Abstract
In the present study, embryotoxicity experiments using the sea urchin Lytechinus variegatus were carried out to better clarify the ecotoxicological effects of tributyltin (TBT) and triphenyltin (TPT) (the recently banned antifouling agents), and Irgarol and Diuron (two of the new commonly used booster biocides). Organisms were individually examined to evaluate the intensity and type of effects on embryo-larval development, this procedure has not been commonly used, however it showed to be a potentially suitable approach for toxicity assessment. NOEC and LOEC were similar for compounds of same chemical class, and IC10 values were very close and showed overlapping of confidence intervals between TBT and TPT, and between Diuron and Irgarol. In addition, IC10 were similar to NOEC values. Regardless of this, the observed effects were different. Embryo development was interrupted at the gastrula and blastula stages at 1.25 and 2.5 μg l−1 of TBT, respectively, whereas pluteus stage was reached with the corresponding concentrations of TPT. Furthermore, embryos reached the prism and morula stages at 5 μg l−1 of TPT and TBT, respectively. The effects induced by Irgarol were also more pronounced than those caused by Diuron. Pluteus stage was always reached at any tested Diuron concentration, while embryogenesis was interrupted at blastula/gastrula stages at the highest concentrations of Irgarol. Therefore, this study proposes a complementary approach for interpreting embryo-larval responses that may be employed together with the traditional way of analysis. Consequently, this application leads to a more powerful ecotoxicological assessment tool focused on embryotoxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antifouling compounds are incorporated into marine paints in order to prevent the settlement and growth of marine organisms on submerged structures. These compounds are particularly important for the naval industry, since the biological settlement onto the hull surfaces impaires fuel efficiency as well as decreases time between each maintenance servicing. Various antifouling strategies have been used to prevent fouling organisms and the most effective were the organotins-based (OTs) paints, such as tributyltin (TBT), which was the principal antifouling compound used for the last four decades, and triphenyltin (TPT) (used mainly in combination with TBT). However, TPT has been mainly used in agriculture as a fungicide in crop protection. Unfortunately, OTs affect adversely the environment, and even extremely low concentrations (ng l−1) can cause sublethal effects (such as poor grow rates and low reproductive success) in a wide range of marine organisms (Alzieu 2000,1998).
As a consequence of OTs environmental impact, International Maritime Organization (IMO) has banned new applications of OTs-based antifouling paints from 2003 and, in January 2008, these coats were worldwide banned from vessels (IMO 2008). Consequently, many new antifouling paints without OTs have been developed. Organotin-free antifouling paints are composed of seawater-soluble matrices containing biologically active ingredients, such as copper and/or organic biocides (Voulvoulis et al. 1999). The copper component alone, in fact, is not effective against diatoms and algae, which are particularly relevant organisms, since they form the initial biofilm and, consequently, promote the ecological succession of biofouling. Therefore, secondary biocides must be incorporated into the paints (Chambers et al. 2006). These compounds are known as ‘‘organic booster biocides’’ and some of them were first developed for agricultural use. Two of the most commonly used organic boosters were Irgarol 1051 (2-methylthio-4-t-butylamino-6-cyclopropylamino-s-triazine) and Diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea) (Mochida and Fujii 2009).
Although a great research effort has been carried out on the study of Irgarol 1051 and Diuron characteristics, little is known about their toxicity and action mechanisms on marine invertebrates so far (Downs and Downs 2007). The introduction of these and other ‘new’ compounds in the antifouling paints obviously generates the necessity to investigate any possible adverse effect on the marine ecosystem. In addition, while the effects of TBT have been largely studied during the last decades (Evans et al. 2000) and received widespread attention, there are few ecotoxicological studies involving TPT, especially effects towards marine invertebrates. Since OTs are considered persistent pollutants in the coastal environment (for a review see Langston et al. 2009), ongoing and widespread monitoring is justified to evaluate the efficacy of the global ban on organotin-based paints, and to appraise their eventual depuration of the marine environment.
Although the majority of standardized toxicity-testing with marine invertebrate run on adult organisms, embryos and larvae are several orders of magnitude more sensitive to toxicants (His et al. 1999; Pinsino et al. 2010; Ringwood 1992). Therefore, embryos of echinoderms have been widely used to evaluate the toxicity of marine pollutants and their high sensitivity make such bioassays a reliable and appropriate component of environmental risk assessment studies (Bellas 2008).
In Brazil, the sea urchin Lytechinus variegatus (Lamarck, 1816) is among the most used test-organism in ecotoxicological studies, presenting standardized protocols for bioassays with gametes and embryos (ABNT 2006; CETESB 1999) which were adapted from international procedures (ASTM 2004; Environment Canada 1997; USEPA 2002). This species is fertile throughout the year and occurs in shallow waters from North Carolina (USA), throughout the Caribbean and Gulf of Mexico, up to Santa Catarina (Southern Brazil) (Serafy 1973), living on rock, shell-hash, sand, and hard bottoms (Watts et al. 2001). L. variegatus is an ecologically important grazer of algae and sea grasses (Valentine and Heck 1991). Since moderate grazing can stimulate vegetal growth, shoot production (Valentine and Duffy 2006) and nutrient recycling (Drifmeyer 1981; Koike et al. 1987), L. variegatus is important to the energy transference between trophic levels and, therefore, considered as an integral part of ecosystem dynamics (Valentine and Duffy, 2006).
Since L. variegatus is a sensitive species, which inhabits coastal environments potentially contaminated by antifouling chemicals, it may be used as a relevant model to evaluate their chronic adverse effects. Therefore, the purpose of the present study was to determine and compare the chronic toxicity of the recently banned antifouling compounds (TBT and TPT) and two of the new commonly used booster biocides (Irgarol 1051 and Diuron) using sea urchin L. variegatus embryo-larval bioassay. Furthermore, in order to evaluate the efficiency of the standard testing in detecting and comparing the toxicity levels and better understand the intensity of effects, the differences in concentration–response levels at each exposure concentration were further analyzed during the early embryonic developmental stages.
Materials and methods
Controls
Seawater used for the dilution of test solutions and controls was sampled in an uncontaminated area at São Sebastião, located in the northern coast of São Paulo (southeastern Brazil). This area was frequently used in the laboratory for ecotoxicological tests. A sufficient amount of seawater was then filtered using cellulose acetate filter (0.45 μm, Millipore). The controls consisted of an untreated negative control (natural filtered seawater) and a solvent control with equivalent volumes of acetone at a final concentration of 0.05% (that is, the higher one in the test solutions).
Test solutions
Stock solutions were prepared by dissolving analytical grade organotins (TBT and TPT; purity >98%) and herbicides (Residue Analysis Pentanal standards of Irgarol; purity >98% and Diuron; purity >99,5%) in acetone. All standards were purchased from Sigma–Aldrich. As acetone was used to prepare the stock solutions, its toxicity on the embryonic development of L. variegatus was previously investigated. The maximum concentration of acetone to dilute the compounds was 0.05%, which was below the toxicity threshold for the species. The stock solutions were chemically analyzed before the toxicity test for confirmation of analytes concentrations. The analytical measurement was performed using gas chromatography/mass spectrometry (GC/MS). The test solutions were prepared by diluting the stock solutions in natural filtered seawater and were based on the concentrations measured by GC/MS. Test concentrations of 0.01, 0.05, 0.25, 0.5, 1.25, 2.5 and 5 μg l−1 were used for TBT and TPT, whereas test concentrations of 125, 250, 500, 1000, 2000, 4000 and 8000 μg l−1 were used for Irgarol and Diuron. These concentrations were chosen based on the literature where these compounds were tested using other species, and these ranges were also confirmed by preliminary tests.
Biological material
Adult L. variegatus individuals were collected by snorkeling from a rocky reef situated at Ilha das Palmas, central coast of São Paulo (southeastern Brazil). Animals were transported to the laboratory in a portable icebox, where they were acclimated for at least 24 h in tanks containing natural seawater, at 25 ± 2 ºC (salinity 35, pH 8 ± 0.2). Sea urchins were fed with the macroalga Ulva lactuca obtained at their collection site.
Gametes were harvested according to Nipper et al. (1993).The sea urchins were induced to spawn by injecting 3 ml of 0.5 M KCl into the coelomic cavity. Eggs were collected in 400 ml beakers containing dilution water, whereas sperm was collected with an automatic pipette, transferred to a small glass flask, and kept on ice until the fertilization procedure. Each eggs batch was observed under a microscope and those presenting abnormalities were discarded. The selected eggs batches were then filtered through nylon cheesecloth (∅ = 350 μm) to remove debris, and pooled together in a glass beaker containing 600 ml seawater. Pooled sperm and eggs from at least three males and three females, respectively, were used.
Fertilization was promoted by the addition of 1 ml of sperm into the beaker containing the eggs. A subsample of the egg solution was diluted 100 times and after ten minutes counted under a microscope in a Sedgwick–Rafter chamber to ensure that 90–100% of the eggs were fertilized. The solution amount was calculated in order to obtain 300 fertilized eggs and this volume was added to the test tubes containing 10 ml of exposure solution (30 eggs per ml). Four replicates for each treatment were prepared. The test was finished after 24 h by adding 5 drops of formalin, when the control embryos should have reached the pluteus stage. Each experiment was performed two times for TBT and three times for TPT, Irgarol and Diuron.
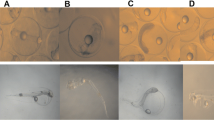
Developmental abnormalities were determined in each replicate by direct observation of 100 individuals, randomly chosen. Normal plutei were considered when the digestive tract is complete and skeleton developed is clearly different from the previous stage such as symmetry, shape and size (Fig. 1). Toxicity of the sample is indicated by a reduction in the number of normal larvae compared with the controls. Tests were accepted if the percent of normal larvae in negative controls were ≥80%. Additionally, in order to provide additional information on the action mechanisms related to the toxicity of the tested compounds, the predominance of developmental stages and abnormalities present in each sample were qualitatively observed. Such types of effect were classified as: (I) retarded pluteus (= young pluteus), when the formation of larval structure such as shape and symmetry was the same as normal, but markedly reduced with respect to their skeletal size (<1/2 N); (II) malformed plutei, appearing mainly affected by skeletal-radialized abnormalities; (III) prisms, embryos showing a transitional stage in which larval differentiation begins; (IV) and the early embryonic stages; (IVa) morula (globular mass of cells); (IVb) blastula, when the outer cells of the morula become more defined (form the blastoderm); and (IVc) gastrula, observed as a hollow cup-shaped structure having three layers of cells. The stages listed above are presented in Fig. 1.
Statistical analyses
The linear interpolation method (Norberg-King 1993) was used to calculate the inhibitory concentration to 50 and 10% of the test organisms (IC50 and IC10). The bootstrap method was used to estimate the 95% confidence interval, because standard statistical methods for calculating confidence intervals are not applicable. The IC50/IC10 and the 95% confidence interval were calculated using the inhibition concentration procedure (ICp) (USEPA 2002). Comparisons among experiments of each compound and between the compounds of same chemical class were tested for significance using the multiple comparison Dunnett’s test.
Prior to the hypothesis tests, Shapiro–Wilks test for normality and Hartley test for homogeneity of variance were used. The raw data were frequently found not to be normally distributed and were therefore arcsine-transformed to achieve normality. Analysis of variance (ANOVA) was applied to each individual test to detect significant differences on effects into the treatments (comparisons among the control group and each of the experimental concentration), and significance level was always set at α = 0.05. Afterwards, the post hoc Dunnett’s test was applied to estimate the NOEC (no observed effect concentration) and LOEC (lowest observed effect concentration).
In order to allow a comparison among the toxicities of different compounds, the results obtained from the exposure experiments (two for TBT and three for TPT, Irgarol and Diuron) were tested using ANOVA followed by the Tukey’s multiple comparison and showed no significant differences. Therefore, the IC50, IC10 and NOEC/LOEC were calculated for each compound considering the replicates of the all experiments together.
Results
Normal pluteus frequency was approximately 90 ± 3.5% in the negative control and 87 ± 2.8% in the solvent control (0.05% acetone). No significant difference (P > 0.05) was observed between the controls, therefore, they were grouped into a single control group.
In general, OTs were more toxic than herbicides and the estimated endpoints were similar for compounds of same chemical class. For both TBT and TPT, the respective NOECs and LOECs were 0.25 and 0.5 μg l−1, respectively. However, when the inhibition concentrations (particularly the IC50) were considered, TBT showed itself as more toxic that TPT; its IC50 was 0.36 (0.33–0.38) μg l1 and IC10 was 0.23 (0.12–0.27) μg l−1. It must be highlighted that at the LOEC (i.e., 0.5 μg l−1) 100% abnormal larvae were observed (Fig. 2a). This concentration was also the highest concentration at which embryos reached the pluteus stage (retarded pluteus). Besides, there is an apparent discrepancy between the IC50 and LOEC, which occurred because the effects raised from non significant to 100% between 0.25 to 0.5 μg l−1, creating a mathematical artifact similar to that previously observed by Mastroti et al. (2001) when evaluating the effects of detergents in L. variegatus. A similar discrepancy was observed for TBT, when IC10 and NOEC were compared. For the TPT, the estimated IC50 was 0.73 (0.66–0.79) μg l−1 and the IC10 was 0.27 (0.21–0.33) μg l−1 (Fig. 2b).
Concentration–response relationship of L. variegatus larvae (% of normal larvae per replicate normalized against the control) after 24 h exposure to different concentrations of TBT (a), TPT (b), Irgarol (c) and Diuron (d). The IC50 and IC10 values (confidence interval) are also expressed for each analite. Solid circles represent replicates for all treatments, while solid lines indicate fits of the experimental data to a logistic model
In the Diuron treatments, no significant differences (P > 0.05) from the controls were observed up to 1,000 μg l−1 (NOEC value), while the LOEC value was 2,000 μg l−1. The estimated IC50 was 3,754 (3,579–3,955) μg l−1 and the IC10 was 994 (466–1,722) μg l−1 (Fig. 2c). The results for Irgarol were similar to those obtained for Diuron, since the frequency of pluteus stage found up to 1,000 μg l−1 (NOEC value) showed no significant differences (P > 0.05) from the controls. However, at 2,000 μg l−1 (LOEC value), larval development was strongly affected by Irgarol, since 100% of pluteus exhibited a decrease in skeleton size. The estimated IC50 for Irgarol was 1485 (1,463–1,500) μg L−1 and the IC10 was 1,073 (1,037–1,100) μg l−1 (Fig. 2d). Due to the same reason mentioned for TBT, a discrepancy between IC50 and LOEC was also seen for Irgarol.
The effects of antifouling agents on L. variegatus developmental stages are shown as representative photomicrographs (magnification 100–200×) for OTs (Table 1) and herbicides (Table 2). The observed anomalies were uniform for all replicates of each TBT and TPT concentration. This means that embryos in each concentration group showed an specific type of effect (stage of development). For the herbicides, as different types of effects occurred in each concentration, the photomicrographs showed the main anomalies observed at each respective exposure concentration. The approximated frequencies of embryo-larval stages observed at each concentration are summarized in Table 3.
The frequency of developmental stages showed that retarded development increased with TBT concentrations, and 100% of retarded pluteus were observed at 0.5 µg L−1. Embryos development was interrupted at the gastrula and blastula stages at 1.25 and 2.5 µg L−1, respectively. Moreover, only the morula stage was found at 5 μg l−1 (Table 1). Although all replicates also showed 100% of effect at concentrations ≥1.25 μg l−1 of TPT (Fig. 2b), pluteus stage was observed up to 2.5 μg l−1, while at 5 μg l−1 the embryos development was interrupted at prism stage (Table 1).
The frequency of anomalies (e.g. malformed plutei) increased progressively with Diuron concentration, starting from 1,000 μg l−1 up to the maximum effect (100% of anomalies) at 8,000 μg l−1, which was the highest tested concentration for this substance. In spite of that, the pluteus stage was always reached at any tested Diuron concentration (Table 2). The situation was different for Irgarol, where early embryonic stages were seen at 4,000 μg l−1. Although pluteus with skeletal anomalies predominated (~55%), prism (~25%) and gastrula (~20%) stages were also seen at this concentration. At the highest exposure concentration (8,000 μg l−1) embryos never reached the pluteus stage and their development was interrupted at blastula stage (Table 2).
Discussion
Embryotoxicity appeared to be in the following decreasing order: TBT > TPT > Irgarol 1051 > Diuron. Additionally, the qualitative assessment of the types of effects helped to evaluate the intensities and modes of action of antifouling compounds that led to damage in embryos of L. variegatus.
In the present study, the toxic effects of antifouling compounds were preliminarily expressed as NOEC/LOEC and IC50 (50% inhibitory concentration to the test organisms). However, due to current criticisms about traditional hypothesis testing (Crane and Newman 2000; Isnard et al. 2001; Van der Hoeven 2004), IC10 (10% inhibitory concentration to the test organisms) was also estimated in order to find out more realistic values of no observed effect concentrations. Despite that, IC10 values were very similar and overlaps between the 95% confidence intervals were seen for compounds of the same chemical class, i.e. 0.23 (0.12–0.27) and 0.27 (0.21–0.33) μg l−1 for TBT and TPT; 994 (466–1,722) and 1,073 (1,037–1,100) μg l−1 for Diuron and Irgarol, respectively. Interestingly, estimated IC10 values were very close to the NOECs calculated by hypothesis test (0.25 μg l−1 for the OTs and 1,000 μg l−1 for the herbicides).
Although TBT and TPT have showed the same values of NOEC and LOEC, relatively similar IC10 values, and clear concentration–response relationships, the morphological analysis of embryos allowed a clearer comparison of the intensity of effects caused by these two OT compounds, showing that their toxicity cannot be considered as equivalent (Table 1). At equivalent concentrations, embryogenesis in the TBT treatments was delayed at earlier stages than in the TPT (Table 1). Embryos stopped their development at the gastrula and blastula stage at 1.25 and 2.5 μg l−1 of TBT, respectively, while the embryos reached the early pluteus stage in the corresponding concentrations of TPT. In addition, embryos reached the prism stage at 5 μg l−1 of TPT, whereas only the morula stage was observed at the same concentration of TBT (Table 1). Moreover, the effects observed through morphological analysis of embryos suggest that concentrations established for TPT as effect-free could still affect the L. variegatus larval cycle and metamorphosis.
Diuron and Irgarol have also showed a progressive concentration-dependent relationship. Again, although both compounds presented the same values of NOEC and LOEC, and similar IC10 values, different larval development responses were also seen for the same exposure concentrations of Irgarol and Diuron (Table 2). In these cases, effects induced by Irgarol were more pronounced than those caused by Diuron. Retarded pluteus rates were very high for 2,000 μg l−1 of Irgarol, and the developmental anomalies (skeletal system malformations) also increased from this concentration. For Diuron, the concentration–response curve exhibited a progressive reduction in normal development until the final concentration (Fig. 2c). The frequency of embryos with development interrupted at the blastula/gastrula stage achieved 45% at 4,000 μg l−1 of Irgarol and 100% at 8,000 μg l−1, whereas embryos always reached the pluteus stage (although retarded and/or malformed) at any exposure concentration of Diuron (Table 2). Additionally, the first concentration of Irgarol with 100% effect was four times lower than Diuron (2,000 and 8,000 μg l−1, respectively) (Fig. 2c, d).
The differences between the intensity of responses after exposure to both herbicides suggest, further than different toxicities, diverse mechanisms of action, which are well known only for photosynthetic organisms (Fernandez-Alba et al. 2002; Hall et al. 1999; Ranke and Jastorff 2000). These compounds act by blocking electron transport in the photosynthesis system, which animal species do not have. However, there is scarce information about the effects of Irgarol and Diuron to more complex cellular systems.
As seen in the present study, OTs and the new organic booster biocides have in common the ability to disrupt embryonic development of L. variegatus. These new organic booster biocides, however, showed three important differences. First, they were less toxic, requiring concentrations of three orders of magnitude higher to produce the same malformations elicited by OTs. Second, while the OTs showed a single embryonic stage for each exposure concentration, the embryos exposed to Irgarol had two or more embryo-larval stages in a given concentration (embryos always reached the pluteus stage at Diuron treatments, and the effects were more related to morphological abnormalities). Third, Irgarol showed adverse effects on embryos morphology at all stages (from the blastula up to pluteus), whereas TBT appear to affect the structures of embryos only at the earliest stages, centered on blastula and gastrula. It is possible that TBT induced malformations on earliest embryonic stages (gastrula and blastula) of sea urchins due to the absence of an appreciable activity of cytochrome P450 system, which is more effective after the gastrula stage (Bresch and Arendt 1977). However, further studies are required to support such hypothesis.
OTs may also act via other modes of action. Inhibition of mitochondrial oxidative phosphorylation and ATP synthesis, genotoxicity and DNA damage, inhibition of enzymes (such as ATPases) and perturbation of calcium homeostasis are among the key biochemical processes (Fent 1996). Calcium ions, for instance, is a key signaling molecule in many developmental processes, which play a critical role in chemical-induced toxic cell killing and programmed cell death (apoptosis) (Reader et al. 1999). According to Raffray et al. (1993), TBT-induced apoptosis occurs independently of inhibition of protein and ATP synthesis, indicating that disruption of Ca2+ homeostasis is involved. As cited in the literature (Girard et al. 1997; Marin et al. 2000; Moschino and Marin 2002), alteration of calcium signaling may be an important cause of developmental toxicity in sea urchin embryos and larvae. According to Girard et al. (1997), TBT provokes a non-specific increase of plasma membrane permeability in sea urchin eggs, as a result of a distribution of organotin molecules in the membrane phospholipids. Furthermore, they proposed that TBT-induced decrease of the intracellular compartmentalization of Ca2+ could be a mainly related to an inhibition of the ATP-driven Ca2+ pump. Therefore, one way which organotin interrupts embryo development could be by preventing these supplies of Ca2+. Thus, embryonic effects observed in the present study, on both skeletal deposition and developmental interruption, could be due to interference of antifouling compounds on the intracellular calcium homeostasis.
Despite TPT has been used since the early 1960 s in agriculture as a broad-spectrum fungicides to combat fungus diseases (Piver 1973), there are few studies about its toxic mode of action on marine invertebrates. Therefore, the retarded embryo-larval development observed after TPT treatments is not so easy to explain, considering the lack of information available in the literature, which is mainly focused on TBT. Nevertheless, it is hypothesized that the mechanisms of action of TPT are very similar to those of TBT, since these molecules are chemically very similar. According to Moschino and Marin (2002), TPT interacts with calcium homeostasis during embryonic and larval development of Paracentrotus lividus, causing alteration of intracellular Ca2+ levels at low concentrations and inhibiting the ion flow during skeletal deposition. Higher TPT concentrations can slow embryonic development further to the point of total inhibition, which causes death of exposed embryos. Cima et al. (1996) reported that both TBT and TPT can interrupt the embryonic development of the ascidian Styela plicata, with irreversible effects, probably due to inhibition of microtubule polymerization during mitosis. Thus, it seems possible that this compound could also interact with calcium homeostasis in sea urchins.
The results obtained in this study confirm the relative toxicity levels of both TPT and TBT (with TBT being more toxic than TPT), as previously reported for other invertebrates embryos (dema-Hannes and Shenker 2008; Dimitriou et al. 2003; Duft et al. 2003; Zhang et al. 2008). Similar toxic effects have been observed in OTs-exposed embryos of other sea urchin species. Moschino and Marin (2002) exposed fertilized eggs to higher levels of TPT (10 μg l−1), and observed that all embryos were interrupted at the gastrula stage, whereas Marin et al. (2000) exposed embryos of the same species to the same concentration of TBT and observed that all embryos were at the earliest morula stage.
The present results showed that Irgarol and Diuron may pose less environmental damage than TBT and TPT, which agrees with previous studies where environmental risk of new antifouling compounds were considered to be lower than OTs (Ranke and Jastorff 2000, 2002). This order of toxicity to embryos of L. variegatus is consistent with previous toxicity data reported for other sea urchin species. Despite the methodological variability among experiments, Bellas et al. (2005) obtained a similar effect on embryos of P. lividus, showing that 0.4 μg l−1 of TBT caused total inhibition of development after 48 h from eggs fertilization. Larval growth of P. lividus was already significantly affected by 0.01 μg l−1 of TBT (after 48 h from eggs fertilization), and 1 μg l−1 was the maximum concentration that allowed embryos to reach the pluteus stage (Marin et al. 2000), whereas in the present investigation, the highest concentration in which embryos of L. variegatus developed to pluteus stage was 0.5 μg l−1 (Table 2). For the herbicides, Manzo et al. (2006) estimated the toxicities of Irgarol and Diuron to P. lividus embryos and found effective concentrations to 50% exposed organisms (EC50) of 2,390 μg l−1 for Diuron and 990 μg l−1 for Irgarol after 48 h exposure.
Despite recent IMO actions towards the banning of organotin compounds, their environmental levels (and consequent effects) will take some time to be reduced. Contamination levels could even increase at some locations where the legislation is ineffective, or where coatings are still being replaced and residues are contaminating the environment (i.e. paint particles from shipyards and docks) (Kotrikla 2009). These compounds are persistent in the marine environment, especially in sediments, due to slow degradation rates (Hoch 2001). Partitioning to solids is reversible and hence sediments may act either as a permanent sink or a source after remobilization of adsorbed organotins (Cornelis et al. 2006). According to Rüdel (2003), this ‘reservoir’ of organotins is likely to remain biologically relevant, since bioavailability is influenced by environmental conditions such as ion composition of the aqueous environment, pH, dissolved organic carbon content, and presence of competing compounds. In addition, studies have reported that contamination is slowly decreasing in countries where TBT antifouling paints have been regulated (Harino et al. 1999), but in some cases such decrease is not significant (Thomas et al. 2001), or even absent (Becker Van-Slooten and Tarradellas 1994; Harino and Langston 2009; Michel et al. 2001; Takeuchi et al. 2004).
The OTs compounds showed high toxicity to L. variegatus at the contamination levels already seen in many coastal areas (Langston et al. 2009), indicating that this sea urchin species may be threatened at such sites, as well as those species equally or more sensitive to OTs. Conversely, sensitivity of sea urchins to Irgarol and Diuron indicated that concentrations necessary to cause toxicity are higher than the reported environmental levels (reviewed by Konstantinou and Albanis 2004). However, this may not indicate absence of risks since interactions and synergic effects with other contaminants were not evaluated in this paper.
Presumably, populations of L. variegatus chronically exposed to antifouling compounds are likely to suffer reduction in juvenile recruitment. As L. variegatus is an important ecological determinant of community structure and a keystone species in near shore algae and sea grass communities (Watts et al. 2001), antifouling-induced changes in population could have a wide repercussion at community and ecosystem levels. Further studies are needed to examine the impacts of antifouling compounds on development and recruitment in the natural environment, as well as future investigations are necessary to better understand how antifouling agents act in association with other stress factors, and how to improve the exactness in predicting their toxicity.
Conclusions
Experimental data are presented here supporting high levels of toxicity of OTs on early life stages of marine invertebrates. Embryotoxicity of the tested compounds was ranked in the following decreasing order: TBT > TPT > Irgarol 1051 > Diuron. Additionally, the qualitative assessment of types of damage in embryos of L. variegatus allowed a better discrimination between types of effects induced by the tested compounds. Thus, the appraisal on the embryogenesis indicated that, although compounds of same chemical class show similar levels of toxicity (by traditional numerical endpoints), the effects induced by TBT and Irgarol are more pronounced in comparison to TPT and Diuron, respectively. Moreover, abnormalities in embryos exposed to OTs consist of retarded rates of development and interruption of embryogenesis, with morphological anomalies occurring less commonly, whereas Diuron and Irgarol may lead to delay and several types of skeletal abnormalities. This indicated that beyond the toxicity ranking (mentioned above), OTs and the new booster biocides produced different types of effects on the sea urchin embryos. In summary, this study proposes a complementary approach for interpreting embryo-larval responses that may employed together with the traditional analysis based solely on the absence/presence of toxicity.
References
ABNT (2006) Ecotoxicologia aquática-Toxicidade crônica de curta duração—“Método de ensaio com ouriço-do-mar (Echinodermata: Echinoidea)”. Norma ABNT-NBR:15350:2006, Associação Brasileira de Normas Técnicas, Rio de Janeiro, 14 p
Alzieu C (1998) Tributyltin: case study of a chronic contaminant in the coastal environment. Ocean Coast Manag 40(1):23–36
Alzieu C (2000) Impact of tributyltin on marine invertebrates. Ecotoxicology 9(1–2):71–76
ASTM (2004) Standard guide for “Conducting static acute toxicity tests with echinoid embryos”. Standard E1563:1995 (2004), ASTM International, West Conshohocken, PA, 20 p
Becker Van-Slooten K, Tarradellas J (1994) Accumulation, depuration and growth effects of tributyltin in the freshwater bivalve Dreissena polymorpha under field conditions. Environ Toxicol Chem 13:755–762
Bellas J (2008) Prediction and assessment of mixture toxicity of compounds in antifouling paints using the sea-urchin embryo-larval bioassay. Aquat Toxicol 88(4):308–315
Bellas J, Beiras R, Marino-Balsa J, Fernandez N (2005) Toxicity of organic compounds to marine invertebrate embryos and larvae: a comparison between the sea urchin embryogenesis bioassay and alternative test species. Ecotoxicology 14(3):337–353
Bresch H, Arendt U (1977) Influence of different organochlorine pesticides on the development of the sea urchin embryo. Environ Res 13(1):121–128
CETESB (1999) Água do mar—“Teste de toxicidade crônica de curta duração com Lytechinus variegatus, Lamarck, 1816”. Norma Técnica L5.250:1999, Companhia de Tecnologia e Saneamento Ambiental, São Paulo, 20 p
Chambers LD, Stokes KR, Walsh FC, Wood RJK (2006) Modern approaches to marine antifouling coatings. Surf Coat Technol 201(6):3642–3652
Cima F, Ballarin L, Bressa G, Martinucci G, Burighel P (1996) Toxicity of organotin compounds on embryos of a marine invertebrate (Styela plicata; Tunicata). Ecotoxicol Environ Saf 35(2):174–182
Cornelis C, Bierkens J, Joris I, Nielsen P, Pensaert S (2006) Quality criteria for re-use of organotin-containing sediments on land. J Soils Sediments 6(3):156–162
Crane M, Newman MC (2000) What level of effect is a no observed effect? Environ Toxicol Chem 19(2):516–519
dema-Hannes R, Shenker J (2008) Acute lethal and teratogenic effects of tributyltin chloride and copper chloride on mahi mahi (Coryphaena hippurus) eggs and larvae. Environ Toxicol Chem 27(10):2131–2135
Dimitriou P, Castritsi-Catharios J, Miliou H (2003) Acute toxicity effects of tributyltin chloride and triphenyltin chloride on gilthead seabream, Sparus aurata L., embryos. Ecotoxicol Environ Saf 54(1):30–35
Downs C, Downs A (2007) Preliminary examination of short-term cellular toxicological responses of the coral Madracis mirabilis to acute irgarol 1051 exposure. Arch Environ Contam Toxicol 52(1):47–57
Drifmeyer J (1981) Urchin Lytechinus variegatus grazing on Eelgrass, Zostera marina. Estuar Coasts 4(4):374–375
Duft M, Schulte-Oehlmann U, Tillmann M, Markert B, Oehlmann J (2003) Toxicity of triphenyltin and tributyltin to the freshwater mudsnail Potamopyrgus antipodarum in a new sediment biotest. Environ Toxicol Chem 22(1):145–152
Environment Canada (1997) Biological Test Method: “Fertilization assay using echinoids (sea urchin and sand dollars)”. Report EPS1/RM/27:(1992)1997, Ottawa, Ontario, Canada, 97 p
Evans SM, Birchenough AC, Brancato MS (2000) The TBT ban: out of the frying pan into the fire? Mar Pollut Bull 40(3):204–211
Fent K (1996) Ecotoxicology of organotin compounds. Crit Rev Toxicol 26(1):3–117
Fernandez-Alba AR, Hernando MD, Piedra L, Chisti Y (2002) Toxicity evaluation of single and mixed antifouling biocides measured with acute toxicity bioassays. Anal Chim Acta 456(2):303–312
Girard JP, Ferrua C, Pesando D (1997) Effects of tributyltin on Ca2+ homeostasis and mechanisms controlling cell cycling in sea urchin eggs. Aquat Toxicol 38(4):225–239
Hall LW, Giddings JM, Solomon KR, Balcomb R (1999) An ecological risk assessment for the use of Irgarol 1051 as an algaecide for antifoulant paints. Crit Rev Toxicol 29(4):367–437
Harino H, Langston, WJ (2009) Degradation of alternative biocides in the aquatic environment. In: Arai T, Harino H, Ohji M, Langston WJ (eds) Ecotoxicology of antifouling biocides. Tokyo,Japan, pp 397–412
Harino H, Fukushima M, Kawai S (1999) Temporal trends of organotin compounds in the aquatic environment of the Port of Osaka, Japan. Environ Pollut 105:1–7
His E, Heyvang I, Geffard O, De Montaudouin X (1999) A comparison between oyster (Crassostrea gigas) and sea urchin (Paracentrotus lividus) larval bioassays for toxicological studies. Water Res 33(7):1706–1718
Hoch M (2001) Organotin compounds in the environment—an overview. Appl Geochem 16(7–8):719–743
IMO (2008) International convention on the control of harmful anti-fouling systems on ships. http://www.imo.org/conventions/mainframe. Accessed 17 September 2008
Isnard P, Flammarion P, Roman G, Babut M, Bastien P, Bintein S, Essermeant L, Ferard JF, Gallotti-Schmitt S, Saouter E, Saroli M, Thiebaud H, Tomassone R, Vindimian E (2001) Statistical analysis of regulatory ecotoxicity tests. Chemosphere 45(4–5):659–669
Koike I, Mukai H, Nojima S (1987) The role of the sea urchin, Tripneustes gratilla (Linnaeus), in decomposition and nutrient cycling in a tropical seagrass bed. Ecol Res 2(1):19–29
Konstantinou IK, Albanis TA (2004) Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review. Environ Int 30(2):235–248
Kotrikla A (2009) Environmental management aspects for TBT antifouling wastes from the shipyards. J Environ Manage 90:S77–S85
Langston WJ, Harino H, Pope ND (2009) Behaviour of organotins in the coastal environment. In: Arai T, Harino H, Ohji M, Langston WJ (eds) Ecotoxicology of antifouling biocides. Springer, New York, pp 75–94
Manzo S, Buono S, Cremisini C (2006) Toxic effects of irgarol and diuron on sea urchin Paracentrotus lividus early development, fertilization, and offspring quality. Arch Environ Con Tox 51(1):61–68
Marin MG, Moschino V, Cima F, Celli C (2000) Embryotoxicity of butyltin compounds to the sea urchin Paracentrotus lividus. Mar Environ Res 50(1–5):231–235
Mastroti RR, Sousa ECPM, Abessa DMS (2001) Toxicidade de tensoativos aniônicos sobre embriões de ouriço do mar Lytechinus variegatus. In: Moraes R, Crapez M, Pfeiffer W, Farina M, Bainy A, Teixeira V (eds) Efeitos de poluentes sobre organismos marinhos. Arte & Ciência Villipres, São Paulo, SP, pp 207–216
Michel P, Averety B, Andral B, Chiffoleau J, Galgani F (2001) Tributiltin along the coasts of Corsica (Western Mediterranean): a persistent problem. Mar Pollut Bull 42(11):1128–1132
Mochida K, Fujii K (2009) Further effects of alternative biocides on aquatic organisms. In: Arai T, Harino H, Ohji M, Langston WJ (eds) Ecotoxicology of antifouling biocides. Springer, Tokyo,Japan, pp 383–395
Moschino V, Marin MG (2002) Spermiotoxicity and embryotoxicity of triphenyltin in the sea urchin Paracentrotus lividus Lmk. Appl Organometal Chem 16(4):175–181
Nipper MG, Prósperi VA, Zamboni AJ (1993) Toxicity testing with coastal species of southeastern Brazil—echinoderm sperm and embryos. Bull Environ Contam Toxicol 50(5):646–652
Norberg-King TJ (1993) A linear interpolation method for sublethal toxicity: the inhibition concentration (Icp) Approach version 2.0. National Effluent Toxicity Assessment Center, Environmental Research Laboratory, Duluth, MN, Technical Report 63–93
Pinsino A, Matranga V, Trinchella F, Roccheri MC (2010) Sea urchin embryos as an in vivo model for the assessment of manganese toxicity: developmental and stress response effects. Ecotoxicology 19(3):555–562
Piver WT (1973) Organotin compounds: industrial applications and biological investigation. Environ Health Persp 4:61–79
Raffray M, Mccarthy D, Snowden RT, Cohen GM (1993) Apoptosis as a mechanism of tributyltin cytotoxicity to thymocytes: relationship of apoptotic markers to biochemical and cellular effects. Toxicol Appl Pharm 119(1):122–130
Ranke J, Jastorff B (2000) Multidimensional risk analysis of antifouling biocides. Environ Sci Pollut Res 7(2):105–114
Ranke J, Jastorff B (2002) Risk comparison of antifouling biocides. Fresen Environ Bull 11(10A):769–772
Reader S, Moutardier V, Denizeau F (1999) Tributyltin triggers apoptosis in trout hepatocytes: the role of Ca2+, protein kinase C and proteases. Biochim Biophys Acta (BBA)—Mol Cell Res 1448(3):473–485
Ringwood AH (1992) Comparative sensitivity of gametes and early developmental stages of a sea-urchin species (Echinometra mathaei) and a bivalve species (Isognomon californicum) during metal exposures. Arch Environ Con Toxcol 22(3):288–295
Rüdel H (2003) Case study: bioavailability of tin and tin compounds. Ecotoxicol Environ Saf 56(1):180–189
Serafy DK (1973) Variation in the polytypic sea urchin Lytechinus Variegatus (Lamarck, 1816) in the Western Atlantic (Echinodermata; Echinoidea). Bull Mar Sci 23:525–534
Takeuchi I, Takahashi S, Tanabe S, Miyazaki N (2004) Butyltin concentrations along the Japanese coast from 1997 to 1999 monitored by Caprella spp. (Crustacea: Amphipoda). Mar Environ Res 57(5):397–414
Thomas KV, Fileman TW, Readman JW, Waldock MJ (2001) Antifouling paint booster biocides in the UK coastal environment and potential risks of biological effects. Mar Pollut Bull 42(8):677–688
USEPA (2002) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to marine and estuarine organisms. EPA-821-R-02-014, 3rd edition, U.S. Environmental Protection Agency, Office of Water, Washington, DC, p 464
Valentine JF, Duffy JE (2006) The central role of grazing in seagrass ecology. In: Larkum AWD, Orth RJ, Duarte C (eds) Seagrasses: biology, ecology and conservation. Springer, The Netherlands, pp 463–501
Valentine JF, Heck KL (1991) The role of sea urchin grazing in regulating subtropical seagrass meadows: evidence from field manipulations in the northern Gulf of Mexico. J Exp Mar Biol Ecol 154(2):215–230
Van der Hoeven N (2004) Current issues in statistics and models for ecotoxicological risk assessment. Acta Biotheor 52(3):201–217
Voulvoulis N, Scrimshaw MD, Lester JN (1999) Alternative antifouling biocides. Appl Organomet Chem 13(3):135–143
Watts SA, McClintock JB, Lawrence JM (2001) The ecology of Lytechinus variegatus. In: John ML (ed) Developments in aquaculture and fisheries science edible sea urchins: biology and ecology. Elsevier, Amsterdam, pp 375–393
Zhang ZB, Hu JY, Zhen HJ, Wu XQ, Huang C (2008) Reproductive inhibition and transgenerational toxicity of triphenyltin on medaka (Oryzias latipes) at environmentally relevant tip levels. Environ Sci & Technol 42(21):8133–8139
Acknowledgments
The authors would like to thank Petrobras S.A. for the infrastructure built (Research grant No. 4600224067) at CONECO Laboratory (FURG-RS), and to colleagues Ítalo Braga de Castro and Luís Alberto Echenique Dominguez for help in the chemical analyses. F. C. Perina (MSc grant No. 134170/2007-5), D. M. S. Abessa (PQ 303620/2008-0) and G. Fillmann (PQ 311459/2006-4 and 314335/2009-9) were sponsored by CNPq (Brazilian National Research Council).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perina, F.C., de Souza Abessa, D.M., Pinho, G.L.L. et al. Comparative toxicity of antifouling compounds on the development of sea urchin. Ecotoxicology 20, 1870–1880 (2011). https://doi.org/10.1007/s10646-011-0725-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0725-y