoes (PURGE) water suppression (Simpson and Brown 2005) and 512 scans, a recycle delay of 3 s, and 65 K time domain points. All 1D NMR spectra were manually phased and calibrated to the DSS internal reference methyl singlet, set to a chemical shift (δ) of 0.00 ppm.
A 2D 1H–13C HSQC NMR experiment was performed on one control earthworm for both the CF and aqueous tissue homogenate samples to aid in spectral interpretation. HSQC was collected in phase-sensitive mode using echo/anti-echo gradient selection, a 1J 1H–13C (145 Hz) and a relaxation delay of 0.5 s. Eight hundred and eighty scans and 2,048 data points were collected for each of the 196 increments in the F1 dimension. The F2 dimension was processed using an exponential function corresponding to a line broadening of 15 Hz while the F1 dimension was processed using a sine-squared function with a π/2 phase shift. Both dimensions were zero-filled by a factor of two while forward linear prediction using 32 coefficients was applied in the F1 dimension. The 2D NMR spectra were manually phased and calibrated to the DSS internal reference methyl singlet, set to a chemical shift (δ) of 0.00 ppm.
Data and statistical analysis
Principal component analysis (PCA) was performed on the 1D NMR spectra using an analysis of mixtures (AMIX) statistics package (version 3.9.8, Bruker BioSpin). The earthworm CF 1H NMR spectra were divided into width bins of 0.02 ppm from the region 0.25 to 9.0 ppm and the region from 4.35 to 5.21 ppm was not analyzed due to residual H2O/HOD signals present in this region. The earthworm tissue extract 1H NMR spectra were divided into width bins of 0.02 ppm from the region 0.25–9.0 ppm and the region from 4.75 to 4.90 ppm was not analyzed due to residual H2O/HOD signals present in this region. The “sum of intensities” was used as the integration mode and the scaling was set to “total intensity” for all the NMR spectra. PCA was performed at the 95 % confidence level and any variances that represented less than 1 % were excluded (Brown et al. 2010). In the earthworm tissue extract dataset, one earthworm at the lowest endosulfan concentration (0.5 μg cm−2) was identified as an outlier through the Hotelling’s T2 ellipse at the 95 % confidence interval and was removed prior to analysis (Ekman et al. 2008; McKelvie et al. 2010). Therefore in this study, each treatment group and unexposed control had 10 earthworms for both CF and tissue extract experiments with one excluded from the lowest concentration of endosulfan (0.5 μg cm−2), which had 9 earthworms. Mean PCA scores and their associated standard errors for control and exposure concentrations were calculated and graphed to understand the differences between the unexposed and exposed earthworm groups. Individual PCA score plots for the earthworm CF and tissue extract 1H NMR spectra were also calculated to understand the differences between each endosulfan exposure concentration and the control earthworm group. PCA is an exploratory data analysis tool to identify general similarities and differences through a multivariate approach. However, PCA scores plots themselves do not determine if the difference between various groups is significant. To achieve this, ANOVA with a Dunnett’s multiple comparison test was used to determine if the separation between each treatment group compared to the control group is significant (α = 0.1 or 0.05) (Weckwerth 2007; Yuk et al. 2011). ANOVA in this study will be reported as: ANOVA, F df = F value, p value, where df is the degrees of freedom. A p value of <0.1 generally indicates significance, whereas a p value >0.1 indicates less statistical significance. ANOVA, t test and Dunnett’s multiple comparison tests were performed using SPSS 19.0 (IBM, Somers, NY, USA).
Multiple t test filtered difference 1H NMR spectra were constructed to identify increases or decreases in the peaks between the control and each exposure concentration set (Ekman et al. 2009). Each difference NMR spectrum was generated by subtracting the averaged bucket intensities of the control group from each of the exposed group concentrations. In addition, a t test was conducted on each bin to determine if the intensity difference was significantly different to the control (p < 0.05). Any intensity values that were significantly different were kept in the spectrum but if not, were replaced with a zero. The final t test filtered difference NMR spectrum allows the identification of potential metabolites from the significant peaks that were increasing/decreasing after exposure. Influential peak signals identified in the 1D difference spectra were then matched with metabolite signals from a previous study which identified the major metabolites in E. fetida (Brown et al. 2008) and were also compared to the Bruker Biofluid Reference Compound Database version 2-0-3 (Bruker BioSpin). The 1H–13C HSQC NMR spectra for the CF and tissue extract also assisted in the identification of the major metabolites through the comparison with the Bruker Biofluid Reference Compound Database version 2-0-3 (Bruker BioSpin). Percent changes for the identified metabolites in the difference spectrum of exposed earthworms relative to control were calculated by the equation: (IE − IC)/IC × 100 where IE is the mean bucket intensity for the exposed earthworm group and IC is the mean bucket intensity for the control earthworm group.
Results and discussions
Comparison of 1H and 1H–13C HSQC NMR spectra of earthworm extracts
A 1D 1H NMR and 2D 1H–13C HSQC NMR spectrum was acquired for both the earthworm CF (Fig. 1a, b) and tissue extracts (Fig. 2a, b) to identify the various metabolites in the samples and to understand their metabolic profile differences. From the 1D NMR spectrum of the earthworm CF (Fig. 1a), carbohydrates are present at trace levels (for example, glucose anomeric peak, detected at δ 5.22 ppm), which is in sharp contrast to the tissue extract, where various sugars including maltose, glucose and melibiose dominate the spectral profile between δ 3.0 and 5.4 ppm. The lower concentration of sugars in the earthworm CF significantly reduces overlap in δ 3.0–5.4 ppm region and permits many obscured metabolites such as myo-inositol (δ 3.53 and δ 4.05 ppm), scyllo-inositol (δ 3.34 ppm), citric acid intermediates (malate (δ 2.33–2.36, δ 2.65–2.68 and δ 4.30 ppm), α-ketoglutarate (δ 2.43 and δ 2.99 ppm) and succinate (δ 2.39 ppm) and polyamines (putrescine (δ 3.04 ppm) and spermidine (δ 3.13 ppm)) to be identified with certainty in the NMR spectrum. In the tissue extract, the intense sugar resonances completely mask these metabolites and are not seen. A larger water signal was present (δ 4.78 ppm) in the earthworm CF because of the natural water content in the sample but did not disrupt the nearby peak resonances and was excluded prior to the multivariate analysis. Many unique metabolite signals were detected in the aromatic region (δ 6.9–8.0 ppm) of the earthworm CF but are as yet unidentified. None of these aromatic signals matched any entries in our standard metabolite database (Bruker Biofluid Reference Compound Database) and will require further investigation in future studies. In the 1D NMR spectrum of the earthworm tissue extract (Fig. 2a), tyrosine (δ 6.90 ppm), tryptophan (δ 7.17 ppm) and phenylalanine (δ 7.32–7.42 ppm) were detected in the aromatic region but was not seen in the earthworm CF.
Most of the metabolites identified in the 1D NMR spectrum of the earthworm CF were confirmed by the cross-peaks in the 2D HSQC NMR spectrum (Fig. 1b) and corresponds to the chemical shifts of the proton and carbon connections. The tissue extract 2D HSQC NMR spectrum (Fig. 2b) confirmed many of the metabolites identified in the 1D NMR spectrum and shows the complexity of this biological medium as many cross-peaks are detected compared to the earthworm CF. However, with the decrease in sugars in the earthworm CF, myo-inositol, scyllo-inositol and malate could be clearly discerned in that region with spermidine, α-ketoglutarate, succinate and fumarate appeared as additional metabolites in other spectral regions of the CF. The analysis of the earthworm CF provides a unique metabolic profile that cannot be completely discerned from the whole tissue extract alone. This difference in the metabolic profile may potentially enable an alternate window into understanding the earthworm’s response to environmental contaminants.
Multivariate statistical analysis of endosulfan exposure on earthworms
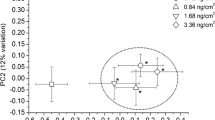
Mean PCA plots (Figs. 3a, b) for the earthworm CF and tissue extract 1H NMR datasets respectively were performed between the endosulfan-exposed earthworms and the control. Individual PCA score plots for the earthworm CF and tissue extract 1H NMR datasets respectively were also performed for each endosulfan exposure concentration and the control (accessory publication, Figs. S1A and S1B of Supplementary material). For the earthworm CF (Fig. 3a), the PC 1 versus PC 2 scores plot showed a trend for separation with the highest separation on the PC 2 axis while high variance was detected on the PC 1 axis. The high variance in PC 1 could be biological variation which is not related to the endosulfan treatment as this is occasionally seen with unsupervised PCA analysis (Parsons et al. 2009). PC 2 had an explained variance of 19.7 % while PC 1 had an explained variance of 48.2 % for a total of 67.9 % for total variance. There was significant separation on the PC 2 axis (ANOVA, F 3,36 = 21.1, p = 4.69E−8) and through a Dunnett’s t test, which compares all the individual concentrations with the control, the two highest concentrations (1.0 and 2.0 μg cm−2) were found to be significantly different than the control (p < 0.05). For the earthworm tissue extract (Fig. 3b), separation was detected in the PC 1 versus PC 3 scores plot with an explained variance of 67.6 and 8.4 % respectively for a total variance of 76.0 %. Higher PCs are commonly analyzed in metabolomic studies using PCA analysis (Brown et al. 2010; Bundy et al. 2004; McKelvie et al. 2011; Rochfort et al. 2009; Whitfield Åslund et al. 2011) as PCs explain the highest amount of total data variation without discrimination in regard to the source of the variation. Some PCs might not be related to the experimental treatment since other factors such as metabolic variations (sampling error and natural biological variation) are incorporated (Verouden et al. 2009). Therefore, it is advantageous to explore higher order PCs to properly represent the metabolomic datasets (Broadhurst and Kell 2006). From ANOVA, separation was found on the PC 1 axis at α = 0.1 (ANOVA, F 3,35 = 2.248, p = 0.100) and PC 3 axis at α = 0.05 (ANOVA, F 3,35 = 4.277, p = 0.011). To further evaluate the PCA separation, a Dunnett’s multiple comparison t test determined that the two highest concentrations (1.0 and 2.0 μg cm−2) were significantly different than the control (p < 0.05). The earthworm CF contains many immunocompetent cells which act as a protective hydrostatic skeleton around the organism (Homa et al. 2008) and is the main communicator between the inner and outer environment (Kurek et al. 2006). Therefore, the earthworm CF was found to be as responsive to the subtle concentrations of contaminants in an environment as the earthworm tissue extract. A concentration dependant exposure was seen in both the earthworm CF and tissue extract from the PCA scores plot as significance of the separation was increased according to the concentration. However, further confirmation will be required using a wider range of sub-lethal concentrations.
Mean PCA scores plot of 1H NMR spectra of endosulfan-exposed E. fetida using their a CF (PC 1 vs PC 2) and b aqueous tissue extract (PC 1 vs PC 3). Each point represents the mean PC score for each exposure concentration and the error bar represents the standard error of the mean. The legend indicates the endosulfan exposure concentration for each point. The “*” represents the mean concentrations that were significantly different from the control (p < 0.05) using Dunnett’s multiple comparison test
Relative metabolite changes in endosulfan-exposed earthworms
To determine the metabolic response differences that contribute to the separation in the earthworm CF and tissue extract of the endosulfan-exposed earthworms PCA scores plots, a t test filtered difference NMR spectrum was made for each exposure concentration from the 1H NMR spectra (Figs. 4, 5 respectively). This method allows the identification of significant metabolites that were specifically perturbed in the earthworm after endosulfan exposure and has been frequently used in other metabolomic studies (Ekman et al. 2008, 2009; Yuk et al. 2011; Lankadurai et al. 2011a). In the earthworm CF (Fig. 4), lactate (δ 1.31 ppm), alanine (δ 1.45 ppm), succinate (δ 2.39 ppm), malate (δ 2.69 ppm and δ 4.29–4.31 ppm), α-ketoglutarate (δ 2.41 and 2.99 ppm), spermidine (δ 3.15 ppm), betaine (δ 3.25 ppm), myo-inositol (δ 3.53 ppm) and glycine (δ 3.55 ppm) were detected as significant (p < 0.05) in the endosulfan-exposed earthworms compared to the control. None of the unidentified aromatic signals in the earthworm CF were determined to be statistically significant in relation to endosulfan exposure. In the earthworm tissue extract (Fig. 5), similar metabolites were detected as significant such as alanine and lactate, but many other metabolites such as glutamate (δ 2.35 ppm), glutamine (δ 2.41 ppm), melibiose (δ 4.95 ppm), maltose (δ 5.40 ppm), fumarate (δ 6.51 ppm) and ATP (δ 8.23 ppm) were identified as well.
t-test filtered 1H NMR difference spectra of E. fetida CF were obtained by subtracting the mean buckets of each endosulfan-exposed earthworm concentration: (a) 0.5 μg cm−2, (b) 1.0 μg cm−2 and (c) 2.0 μg cm−2 with the mean buckets of the control earthworms. Signals that were significantly different from the control (p < 0.05) were retained while others are excluded. Only the major metabolites are labeled for clarity
t-test filtered 1H NMR difference spectra of E. fetida tissue extracts were obtained by subtracting the mean buckets of each endosulfan-exposed earthworm: (a) 0.5 μg cm−2, (b) 1.0 μg cm−2 and (c) 2.0 μg cm−2 with the mean buckets of the control earthworms. Signals that were significantly different from the control (p < 0.05) were retained while everything else were excluded. Only the major metabolites are labeled for clarity
To understand the metabolite level changes from each exposure concentration, the relative percent change was calculated for each significant metabolite in the earthworm CF (Fig. 6) and tissue extract (Fig. 7). In the earthworm CF, spermidine decreased to significant levels at the lowest and highest endosulfan concentrations (0.5 and 2.0 μg cm−2). Spermidine is part of a class of aliphatic nitrogenous bases called polyamines which are found in all organisms (Gerner and Meyskens 2004). Polyamines have gained popularity in the last 15 years due to their role in gene regulation (Montañez et al. 2007). In addition, polyamines are involved in cell growth and differentiation processes which mediate cell apoptosis (Monti et al. 2004; Seiler and Raul 2005). Apoptosis is a gene-controlled process of cellular destruction where the apoptotic cell is digested by phagocytes (Monti et al. 2004). As DNA or other critical components are damaged in organisms, death programs in affected cells are activated in order to prevent further insult (Seiler and Raul 2005). Studies have shown that the decrease in polyamines can cause apoptosis activation in organisms especially spermidine (Monti et al. 2004; Gerner and Meyskens 2004). Putrescine, a precursor metabolite to spermidine and another polyamine in the earthworm CF, decreased consistently in all three endosulfan concentrations (~14 %) but was not significant compared to the control (data not shown). Polyamines are known to influence processes in carcinogenesis (Gerner and Meyskens 2004). Cells with elevated levels of polyamines express higher cell proliferation, decreased apoptosis and expressions of genes that allow tumor invasion and metastasis (Gerner and Meyskens 2004). On the contrary, with decreased polyamine levels, higher apoptosis will be exhibited with less gene expression for tumor formation (Ignatenko et al. 2004). In a recent study (Liu et al. 2009), comet assays were conducted on E. fetida after sub-lethal exposure to endosulfan in soil and detected significant DNA damage (p < 0.01) from both concentration and length of exposure. This study concluded that the early detection of DNA damage by comet assays could be an early detection for endosulfan exposure in earthworms. In our study, the decrease of spermidine in the earthworm CF could be an important biological indicator of a protective response from the earthworm to increase apoptosis due to the genotoxic potential by endosulfan.
Percent change (%) of identified metabolites from the t test filtered 1D NMR difference spectra of endosulfan-exposed E. fetida tissue extracts. Percent changes that were significantly different from the control (p < 0.05) were labelled with “*”. Each percent change is shown with their associated standard error
Amino acids, alanine and glycine, were increased to significant levels at the highest endosulfan concentration (2.0 μg cm−2) compared to the controls in the earthworm CF. Alanine was also identified in the earthworm tissue extract and similar to the earthworm CF, had the same relative percent change. Alanine and glycine are both universal stress indicators and have been known to provide a cytoprotective action against stress damage (Nissim et al. 1992). The increase in alanine and glycine in separate studies have been found to increase the gene expression for stress protein synthesis in organisms (Forcella et al. 2007; Howard et al. 2010). This significant increase in both of these amino acids could elicit a defensive mechanism from the exposure to endosulfan. Other amino acids, glutamine and glutamate were detected in the earthworm tissue extract at 2 sub-lethal concentrations (0.5 and 1.0 μg cm−2) and confirms our previous endosulfan exposure results (Yuk et al. 2011). Both of these amino acids are crucial in the excitatory and inhibitory synapses. Glutamine is the precursor for the synthesis of γ-aminobutyric acid (GABA), a inhibitory neurotransmitter and also for glutamate which is the excitatory neurotransmitter (Behar and Rothman 2001). Endosulfan is a neurotoxin and is known to inhibit the GABA-gated chloride channels which antagonize the action of GABA (Jia and Misra 2007). This inhibition by endosulfan causes a partial repolarization of the neuron which leads to involuntary muscle contractions and convulsions (Stanley et al. 2009). The end result can potentially cause the earthworms to be unable to regulate the flux of neurotransmitters, causing a large increase of these metabolites in their system.
The increased muscle activity due to the neurotoxic response from endosulfan will cause a large increase in the earthworm’s energy expenditure. The disaccharides, maltose and melibiose detected in the earthworm tissue extract, were decreased after endosulfan exposure with maltose decreasing significantly at the highest concentration (2.0 μg cm−2) and melibiose decreasing significantly at the 2 highest concentrations (1.0 and 2.0 μg cm−2). The large decrease in disaccharides will increase the production of glucose by glucogenolysis to increase glycolysis. The breakdown of glucose to pyruvate by glycolysis would then be linked to the citric acid cycle (CAC), which is the main energy transfer cycle for energy production in living organisms (Veech 2004). The CAC intermediate, fumarate identified in the earthworm tissue extract, had a significant decrease at the highest endosulfan concentration (2.0 μg cm−2). The increase in CAC activity is further confirmed in the earthworm CF as other major (CAC) intermediates, malate, α-ketoglutarate and succinate were all decreasing to significant levels after endosulfan exposure. The heightened activity in glycogenolysis, glycolysis and CAC would be used to increase ATP production which was confirmed in the earthworm tissue extract with significant increases at the two highest concentrations (1.0 and 2.0 μg cm−2). Lactate detected in both the earthworm CF and tissue extract had similar increases in all endosulfan exposure concentrations. Lactate which is the byproduct from anaerobic metabolism from pyruvate, is an additional energy pathway when the muscle activity in an organism is beyond steady state and O2 levels are not enough for oxidation phosphorylation after CAC (Robergs et al. 2004). The increase of lactate in the earthworm is an indication for a greater dependency on ATP production by the toxic response to endosulfan where the overactivity in the aerobic energy cycles such as CAC and anaerobic pathways such as glycolysis (Robergs et al. 2004) are insufficient.
Betaine and myo-inositol, identified in the earthworm CF, were both significantly increased after endosulfan exposure. Betaine and myo-inositol are well known osmolytes to regulate cell volume due to the loss of inorganic ions and organic solutes (Strange et al. 1991). Exposure to high salinity or hydrophobic contaminants could cause an imbalance in the intracellular solute content or extracellular osmolality (Nelson et al. 1990; Yancey et al. 1982). Past studies have reported the binding of hydrophobic contaminants such as PAHS to biological membranes which can decrease the stability and alter the fluidity of molecules across the membrane (Nelson 1987; Nelson et al. 1990). Even though the increase or decrease of any organic solute can bring a cell to osmotic balance, only a set of organic osmolytes such as betaine and myo-inositol are utilized in biological systems (Strange et al. 1991; Yancey et al. 1982). In addition, studies have shown that the increase in osmolytes in the cell during external stress does not affect the cellular architecture and function (Somero 1986; Yancey et al. 1982). Endosulfan is considered a hydrophobic compound due to its high log n-octanol/water partition coefficient (Kow) of 4.94 (Weber et al. 2010) and log organic carbon adsorption coefficient (Koc) of 3.6 (Weber et al. 2010). The exposure of endosulfan could potentially disrupt the fluidity of the cell membrane and the increase in betaine and myo-inositol help maintain osmotic balance in the earthworm.
Conclusions
Our study demonstrates the potential of using the earthworm’s CF and tissue extract in combination to further our understanding of the sub-lethal exposure to endosulfan. The 1H and 1H–13C HSQC NMR spectrum of the earthworm CF displayed a unique metabolic profile compared to the tissue extract. The absence of sugars (maltose and melibiose) in the earthworm CF decreased the signal overlap in the chemical shift region (3.0–4.5 ppm) and along with the different chemical composition of CF compared to tissue extracts, allowed a range of unique endogenous metabolites to be identified. The different metabolic profile of the earthworm CF allowed a new perspective on the earthworm’s response to endosulfan. The earthworm CF was as sensitive to endosulfan exposure as the earthworm tissue extract as significant separation between the exposed and control was seen using PCA multivariate analysis in the highest two sub-lethal endosulfan exposures. The t test filtered difference NMR spectrum of the earthworm CF and tissue extract detected a plethora of metabolites that had significant fluctuations compared to the controls. The decrease of spermidine in the earthworm CF explains an apoptotic response after endosulfan exposure due to its potential genotoxicity and can be an important biological indicator for DNA damage. The decrease in CAC intermediates, α-ketoglutarate, malate and succinate in the earthworm’s CF and fumarate in the tissue extract enabled a clearer depiction of the high activity of the CAC for the increase production of ATP. The binding of cell membranes by endosulfan could potentially occur as increases in osmolytes, betaine and myo-inositol in the earthworm CF were detected. Alanine and lactate were both identified in the earthworm CF and tissue extract and their similar increases in both biological mediums deter any differences in their biochemical response. Even though the earthworm tissue extract is commonly used as the main biological medium for earthworm metabolomic studies (Brown et al. 2010; Jones et al. 2008; McKelvie et al. 2009, 2010), this study demonstrates the potential of using both the earthworm CF and tissue extract to describe a more complete picture of the subtle responses to environmental contaminants. Contact tests were used in this study as an initial basis for method development and direct analysis of pesticide exposure. Future earthworm metabolomic experiments using both biological mediums will transition to soil environments to understand other important factors such as ingestion and pesticide–soil interactions.
References
Ayres RU, Ayres LW (1999) The life-cycle of chlorine, Part IV: accounting for persistent cyclic organo-chlorines. J Ind Ecol 3(2–3):121–159
Barthe M, Pelletier I (2007) Comparing bulk extraction methods for chemically available polycyclic aromatic hydrocarbons with bioaccumulation in worms. Environ Chem 4(4):271–283
Behar KL, Rothman DL (2001) In vivo nuclear magnetic resonance studies of glutamate-γaminobutyric acid-glutamine cycling in rodent and human cortex: the central role of glutamine. J Nutr 131(9 Suppl.):2498S–2504S
Broadhurst DI, Kell DB (2006) Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2(4):171–196
Brown SAE, Simpson AJ, Simpson MJ (2008) Evaluation of sample preparation methods for nuclear magnetic resonance metabolic profiling studies with Eisenia fetida. Environ Toxicol Chem 27(4):828–836
Brown SAE, Simpson AJ, Simpson MJ (2009) 1H NMR metabolomics of earthworm responses to sub-lethal PAH exposure. Environ Chem 6(5):432–440
Brown SAE, McKelvie JR, Simpson AJ, Simpson MJ (2010) 1H NMR metabolomics of earthworm exposure to sub-lethal concentrations of phenanthrene in soil. Environ Pollut 158(6):2117–2123
Bundy JG, Osborn D, Weeks JM, Lindon JC, Nicholson JK (2001) An NMR-based metabonomic approach to the investigation of coelomic fluid biochemistry in earthworms under toxic stress. FEBS Lett 500(1–2):31–35
Bundy JG, Spurgeon DJ, Svendsen C, Hankard PK, Osborn D, Lindon JC, Nicholson JK (2002) Earthworm species of the genus Eisenia can be phenotypically differentiated by metabolic profiling. FEBS Lett 521:115–120
Bundy JG, Spurgeon DJ, Svendsen C, Hankard PK, Warne MA, Osborn D, Lindon JC, Nicholson JK (2004) Environmental metabonomics: applying combination biomarker analysis in earthworms at a metal contaminated site. Ecotoxicology 13:797–806
Bundy JG, Keun HC, Sidhu JK, Spurgeon DJ, Svendsen C, Kille P, Morgan AJ (2007) Metabolic profile biomarkers of metal contamination in a sentinel terrestrial species are applicable across multiple sites. Environ Sci Technol 41(12):4458–4464
Bundy JG, Sidhu JK, Rana F, Spurgeon DJ, Svendsen C, Wren JF, Stürzenbaum SR, Morgan AJ, Kille P (2008) ‘Systems toxicology’ approach identifies coordinated metabolic responses to copper in a terrestrial non-model invertebrate, the earthworm Lumbricus rubellus. BMC Biol 6(25):1–21
Bundy JG, Davey MP, Viant MR (2009) Environmental metabolomics: a critical review and future perspectives. Metabolomics 5(1):3–21
Ekman DR, Teng Q, Villeneuve DL, Kahl MD, Jensen KM, Durhan EJ, Ankley GT, Collette TW (2008) Investigating compensation and recovery of fathead minnow (Pimephales promelas) exposed to 17α-ethynylestradiol with metabolite profiling. Environ Sci Technol 42(11):4188–4194
Ekman DR, Teng Q, Villeneuve DL, Kahl MD, Jensen KM, Durhan EJ, Ankley GT, Collette TW (2009) Profiling lipid metabolites yields unique information on sex- and time-dependent responses of fathead minnows (Pimephales promelas) exposed to 17α-ethynylestradiol. Metabolomics 5(1):22–32
Forcella M, Berra E, Giacchini R, Rossaro B, Parenti P (2007) Increased alanine concentration is associated with exposure to fenitrothion but not carbamates in Chironomus riparius larvae. Ecotoxicol Environ Saf 66(3):326–334
Gerner EW, Meyskens FL Jr (2004) Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4(10):781–792
Heimbach F (1988) A comparison of laboratory methods for toxicity testing with earthworms. In: Edwards CA, Neuhauser EF (eds) Earthworms in waste and environmental management. SPB Academic Publ, The Hague, pp 329–335
Hendawi M, Sauve S, Ashour M, Brousseau P, Fournier M (2004) A new ultrasound protocol for extrusion of coelomocyte cells from the earthworm Eisenia fetida. Ecotoxicol Environ Saf 59(1):17–22
Homa J, Bzowska M, Klimek M, Plytycz B (2008) Flow cytometric quantification of proliferating coelomocytes non-invasively retrieved from the earthworm, Dendrobaena veneta. Dev Comp Immunol 32(1):9–14
Howard A, Tahir I, Javed S, Waring SM, Ford D, Hirst BH (2010) Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J Physiol 588(6):995–1009
Ignatenko NA, Zhang H, Watts GS, Skovan BA, Stringer DE, Gerner EW (2004) The chemopreventive agent α-difluoromethylornithine blocks Ki-ras-dependent tumor formation and specific gene expression in Caco-2 cells. Mol Carcinog 39(4):221–233
Jia Z, Misra HP (2007) Developmental exposure to pesticides zineb and/or endosulfan renders the nigrostriatal dopamine system more susceptible to these environmental chemicals later in life. Neurotoxicology 28(4):727–735
Jones OAH, Spurgeon DJ, Svendsen C, Griffin JL (2008) A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus. Chemosphere 71(3):601–609
Kauschke E, Mohrig W, Cooper EL (2007) Coelomic fluid proteins as basic components of innate immunity in earthworms. Eur J Soil Biol 43:S110–S115
Kroeger M (2006) How omics technologies can contribute to the ‘3R’ principles by introducing new strategies in animal testing. Trends Biotechnol 24(8):343–346
Kurek A, Homa J, Kauschke E, Plytycz B Characteristic of coelomocytes of the stubby earthworm, Allolobophora chlorotica (Sav.). In: 8th International symposium on earthworm ecology (ISEE8), Cracow, Poland, 4–9 Sep 2006. Elsevier France-Editions Scientifiques Medicales Elsevier, Paris, pp S121–S126
Lankadurai BP, Wolfe DM, Simpson AJ, Simpson MJ (2011a) 1H NMR-based metabolomic observation of a two-phased toxic mode of action in Eisenia fetida after sub-lethal phenanthrene exposure. Environ Chem 8(2):105–114
Lankadurai BP, Wolfe DM, Simpson AJ, Simpson MJ (2011b) 1H NMR-based metabolomics of time-dependent responses of Eisenia fetida to sub-lethal phenanthrene exposure. Environ Pollut 159(10):2845–2851
Lenz EM, Weeks JM, Lindon JC, Osborn D, Nicholson JK (2005) Qualitative high field 1H-NMR spectroscopy for the characterization of endogenous metabolites in earthworms with biochemical biomarker potential. Metabolomics 1(2):123–136
Liu W, Zhu LS, Wang J, Wang JH, Xie H, Song Y (2009) Assessment of the genotoxicity of endosulfan in earthworm and white clover plants using the comet assay. Arch Environ Contam Toxicol 56(4):742–746
McKelvie J, Yuk J, Xu Y, Simpson A, Simpson M (2009) 1H NMR and GC/MS metabolomics of earthworm responses to sub-lethal DDT and endosulfan exposure. Metabolomics 5(1):84–94
McKelvie JR, Wolfe DM, Celejewski M, Simpson AJ, Simpson MJ (2010) Correlations of Eisenia fetida metabolic responses to extractable phenanthrene concentrations through time. Environ Pollut 158(6):2150–2157
McKelvie JR, Wolfe DM, Celejewski MA, Alaee M, Simpson AJ, Simpson MJ (2011) Metabolic responses of Eisenia fetida after sub-lethal exposure to organic contaminants with different toxic modes of action. Environ Pollut 159(12):3620–3626
Montañez R, Sánchez-Jiménez F, Aldana-Montes JF, Medina MÁ (2007) Polyamines: metabolism to systems biology and beyond. Amino Acids 33(2):283–289
Monti MG, Ghiaroni S, Marverti G, Montanari M, Moruzzi MS (2004) Polyamine depletion switches the form of 2-deoxy-d-ribose-induced cell death from apoptosis to necrosis in HL-60 cells. Int J Biochem Cell Biol 36(7):1238–1248
Moore MN (2002) Biocomplexity: the post-genome challenge in ecotoxicology. Aquat Toxicol 59(1–2):1–15
Nelson A (1987) Penetration of mercury-adsorbed phospholipid monolayers by polynuclear aromatic hydrocarbons. Anal Chim Acta 194(C):139–149
Nelson A, Auffret N, Borlakoglu J (1990) Interaction of hydrophobic organic compounds with mercury adsorbed dioleoylphosphatidylcholine monolayers. Biochim Biophys Acta Biomembr 2:205–216
Nissim I, Hardy M, Pleasure J, States B (1992) A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int 42(3):775–782
OECD (1984) OECD guidelines for testing of chemicals: earthworm, acute toxicity test. OECD Guidel 207:15
Paoletti MG (1999) The role of earthworms for assessment of sustainability and as bioindicators. Agric Ecosyst Environ 74(1–3):137–155
Parsons HM, Ekman DR, Collette TW, Viant MR (2009) Spectral relative standard deviation: a practical benchmark in metabolomics. Analyst 134(3):478–485
Presley ML, McElroy TC, Diehl WJ (1996) Soil moisture and temperature interact to affect growth, survivorship, fecundity, and fitness in the earthworm Eisenia fetida. Comp Biochem Physiol A 114(4):319–326
Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287(3):R502–R516
Rochfort SJ, Ezernieks V, Yen AL (2009) NMR-based metabolomics using earthworms as potential indicators for soil health. Metabolomics 5(1):95–107
Saint-Denis M, Narbonne JF, Arnaud C, Thybaud E, Ribera D (1999) Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of benzo(a)pyrene. Soil Biol Biochem 31(13):1837–1846
Sanchez-Hernandez J (2006) Earthworm biomarkers in ecological risk assessment. In: Ware GW, Whitacre DM, Albert LA et al (eds) Reviews of environmental contamination and toxicology, vol 188. Reviews of environmental contamination and toxicology. Springer, New York, pp 85–126
Scheu S, Schlitt N, Tiunov AV, Newington JE, Jones TH (2002) Effects of the presence and community composition of earthworms on microbial community functioning. Oecologia 133(2):254–260
Seiler N, Raul F (2005) Polyamines and apoptosis. J Cell Mol Med 9(3):623–642
Siddique T, Okeke BC, Arshad M, Frankenberger WT Jr (2003) Enrichment and isolation of endosulfan-degrading microorganisms. J Environ Qual 32(1):47–54
Simpson AJ, Brown SA (2005) Purge NMR: effective and easy solvent suppression. J Magn Reson 175(2):340–346
Simpson MJ, McKelvie JR (2009) Environmental metabolomics: new insights into earthworm ecotoxicity and contaminant bioavailability in soil. Anal Bioanal Chem 394(1):137–149
Somero GN (1986) Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol 251(2 Pt 2):R197–R213
Stanley KA, Curtis LR, Massey Simonich SL, Tanguay RL (2009) Endosulfan I and endosulfan sulfate disrupts zebrafish embryonic development. Aquat Toxicol 95(4):355–361
Strange K, Morrison R, Heilig CW, DiPietro S, Gullans SR (1991) Upregulation of inositol transport mediates inositol accumulation in hyperosmolar brain cells. Am J Physiol-Cell Ph 260(4):C784–C790
Veech RL (2004) Tricarboxylic acid cycle. In: William JL, Lane MD (eds) Encyclopedia of biological chemistry. Elsevier, New York, pp 256–262
Verouden MPH, Westerhuis JA, van der Werf MJ, Smilde AK (2009) Exploring the analysis of structured metabolomics data. Chemom Intell Lab Syst 98(1):88–96
Weber J, Halsall CJ, Muir D, Teixeira C, Small J, Solomon K, Hermanson M, Hung H, Bidleman T (2010) Endosulfan, a global pesticide: a review of its fate in the environment and occurrence in the Arctic. Sci Total Environ 408(15):2966–2984
Weckwerth W (2007) Metabolomics: methods and protocols. Humana Press Inc., Totowa
Whitfield Åslund M, Simpson A, Simpson M (2011) 1H NMR metabolomics of earthworm responses to polychlorinated biphenyl (PCB) exposure in soil. Ecotoxicology 20(4):836–846
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217(4566):1214–1222
Yuk J, McKelvie JR, Simpson MJ, Spraul M, Simpson AJ (2010) Comparison of 1-D and 2-D NMR techniques for screening earthworm responses to sub-lethal endosulfan exposure. Environ Chem 7(6):524–536
Yuk J, Simpson MJ, Simpson AJ (2011) 1-D and 2-D NMR metabolomics of earthworm responses to sub-lethal trifluralin and endosulfan exposure. Environ Chem 8(3):281–294
Acknowledgment
Funding was provided by the Natural Sciences and Engineering Research Council Strategic Grants Program (NSERC). André Simpson would like to thank the government of Ontario for an Early Researcher Award. We would also like to extend to Dr. Melissa Whitfield Åslund and Brian Lankadurai for technical assistance and valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuk, J., Simpson, M.J. & Simpson, A.J. Coelomic fluid: a complimentary biological medium to assess sub-lethal endosulfan exposure using 1H NMR-based earthworm metabolomics. Ecotoxicology 21, 1301–1313 (2012). https://doi.org/10.1007/s10646-012-0884-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-012-0884-5