Abstract
Irgarol and Diuron are the most representative “organic booster biocides” that replaced organotin compounds in antifouling paints. It cannot be assumed beforehand that their use will have no environmental impact: more ecotoxicological data and a significant environmental monitoring are required. Spermio and embryotoxicities of the biocides Irgarol and Diuron were investigated on Paracentrotus lividus, the dominant echinoid species of the Mediterranean Sea. Spermiotoxicity was studied by assessing the effects of sperm exposure on fertilization rate as well as on the induction of transmissible damages to the offspring. Embryotoxicity was studied by assessing the developmental defects in the exposed larvae. The experimental results show a Diuron EC50 of 2.39 (± 0.21) mg/L with a NOEL of 0.25 mg/L for embryos, and of 5.09 (± 0.45) mg/L with a NOEL of 0.5 mg/L for sperms, respectively. Data obtained from the embryotoxicity test on Irgarol [EC50 0.99 (± 0.69) mg/L] are of the same order of magnitude as the literature data about Japanese urchins. Spermiotoxicity tests show an Irgarol EC50 of 9.04 (± 0.45) mg/L with a NOEL of 0.1 mg/L. These data show the different sensitivities of the two tests: embryos are more sensitive than sperms for both the tested chemicals and Diuron seems to be the less toxic one. Moreover, as a major output of the experimental work, tested herbicides exert transmissible damage to spermatozoa evidenced by larval malformations in the offspring, mainly P1 type (skeletal alterations). The comparison of the endpoints results offers an interesting indication of a probable different mode of action (Irgarol seems to interact with calcium homeostasis) of the two biocides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
TBT-based antifouling paints were largely used to inhibit the growth of fouling organisms, i.e., barnacles and tubeworms among others. Since the early 1980s, it was pointed out that TBT and its derivatives had deleterious effects on non-target organisms, some of which (e.g., oysters) were economically important (Maguire 1987; Thompson et al. 1985). Furthermore, many authors found that even very low TBT concentrations (ng/L) caused sublethal effects (such as poor grow rates and low reproductive success) in a wide range of marine organisms (Alzieu et al. 1982; Andreae et al. 1983; Chiavarini et al. 1996; Hall and Bushong 1996; Laughlin et al. 1996; Marin et al. 2000; Waldock and Thain 1983). As an example, these compounds were held responsible for the near collapse of oyster farming in France and declines in the population of dogwhelks in British waters (Evans 1999). However, regulations, which were introduced by many countries in the last decade, restricting the use of these antifoulings, have greatly reduced the TBT contamination levels (Evans 1999). Furthermore, in 2001 the IMO (International Maritime Organization) decided that the use of organotin antifoulants would be totally banned by the year 2003 and the TBT coatings on ships should be prohibited from 2008 onwards (international convention on the control of harmful antifouling system on ships, adopted on 5/10/2001). Consequently, many organotin-free antifouling paints have been developed.
Tin-free antifouling paints, composed of seawater-soluble matrices containing tin-free biologically active ingredients (a copper compound and/or organic biocides), are now available. The copper component (typically cuprous oxide or even metallic copper) alone, in fact, is not effective against diatoms and algae. Therefore, secondary biocides must be incorporated into the paints (Brady 2000). These compounds are termed “organic booster biocides” and some of them originated for agricultural use (Voulvoulis et al. 1999). The mainly used organic boosters are, until now, Irgarol (2-methylthio-4-t-butylamino-6-cyclopropylamino-s-triazine) and Diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea) (Voulvoulis et al. 1999).
The introduction of these and other compounds in the antifouling products obviously generates the necessity to investigate any possible adverse effect on the marine ecosystem. It seems, in fact, that Irgarol does not undergo to rapid biodegradation and the persistence in water is probably due to the low affinity to partition onto sedimentary material and high resistance to degradation (Thomas et al. 2002).
Irgarol residues have been found in European coastal and lake waters and in Japanese coastal waters suggesting the possibility that Irgarol may become a ubiquitous contaminant of the aquatic environment (Okamura et al. 2000), mainly in the areas of high yachting activity and low water exchange rates (Omae 2003; Thomas et al. 2002).
In 2001, Thomas et al. wrote “a simple risk assessment indicates that at present Irgarol 1051 and Diuron represent a lower threat to the environment than TBT. However, it cannot be assumed that their use will have no environmental harm, and further data are required on the fate and effects of all antifouling paint booster biocides.”
Van Wezel and van Vlaardingen (2004) proposed environmental risk limits for Irgarol 1051 and some other TBT substitutes, derived from data on ecotoxicology and environmental chemistry of these substances. For Irgarol 1051, plants appear to be especially sensitive. The authors concluded that the plant species composition, and thereby the complete ecosystem functioning, cannot be considered as protected.
A comprehensive review of very recent Irgarol and Diuron toxicity data for aquatic organisms was proposed by Kostantinou and Albanis (2004); other studies about the toxic effects of Diuron have been recently published about corals (Jones et al. 2003), sea grass (Maccinnis-Ng et al. 2003), V. fisheri and D. magna (Hernando et al. 2003). Other data are reported in the U.S. EPA Pesticide Database.
Gaps in available data make difficult the evaluation of biocides impact on the aquatic environment. Therefore, further ecotoxicological studies especially about marine non-target organisms still seems to be necessary.
In 2002 Kobayashi and Okamura assessed, comparatively, the effects of various biocides on Japanese H. pulcherrimus and A. crassispina sea urchin eggs and embryos. In this study, the maximum concentration at which Irgarol showed no toxic effects (No Observed Effect Concentration: NOEC) on the early juvenile stages of sea urchin was 10 μg/L. The NOEC value (1 mg/L) was reported also for Diuron, in the same study.
In this report, we show our experimental data on the toxic effects of the two booster biocides Irgarol and Diuron on sea urchin Paracentrotus lividus early development, fertilization, and offspring quality. The sea urchins toxicity test has been utilized for several decades to evaluate a number of xenobiotics and their future in the marine ecosystem (Bay et al. 1993; His et al. 1999; Kobayashi and Okamura 2002; Manzo 2004; Marin et al. 2000; Pagano et al. 1996a, 1996b). P. lividus is one of the most commonly used organisms in biomonitoring studies, which require simple, rapid, and inexpensive but sensitive methods (Bougis et al. 1979; Chapman and Long 1983; Kobayashi 1991; Manzo and Torricelli 2000; Pagano et al. 1989). In particular P. lividus early life stages are very sensitive to many pollutants (His et al. 1999; Ringwood Huffman 1992).
Materials and Methods
Test Organisms
Adult Paracentrotus lividus (Lamark) were collected from the Tyrrhenian Sea (Bay of Naples) by the staff of the Zoological station. The sea urchins were then stabulated for 24 h in natural Filtered SeaWater (FSW) at 18 ± 1°C (Salinity 38%, pH 8 ± 0.2).
In fact, it was noted that the utilization of the animals immediately after the collection produces a decrease of normal plutei in the control, probably due to the stress induced by the collection activity itself. An abrut increase in temperature or salinity might not only induce spawning, but seriously harm the gametes (ASTM 2004).
Test Solutions
The herbicides Irgarol (2-(tert-butylamino)-4-(cyclopropylamino)-6-(methylthio)-s-triazine), and Diuron (3-(3,4-dichlorophenyl)-1,1dimethylurea) at the highest available purity (Residue Analysis-Pestanal®) were purchased by Sigma Aldrich, Milano, Italy, from a Fluka catalogue. The choice of pure chemicals was based on the fact that the technical products mentioned in the literature always have a quite high level of purity (97–99,8%). This substantially reduces the problem to the possible presence of highly toxic intermediates of synthesis or metabolites; on the other hand, pure chemicals guarantee a higher reproducibility and comparability of the results.
Since both chemicals have a relatively low water solubility (7 mg/L for Irgarol and 42 mg/L for Diuron), the stock solutions were prepared dissolving 10 mg of the herbicide in 1 mL of dimethyl sulfoxide (DMSO, 97% purchased from Merk, Dusseldorf, Germany).
Test solutions were then obtained diluting the stock solution in natural FSW as follows. For Irgarol the following concentrations were used for test solutions: 0.01, 0.1, 0.5, 1, 5, 7.5, and 10 mg/L for the embryotoxicity test; 0.01, 0.1, 0.5, 1, 5, and 10 mg/L for the spermiotoxicity test.
For Diuron the following concentrations were used for test solutions: 0.2, 0.25, 0.5, 1, 2, 2.5, 5, and 7.5 mg/L for the embryotoxicity test and 0.25, 0.5, 1, 2, 2.5, 5, and 7.5 mg/L for the spermiotoxicity test.
Controls
Seawater used for the test solutions was sampled in an uncontaminated area far from the coast and was already frequently used in the laboratory for ecotoxicological tests and optimisation of analytical methods. As a consequence, seawater samples from this area were analysed several times for trace elements and organic micropollutants using wide-spectrum-screening analytical methods. Test solutions were always controlled for Irgarol and Diuron concentrations before the test execution, checking those at the lowest and highest concentration. The analytical control was performed using solvent extraction and GC/MS determination for Irgarol, and solvent extraction and LC/MS for Diuron according to Lamoree et al. (2002). A sufficient amount of seawater, necessary for the experiments and negative (blank) controls, was then filtered (0.45 μm). Standard control was carried out with equivalent volumes of DMSO at a final concentration of 0.1% (that is, the higher one in the test solutions) and exhibited no observable effect on the studied organisms.
Toxicity Test
Gametes were harvested and embryos were reared as described by Pagano et al. (1986). Spawning was induced in sea urchin by injection of 1 mL of 0.5 M KCl through the perioral membrane. Eggs were collected by separately placing each spawning female in a different 250-mL beaker with FSW while “dry” sperm from each male was collected with an automatic pipette and stored in a sterile tube placed on ice. For each experiment, six individual females were selected for their appropriate egg quality (no immature forms, no debris, no fertilised eggs) and amount. Males were selected for sperm motility (checked under the microscope) and amount. Then, the best three male and three females gametes were pooled and filtered through nylon cheesecloth (∅ = 200 μm for eggs and 50 μm for sperm). The eggs suspension (stock solution) was diluted in order to obtain the final concentration of 250–300 eggs/mL.
Embryotoxicity Test
In the embryotoxicity protocols, fertilization was carried out by adding 1 mL of pooled-sperm, diluted 1:1000 in FSW, to the egg suspension and by incubating if at 18 ± 1°C for 20 min. Fertilisation success in the stock solution was verified by the presence of the fertilization membrane in a random sample of 100 eggs. An excess of sperm was removed by decanting zygotes and resuspending them in FSW.
The experiments consisted of the exposition of a volume of the egg suspension corresponding to 250–300 fertilized eggs to the test solution (10 mL). Three replicates for each treatment were prepared. Each experiment was performed at least three times. The eggs were incubated at 18 ± 1°C, for 48–50 h. After this period, 100 μL of 40% buffered formalin was added in each vessel and developmental abnormalities were determined in each replicate by direct observation of 100 individuals, randomly chosen.
For each treatment schedule, 100 plutei were scored for the frequencies of: (1) normal (N) larvae, according to their symmetry, shape, and size; (2) retarded (R) larvae with shape and symmetry the same as normal, but with reduced size (<1/2 N); (3) malformed larvae (P1), affected in skeletal and/or gut differentiation and/or pigmentation; (4) pre-larval arrest (P2), embryos unable to go to larval differentiation, as abnormal blastula or gastrulae (Pagano et al. 1986, US EPA 1995).
Spermiotoxicity Test
Aliquots (10 μL) of concentrated sperm from the pool of three males were diluted in 10 ml of testing solutions. After the exposition of 30 min at room temperature, 50 μL of treated sperm suspension were added to 10 ml of FSW containing untreated eggs (20/30 eggs/mL) obtained from stock solution. Experimental wells, three replicates for each treatment, were incubated at 18 ± 1°C for 20 min. Each experiment was performed at least three times. The fertilization rate was determined on a random sample of 100 eggs. The zygote cultures were incubated at 18 ± 1°C, for 48–50 h. Then offspring quality, expressed as frequency of developmental defects, was assessed as described before.
Statistical Analysis
Differences in fertilization success (comparisons between the control group and each of the experimental groups) were tested for significance using the multiple comparison Dunnett’s test.
Mean percentage abnormalities and 95% confidence limits were calculated for all the samples and compared to the results obtained for the controls. If abnormalities in the controls were 20% or more, the test was judged invalid and repeated.
The EC50 was calculated using the Linear Interpolation Method (Inhibition Concentration procedure or ICp) (Cesar et al. 2004, US EPA 1993). The bootstrap method is used to obtain the 95% confidence interval, because standard statistical methods for calculating confidence intervals are not applicable, Analysis of variance (ANOVA) was applied, using raw data, to test for significant differences in effects among treatments (significance level was always set at α = 0.05); then NOEC (No Observed Effect Concentration) and LOEC (Lowest Observed Effect Concentration) were calculated with Dunnett’s procedure.
Results
Diuron
Embriotoxicity
The effect mean percentage (Figure 1A) shows values with an increasing trend up to the maximum effect (SD = 0) at 7.5 mg/L. EC50 is 2.39 (± 0.21) mg/L and NOEL 0.25 mg/L. The developmental defects in treated P. lividus larvae (Figure 1B) are mainly of the P1 type (larvae affected in skeletal or gut differentiation), with an increasing trend until 2.5 mg/L, at 5 mg/L, the effect becomes drastic, with the total arrest at prelarval stadium (P2) at 7.5 mg/L.
Diuron embryotoxicity in P. lividus. A: % of malformed out of 100 individuals normalized with respect to control, as a function of tested concentrations (mean of at least three experiments, each in triplicate ± SE,). B: Number of individuals with different developmental anomalies obtained after 48-h exposure. N = normal plutei; R = retarded larvae; P1 = malformed larvae; P2 = blastulae or gastrulae (developmental arrest). See also Materials and Methods.
Spermiotoxicity
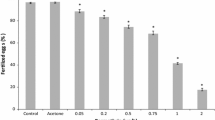
Significant effects on fertilization rate (FR) were observed for this herbicide (Figure 2A). FR shows a significant progressive decrease due to a reduction of the fertilization ability of exposed sperms. The EC50 is 5.09 (± 0.45) mg/L, and NOEL 0.5 mg/L. However, it can be noted that the spermiotoxic effect is not complete, thus permitting the assessment of embryo cultures. The offspring of treated sperm (Figure 2B) shows mainly malformations of P1 type and a dose-dependent increase in the effect percentage curve. So the treated sperm that retain fertilization ability produce offspring that develop abnormally. Diuron first produces toxic effects on the fertilization process and, then, offspring damage. However, at the last two concentrations, 7.5 and 10 mg/L, the data were biased by quite low FR (7% and 17%, respectively).
Diuron spermiotoxicity in P. lividus. A: % of unfertilised eggs out of 100 eggs, as a function of tested concentrations (mean of at least three experiments, each in triplicate ± SE). B: Offspring quality: % of developmental anomalies in the fertilized eggs after 48-h exposure. N = normal plutei; R = retarded larvae; P1 = malformed larvae; P2 = blastulae or gastrulae (developmental arrest). See also Materials and Methods.
Irgarol
Embryotoxicity
The Irgarol toxic effect exerted on P.lividus embryos is reported in Figure 3A. The values quickly increase up to EC50 0.99 (± 0.69) mg/L, and seem to stabilize from 1 to 5 mg/L dose (laying around 60% toxic effects) and at higher concentrations a corresponding increase is observed, with the maximum at 10 mg/L. The induced developmental defects are reported in Figure 3B. The abnormal individuals are mainly P1 malformed larvae, showing a dose-dependent trend up to 7.5 mg/L (90%). At a higher tested concentration (10 mg/L), massive prelarval arrest (P2) effects are observed (80%).
Irgarol embryotoxicity in P. lividus. A: % of malformed out of 100 individuals normalized with respect to control, as a function of tested concentrations (mean of at least three experiments, each in triplicate ± SE). B: Number of individuals with different developmental anomalies, obtained after 48-h exposure. N = normal plutei; R = retarded larvae; P1 = malformed larvae; P2 = blastulae or gastrulae (developmental arrest). See also Materials and Methods
Spermiotoxicity
The toxicity pattern of this herbicide on sperm fertilization ability is shown in Figure 4A. Significant (α = 0.05) effects can be evidenced already at 0.01 mg/L concentration (NOEL < 0.01 mg/L), but then the effects remain under 25% up to 5 mg/L. The EC50 is 9.04 ( ± 0.45) mg/L.
Irgarol spermiotoxicity in P. lividus. A: % of unfertilised eggs out of 100 eggs, as a function of tested concentrations (mean of at least three experiments, each in triplicate ± SE). B: Offspring quality: % of developmental anomalies in the fertilized eggs after 48-h exposure. N = normal plutei; R = retarded larvae; P1 = malformed larvae; P2 = blastulae or gastrulae (developmental arrest). See also Materials and Methods.
Irgarol-induced effects on offspring quality following sperm exposure are reported in Figure 4B. Malformed larvae (P1) are almost exclusively present. It is interesting to note the increment of P1 also at the lowest concentration even if the fertilization rate is not affected. Then, the rate between P1 malformed larvae and normal offspring is almost the same (and only the decrease of fertilization success can be seen). So, the maximum defect on offspring is already obtained at the lowest test concentration (0.01 mg/L). After that, the herbicide affects only the sperm fertilization ability.
Discussion
Sea urchin embryos seem to be very sensitive to Irgarol. The measured embryotoxicity on P. lividus (Table 1) is comparable with raw data reported by Kobayashi and Okamura (2002) for the Japanese urchin embryos (EC50 is not reported). Spermiotoxicity test shows an EC50 value in the same range as those reported for crustacean (Kostantinou and Albanis 2004) and a NOEL of 0.10 mg/L.
Being an herbicide, Irgarol is much more toxic to algae, its prevalent effect being the inhibition of photosynthesis, in particular acting on Photosystem-II (PSII) (Dahl and Blanck 1996). Toxicity effects are particularly evident for marine species (Enteromorfa intestinalis zoospores) for which growth inhibition is significant at concentrations as low as 100 ng/L (Scarlett et al. 1997). A moderate toxicity is reported for the marine bacterium V. fischeri EC50 = 50.8 ± 7.8 mg/L (Fernandez-Alba et al. 2002) and for marine crustacean A. salina (> 40 mg/L, Okamura et al. 2000).
To our knowledge, the mode of action of triazine upon aquatic invertebrates is not well known. In our spermiotoxicity and embriotoxicity tests, we observed a predominance of P1 malformed larva, mainly affected by skeletal alterations.
From these results, it seems possible to hypothesize that in P. lividus, this compound could interact with calcium homeostasis. At higher concentrations, it could modify cytoskeleton assembly during blastomere division, producing P2-type alterations, while, at lower concentrations, it could alter the deposition of the larval skeleton giving rise to malformed larvae (P1).
Exposed sperms show a dose-related decrease in fertilization ability (Figure 2A) but with less sensitivity than for embryos, probably because they are differentiated cells. On the contrary, the maximum defect in offspring is obtained at the lowest test concentration (0.01 mg/L). After that, the herbicide affects only the sperm fertilization ability; it actually exerts an acute spermiotoxicity.
Diuron, even if belonging to a different chemical class of pesticide (phenylureas) than Irgarol, is a photosynthesis inhibitor too, but the mode of action at the biochemical level, up to now, has not been precisely determined.
Diuron ecotoxicological data in the recent literature are quite scarce, particularly with reference to marine species. Hernando et al. (2003) reported toxicity effects on D. magna (EC50 8.6 mg/L ± 1.3) and V. fisheri (EC50 100 mg/L ± 7.8). The chronic effect upon algae (S. capricornutum, 72 h test) was reported by Fernandez Alba et al. (2002) (0.0045 mg/L ± 0.0079). Kobayashi and Okamura (2002) reported only the NOEL for the Japanese sea urchin (1 mg/L). P. lividus NOEL (0.5 mg/L, Table 1) is lower than those sea urchin species and LOEL and EC50 higher only than those reported in the literature for algae.
Diuron is less toxic (LOEL 0.5 mg/L) to P. lividus embryos than Irgarol but its toxic effects appear earlier. In fact, a 65% effect level can be observed at a concentration of 2. 5 mg/L (Figure 3A) and almost exclusively P2 damage can be seen at a concentration of 5 mg/L (this happens only for Irgarol concentrations >10 mg/L: 80% P2).
The spermiotoxicity test is less sensitive than the embryotoxicity test for Diuron (NOEL 0.5 mg/L). It is possible to note from the comparison of the two herbicide spermiotoxicity effect curves (Figures 2A, 4A) that Diuron is more toxic from a concentration of 1 mg/L onwards. The developmental defects on exposed sperms is mainly of the P1 type for both herbicides, but for Diuron we can read a dose-related trend of P1 malformations as well as for the inhibition of fertilization rate. So, this biocide exerts quickly, producing on exposed sperm populations toxic effects such as fertilization inhibition and permanent damage transmissible to offspring, that is to say acute and chronic effects.
Fortunately, the NOECs determined are much higher than the concentrations reported in different world seawaters up to now (for a review, see Kostantinou and Albanis 2004). It is important to consider that, from 2008 onwards, tributyltin-based paints will be totally banned and the replacing organotin-free biocide environmental levels can considerably increase. Moreover, these active compounds can accumulate in marine sediments especially if introduced as paint particles (Thomas et al. 2002).
As for antifouling, various chemicals are used. Mixture toxicity, in particular at low concentrations, certainly plays a role in the environmental situation.
Conclusions
The known sensitivity of sea urchins to numerous environmental pollutants environmental is noticeable also in the chemicals (Irgarol and Diuron) tested in this report.
Diuron is less toxic (LOEL 0.5 mg/L) to P. lividus embryos than Irgarol but its toxic effects appear earlier and produce pre-larval arrest (P2).
Irgarol induces a predominance of larvae with skeletal alterations (P1). It seems possible to hypothesize that in P. lividus, this compound could interact with calcium homeostasis. Further experiments are in progress in our laboratory in order to assess the toxicity effects on P. lividus of Irgarol and Diuron mixtures at real contamination levels.
References
Alzieu C, Heral Y, Thibaud J, Dardignac MJ, Feuillet M (1982) Influence of organostannic-based antifouling paints on the cockle calcification of the oyster Crassostrea gigas. Rev Trav Inst Peches Marit 4:101–116
Andreae MO, Byrd JT, Froelich PN (1983) Arsenic, antimony, germanium, and tin in the Tejo Estuary, Portal; modeling a polluted estuary. Environ Sci Technol 17:731–737
Bay S, Burgess R, Nacci D (1993) Status and applications of echinoid (Phylum Echinodermata) toxicity test methods. In: Landis WG, Hughes JS, Lewis MA (eds) Environmental toxicology risk assessment. ASTM STP 1179, pp 281–302. American Society for Testing and Materials. Philadelphia, PA, USA
Bougis P, Corre MC, Etienne M (1979) Sea urchin as a tool for assessment of the quality of seawater, Ann Inst Oceanog Paris 55:21–26
Brady RF Jr (2000) No moretin: What now for fouling control? J Protect Coat Linings (June) 42:79–83
Cesar A, Marìn sA, Marìn-Guirao L, Vita R (2004) Amphipod and sea urchin tests to assess the toxicity of Mediterranean sediments: the case of Portmàn Bay, Sci Mar 68(Suppl 1):205–213
Chapman PM, Long ER (1983) The use of bioassays as part of a comprehensive approach to marine pollution assessment. Mar Pollut Bull 14:81–84
Chiavarini S, Cremisini C, Morabito R (1996) Organotin compounds in marine organisms In: Element speciation in Biorganic Chemistry. Chemical Analysis series, Vol. 135 John Wiley & Sons Inc. (ed), New York, pp 287–329
Dahl B, Blanck H (1996) Toxic effects of the antifouling agent Irgarol 1051 on periphyton communities in coastal water microcosms. Marine Pollut Bull 32:342–350
ASTM (American Society for Testing and Materials) (2004) Standard guide for conducting static acute toxicity tests with echinoid embryos. ASTM Standard Guide E 1563–98. In: Annual Book of ASTM Standards, Section 11, Vol. 115: Biological effects and environmental fate; biotechnology; pesticides. ASTM, West Conshohochen, PA, USA
Evans SM (1999) TBT or not TBT? That is the question. Biofouling 14 (2):117–129
Fernandez-Alba AR, Hernando MD, Piedra L, Chisti Y (2002) Toxicity evaluation of single and mixed antifouling biocides measured with acute toxicity bioassay. Anal China Acta 456:303–312
Hall Jr LW, Bushong SJ (1996) In: Champ MA, Seligman PF (eds) Organotins: environmental fate and effects. Chapman and Hall, London, pp 157–190
Hernando MD, Ejerhoon M, Fernandez-Alba AR, Chisti Y (2003) Combined toxicity effects of MTBE and pesticides measured with Vibrio fisheri and Daphnia magna bioassays. Wat Res 37:4091–4098
His E, Heyvang I, Geffard O, De Mountadouin X (1999) A comparison between oyster (Crassostrea gigas) and sea urchin (Paracentrotus lividus) larval bioassay for toxicological studies. Wat Res 7:1706–1718
Jones RJ, Muller J, Haynes D, Schreiber U (2003) Effects of herbicides diuron and atrazine on corals of the Great Barrier Reef. MEPS, Australia, 251:153–167
Kobayashi N (1991) Marine pollution bioassay by using sea urchin eggs in the Tanabe Bay, Wakayama Prefecture, Japan, 1970–1987. Marine Pollut Bull 23:709–713
Kobayashi N, Okamura H (2002) Effects of new antifouling compounds on the development of sea urchin. Marine Pollut Bull 44:748–751
Kostantinou IK, Albanis TA (2004) Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review. Environ Int 30:253–248
Lamoree M, van der Horst A, Swart CP, van Hattum B (2002) Determination of diuron and the antifouling paint biocide Irgarol 1051 in Dutch marinas and coastal waters. J Chromatogr A 970:183–190
Laughlin RB Jr, Thain J, Davidson B, Valkirs AO, Newton FC (1996) In Champ MA, Seligman PF (eds) Organotins: environmental fate and effects. Chapman and Hall, London, p 192-217
Maccinis-Ng CMO, Ralph PJ (2003) Short term response and recovery of Zostera capricorni photosyntesis after hebicide exposure. Aquat Bot 76(1):1–15
Maguire RJ (1987) Environmental aspect of tributyltin. Appl Organomet Chem 1:475–498
Manzo S (2004) Sea urchin embryotoxicity test: proposal for a simplified bioassay. Ecotox Environ Safety 57(2):123–128
Manzo S, Torricelli L (2000) Preliminary findings about Regione Campania (South Italy) coastal water ecotoxicological data. Abstract book of II, National Conference of Sea Science, Geneva, 21/25 November, p 223
Marin MG, Moschino V, Cima F, Celli C (2000) Embryotoxicity of butyltin compounds to the sea urchin Paracentrotus lividus. Mar Environ Res 50:231–235
Okamura H, Aoyama I, Liu D, Maguire J, Pacepavicius CG, Lau YL (2000) Fate and ecotoxicity of the new antifouling compound Irgarol 1051 in the aquatic environment. Wat Res 34(14):3523–3530
Omae I (2003) Organotin antifouling paints and their alternatives. Appl Organometal Chem 17:81–105
Pagano G, Cipollaro M, Corsale G, Esposito A, Ragucci E, Giordano GG, Trieff NM (1986) The sea urchin: Bioassay for the assessment of damage from environmental contaminants. In: Cairns Jr (ed) Community Toxicity Testing. ASTM STP920, pp, 66–92, American Society for Testing and Materials. Philadelphia, PA, USA
Pagano G, Corsale G, Esposito A, Dinnel PA, Romana LA (1989) Use of sea urchin sperm and embryo bioassay in testing the sublethal toxicity of realistic pollutant level. Adv Appl Biotech Ser 5:153–163
Pagano G, Iaccarino M, Guida M, Manzo S, Oral R, Romanelli R, Rossi M (1996a) Cadmium Toxicity in Spiked Sediment to sea urchin embryos and sperm. Mar Environ Res 42(1–4): 54–55
Pagano G, His E, Beiras R, De Biase A, Korkina LG, Iaccarino M, Oral R, Qiuniou, Warnau M, Trieff NM (1996b) Cytogenetic, developmental, and biochemical effects of aluminium, iron, and their mixture in sea urchins and mussels. Arch Environ Contam Toxicol 31:466–474
Ringwood Huffman A (1992) Comparative sensitivity of gametes and early developmental stages of a sea urchin species (Echinometra mathei) and a bivalve species (Isognomon californicum) during metal exposures. Arch Environ Contain Toxicol 22:288–265
Scarlett A, Donkin ME, Fileman TW, Donkin P (1997) Occurrence of the marine antifouling agent Irgarol 1051 within Plymouth Sound locality: implications for the green macroalga Enteromorpha intestinalis. Marine Pollut Bull 34(8):645–651
Thomas KV, Fileman TW, Readman J, Waldock MJ (2001) Antifouling paint booster biocides in the U.K. coastal environment and potential risks of biological effects. Mar Pollut Bull 42:677–688
Thomas KV, McHugh M, Waldock M (2002) Antifouling paint booster biocides in UK coastal waters: inputs, occurrence and environmental fate. Sci Total Environ 293:117–127
Thompson JAJ, Sheffer MG, Pierce RC, Chau YK, Cooney JJ, Cullen WR, Maguire RJ (1985) Organotin compounds in the aquatic environment: Scientific criteria for assessing their effects on environmental quality. Nat Res Counc Can Ottawa Publ NRCC 22494, p 284
US EPA (1993) A linear interpolation method for sublethal toxicity: the inhibition concentration (ICp) approach. National Effluent Toxicity Assessment Center Technical Report 03-93, Environmental Research Laboratory, Duluth, Minnesota
US EPA (1995) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to weast coast marine and estuarine organisms. EPA/600R95136. Cincinnati, OH, USA
US EPA Office of Pesticide Programs (2000) Pesticide ecotoxicity database (Formerly: Environmental Effects Database [EEDB]). Environmental Fate and Effects Division, Washington, DC
Van Wezel AP, Vlaardingen P (2004) Environmental risk limits for antifouling substances. Aquat Toxicol 66:427–444
Voulvoulis N, Scrimshaw MD, Lester JN (1999) Alternative antifouling biocides. Appl Organomet Chem 13:135
Waldock MJ, Thain JE (1983) Shell thickness in Crassostrea gigas: Organotin antifouling of sediment induced. Marine Pollut Bull 14:411–415
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manzo, S., Buono, S. & Cremisini, C. Toxic Effects of Irgarol and Diuron on Sea Urchin Paracentrotus lividus Early Development, Fertilization, and Offspring Quality. Arch Environ Contam Toxicol 51, 61–68 (2006). https://doi.org/10.1007/s00244-004-0167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0167-0