Abstract
Genotoxicity of crude cyanobacteria extracts (CBE) from blooms in Taihu Lake, China toward common carp (Cyprinus carpio) was measured. The primary extracellular product was determined by HPLC to be Microcystin-LR (MC-LR, L for leucine and R for arginine) with an average concentration of 2.4 × 102 μg MC g−1 dry weight of cyanobacteria. Acute toxicity to carp, expressed as the 72-h LC50, was 53 mg, dw cyanobacteria L−1. Genotoxicity, as determined by the micronucleus (MN) and comet assays were both dose- and time-depended. Deformities of cellular organelles in liver and gill were observed by use of transmission electron microscopy (TEM). The results showed that MC-LR from cyanobacteria from Taihu Lake could induce genotoxic response and tissue-level morphological changes in common carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the primary producers in aquatic ecosystems, microalgae play an important role in biogeochemical cycling (Morel and Price 2003). Algae can release materials into the surrounding water and approximately 40 microalgae species can secrete toxic extracellular products (Sournia et al. 1991). Some of the most commonly produced cyanotoxins are microcystins (MCs) (Chorus and Bartram 1999). MCs are toxic heptapeptides with a basic cyclic structure which share a common moiety composed of seven amino acids. They are named according to their variable l-amino acids and nearly 80 different MCs have been identified (Hoeger et al. 2005; Djediat et al. 2010). MCs can be produced by several species of cyanobacteria in the genera Microcystis, Anabaena and Planktothrix especially under conditions of eutrophication (Chorus and Bartram 1999) and they can be released from lysed cells during natural senescence, physical pressure, or exposure to herbicides (Dietrich and Hoeger 2005; Ross et al. 2006; Gérard et al. 2009). The most toxic of the MCs is Microcystin-LR (MC-LR, L for leucine and R for arginine; Chorus and Bartram 1999) which can cause (1) strand breaks of DNA in different types of cells (Mankiewicz et al. 2002; Zegura et al. 2003), (2) oxidative damage of DNA in hepatocytes (Maatouk et al. 2004), and (3) formation of micronuclei in human lymphoblastoid cells (Zhan et al. 2004).
MCs are specific inhibitors of serine/threonine phosphatases (Runnegar et al. 1995) and thus can produce reactive oxygen species (ROS) which subsequently can cause DNA damage (Ding et al. 1998), and thus pose a potential hazard to birds and mammals as well as aquatic animals (Puschner et al. 1998; Gulland et al. 2002; Shumway et al. 2003; Schnetzer et al. 2007). Traditionally, MCs have been considered to be hepatotoxic due to their accumulation in hepatocytes by organic anion transporting polypeptide (OATP) type specific transporters (Fischer et al. 2005). However, OATP are not only expressed in the liver, but also in the gastrointestinal tract, kidney, brain, and across the human blood–brain barrier (Dietrich and Hoeger 2005; Gaudin et al. 2008).
The comet assay and micronucleus assay (MN assay) have been used to investigate DNA damage and chromosomal damage, respectively (Steinert 1999; Fenech et al. 2003). Both of these assays are routinely used as indices of effects on chromatin because they are easy to perform and sensitive (Steinert 1999; Fenech et al. 2003). The alkaline comet assay, also known as the Single Cell Gel Electrophoresis (SCGE) assay, is used to detect single- and double-strand breaks, alkali-labile sites, and DNA–DNA and DNA–protein cross-links in individual cells (Tice et al. 2000; Gaudin et al. 2008). In the comet assay, several endpoints can be used. The Olive tail moment (OTM), which is defined as the product of the tail length and the percentage of tail DNA, has been recommended as an effective index of DNA damage. OTM is an indication of the relative fluorescent intensity in the head and tail (Lovell and Omori 2008). The MN assay is used to detect micronuclei which are formed by chromosome fragments or whole chromosomes that are not reassembled into the nucleus during cell division (Heddle et al. 1991).
Taihu Lake, the third largest freshwater lake in China, is located in the Yangtze River delta of southeastern China. It has a large human population density and some of the greatest agricultural and industrial production in China. Eutrophication of the lake over the past few decades has lead to the occurrence of cyanobacteria blooms. Average concentrations of MCs in Taihu Lake range from 0 to 16 μg L−1, with the greatest concentrations occurring in Meiliang Bay (Xu et al. 2008), and the most common type of MCs in Taihu Lake is MC-LR (Qi et al. 2009). The current study evaluated the genotoxicity of cyanobacteria extracts (CBE, mainly MCs) from Taihu Lake on the common carp (Cyprinus carpio Linnaeus; Cyprinidae) by use of the MN and comet assays. Transmission electron microscopy (TEM) was also used to evaluate the effects of MCs on the ultrastructure of carp liver and gill cells.
Materials and methods
Chemicals and animals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified. Standard MC-LR material was purchased from the Institute of Hydrobiology, Chinese Academy of Science (Wuhan, China).
150 carp with an average length of 10.4 ± 0.7 cm and average weight of 8.0 ± 2.3 g were purchased from the same supplier in a local fish market (Nanjing, China). All fish had the same histories and were acclimated under laboratory conditions at 18 ± 1°C, 12/12 h dark/light cycle for 7 days before testing. During the acclimation period, all fish were placed in the same container (40 L) and fed with fish food that had been shown previously to contain no MCs. One-fourth of the water in the container was changed every day during acclimation and air was bubbled through the container continuously. Fish were not fed during the experimental period. Tap water used in this study was dechlorinated for 3 days before use.
Collection of cyanobacteria and identification and quantification of MCs
Cyanobacteria were collected from Meiliang Bay, Taihu Lake during a bloom that occurred in early autumn 2008. Cyanobacteria were filtered from water and lyophilized (FreeZone12, Labconco) to produce dry cyanobacteria products. The dry cyanobacterial powder was then stored at −20°C. Dry cyanobacteria powders were mixed with dechlorinated tap water and sonicated at 150 W for 5 min in an ice-water bath with an ultrasonic processor (JY-250, Zhejiang, China) to release MCs into solution and to attain a CBE solution. Microscopic examination of supernatants indicated more than 90% of the cyanobacteria cells were lysed. The extract was centrifuged at 10,000×g for 30 min and filtered. The solution was then evaporated and pulled through a Sep-Pak C18 column and washed with 20% methanol. MCs were eluted with 90% methanol containing 0.1% TFA according to previously published methods (Harada et al. 1988; Aranda-Rodriguez et al. 2005; Qi et al. 2009). Concentrations of MCs in the extracts were analyzed by high performance liquid chromatography (HPLC) (Agilent 1100 Series, Agilent Technologies, Waldbronn, Germany) equipped with a Zorbax Eclipse SB-C18 column (250 mm × 4.6 mm, 5 μm, Agilent Technologies). The mobile phase consisted of water (0.05% TFA) as solvent A and acetonitrile (0.05% TFA) as solvent B. The gradient elution program was performed as follows: % solvent A/solvent B, 10/90 at 0 min, 35/65 at 20 min, 35/65 at 25 min. The flow rate was 1.0 mL min−1 and the injection volume was 20 μL. Chromatograms at 238 nm were recorded with an Agilent 1100 Series PDA detector. Quantification was based on external calibrations of standard MC-LR. Recovery experiments were conducted in quadruplicates by adding standard MC-LR in Milli-Q water to 0.5 μg L−1. The recovery rate was 86.6% ± 4.9%. Concentrations of MCs were reported as μg g−1 dry cyanobacteria.
Acute toxicity
Forty common carp were randomly assigned to four groups (ten carp group−1) and each group was placed in one of four 10-L fish tanks. Carp groups were exposed to 0, 37.5, 75, or 150 mg CBE L−1. The 72 h median lethal concentration (72 h LC50) was determined by the method previously reported by Fawell et al. (1999). The calculated 72 h LC50 was used to determine fish exposure concentrations in subsequent toxicity assays.
MN assay and comet assays
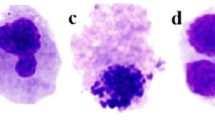
One hundred common carp were randomly assigned to two groups (50 carp each) and exposed to CBEs for 3 or 7 days. Within each of the exposure periods, subgroups of ten common carp, each, were exposed to 0, 6.25, 12.5, 25.0 or 50.0 mg CBE L−1. At the end of the exposure period, peripheral blood was collected from the caudal vein and smeared onto pre-cleaned slides. Three fish were randomly selected from each concentration of each exposure period and three slides were prepared for each fish. After fixation in pure methanol for 10 min, the slides were allowed to air-dry and then stained with 10% Giemsa solution for 25 min. All slides were coded and scored blind. Five thousand cells were scored from each concentration (Zhu et al. 2004) when observed under ×1,000 magnification (Chen et al. 2006). Small, nonrefractive, circular or ovoid chromatin bodies that display the same staining and focusing pattern as the main nucleus were classified as micronuclei (Al-Sabti and Metcalfe 1995).
The alkaline comet assay was performed according to the methods of Tice et al. (2000) with some modifications. Carp kidneys of three fish randomly selected from each of the exposure groups were removed and rinsed twice with ice-cold phosphate-buffered saline (PBS). Kidneys were then cut into pieces, transferred into glass tubes, homogenized using a glass rod for 1 min in an ice bath and passed through a 110 mesh sieve to remove suspended materials. The kidney cell suspensions were centrifuged at 1,500×g for 10 min (Beckman J2-MC), and collected cells were stored at 4°C for comet assay (Li et al. 2004). Frosted microscope slides, on which cells were embedded in an agarose sandwich, were immersed in cold lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 1% N-Lauroyl Sarcosine Na, adjusted to pH 10 with NaOH, 1% Triton X-100 and 10% DMSO). To remove cellular proteins, slides were stored in the dark at 4°C for at least 1 h. To allow DNA unwinding before electrophoresis, slides were placed on a horizontal gel electrophoresis unit filled with fresh electrophoresis buffer (300 mM NaOH, 1 mM EDTA) at 20°C for 30 min. Electrophoresis was conducted at 20°C under 20 V and 200 mA for 20 min. To avoid DNA damage, the above steps were conducted under red light. After electrophoresis, the slides were washed three times with a neutralizing buffer (0.5 M Tris, pH 7.5), then DNA was stained with 50 μg mL−1 ethidium bromide (EB) and the slides were examined with a fluorescence microscope (BX41, Olympus, Japan). Each group consisted of at least five parallel samples and at least 50 cells were analyzed for each slide. Photos were taken with a digital camera (Olympus C-5050 ZOOM) and images were analyzed according to Collins et al. (1997) with the comet assay software project CASP (1.2.2).
TEM analysis
In the 7 day exposure group, livers and gills of controls or carp exposed to 50.0 mg L−1 were cut into pieces, fixed in 2.5% glutaraldehyde and made into paraffin-embedded sections following Datta et al. (2007). Sections were examined under TEM (Hitachi H7650, Japan) to observe changes in cell organelles.
Statistical analysis
Results were expressed as mean ± standard deviation (SD). Differences between control and treated samples were analyzed by one-way ANOVA using Origin Pro 7.0 software. For significant ANOVAs, a Student’s t-test was used to determine which groups were significantly different from each other. A p < 0.05 was considered statistically significant.
Results
MCs concentrations and acute toxicity
The mean concentration of MC-LR from Meiliang Bay, Taihu Lake was 244.26 μg g−1 dry cyanobacteria. Based on this relationship, concentrations of MC-LR in the treatment solutions of 0, 6.25, 12.5, 25 and 50 mg CBE L−1 contained 0, 1.5, 3.0, 6.0 and 12.0 μg MC-LR L−1, respectively. The 72-h LC50 for common carp was 50 mg CBE L−1. After 72 h exposure, no fish died in the control group and all fish died in the group exposed to 150 mg CBE L−1.
MN assay
Both dose- and time-dependent responses were observed in MN formation (Fig. 1). MN frequency was directly proportional to concentrations of cyanobacteria equivalents. After 3 days exposure, there were significantly more MN in blood cells of carp exposed to 50 mg L−1 than in blood cells of carp not exposed to CBE. After 7 days exposure, there were significantly more MN in blood cells of carp exposed to 12.5 and to 50 mg L−1, but not to 25 mg L−1 (p < 0.005) than in blood cells of unexposed carp (controls). The greater concentrations of CBE also caused damage to chromosomes. Frequencies of MN were 10- and 12-fold greater than the control when exposed to the greatest concentration of CBE for 3 and 7 days, respectively.
Comet assay
Dose- and time-depended effects of CBE on OTM of kidney cells were observed in the comet assay (Fig. 2). The OTM was proportional to concentration of CBE except for the 12.5 mg L−1 in the 7 days group. The OTM after 7 days exposure was greater than 3 days exposure. After 3 days exposure, the OTM of kidney cells from carp exposed to 25 or 50 mg CBE L−1 were significantly greater than that of the unexposed control carp. After 7 days exposure, DNA damage, as measured by the OTM was 5.8- and 11.5-fold greater than that of controls for the two greatest concentrations, respectively.
TEM analysis
There was severe cell damage in exposed carp. The ultrastructure of both gill (Fig. 3b) and liver (Fig. 4b) cells changed after exposure to CBE compared to unexposed carp (Figs. 3a, 4a). When exposed to MCs, fish liver cells were smaller than those of hepatocytes of untreated carp. The edges of the cell membrane became unclear and mitochondria swelled and some of the contents were missing. Gill cells from unexposed carp had a clear and complete cell structure with obvious cell membrane and nuclei. The mitochondria and endoplasmic reticulum (ER) were intact without any deformation. Gill cells from carp exposed to 50 mg CBE L−1 became smaller and were misshapen and membranes were less distinguishable. The nuclei and ER atrophied and nuclear membranes were broken. Mitochondria were swollen and the contents were often missing.
Discussion
CBE from Taihu Lake are genotoxic to carp and can lead to the formation of micronuclei and cause DNA strand breaks in a time- and dose-depended manner. The significant genotoxic effects of CBE observed in the MN and comet studies were consistent with the finding that CBE can cause DNA damage, nuclear condensation and fragmentation in rats exposed to MCs (Ding et al. 1998). MC-LR induce DNA stand breaks, in HepG2 cells, which were similar to the finding reported here (Zegura et al. 2003). Those authors suggested that DNA breaks were transiently present as intermediates formed during DNA repair. It has also been reported that MC-LR can cause genomic DNA fragmentation and DNA stand breaks in mouse liver in vivo (Rao and Bhattacharya 1996). Based on the results of the comet assay, Lankoff et al. (2006) concluded that nodularin, a MC belonging to the same class of MC-LR, that can cause oxidative modifications of DNA in a dose- and time-dependent manner.
According to Bolognesi et al. (2006) and Binelli et al. (2009), peripheral erythrocytes are the most appropriate tissues for evaluating the formation of MN in fish. While the MN test was able to demonstrate effects of CBE on fish cells, a disadvantage of using peripheral blood is that blood cells are generally less responsive to effects of genotoxic agents (Buschini et al. 2004). Also, it has been suggested that the comet assay, which is a sensitive indicator, should be combined with other biomarkers to determine DNA damage and genotoxicity (Mitchelmore and Chipman 1998). It was determined that the simultaneous use of the MN and comet assays was a useful battery of tests to determine the genotoxicity of CBE to the common carp.
The results of the current study indicate the ultrastructure of carp liver and gill cells were changed by exposure to CBE. As reported by Dietrich and Hoeger (2005), MCs were found to form non-covalent and covalent interactions with the target enzymes PPs (PP1, 2A, 4, and 5). These interactions could then inhibit the catalytic subunits of the PPs and cause disorganization of cellular architecture and degeneration, and usually followed by cell death (Dietrich and Hoeger 2005). A recent study on the histopathology of snails exposed to dissolved MC-LR (Lance et al. 2010) indicated severe pathological changes including cell lysis and necrosis, vacuolization as well as cell shape alteration, in the digestive gland. Those authors also detected MCs in spermatozoids and oocytes of the snails, which suggested MCs may have potential adverse effects on reproduction functions. This is similar to the TEM reported here, that MCs extracted from cyanobacteria in Taihu Lake caused damage to cells of carp and malformations in organelles. MCs led to the formation of digestive vacuoles in cytoplasm, fusion of lysosomes, swelling of the ER and vacuolization of the Golgi apparatus. This is consistent with the results of other studies that have found that ER in hepatic cells of rats and carp were the most sensitive organelle when exposed to crude MC-LR extracted from CBE (Berg et al. 1988; Li et al. 2001). The results of those studies suggested that the structural deformations were related to modifications of the cytoskeleton. ER and the Golgi apparatus have detection and signaling mechanisms that respond to stressors by either activating cell repair or death pathways (Maag et al. 2003; Hicks and Machamer 2005). In another study with ovary and kidney cells, a causal relationship between ultra structural changes of the ER and Golgi in response to CBE was observed (Davidson et al. 1992). The TEM observations made in our study also found that ER and mitochondria in cells of carp swelled and the nucleus became condensed. These results indicated that CBE alter cell structures which will then affect cell functions. All of these observations are consistent with cellular organelles being the intracellular target for effects of crude extracts of cyanobacteria. However, the mechanism for this response remains unknown.
The occurrence of toxic cyanobacterial blooms in eutrophic lakes, reservoirs, and recreational waters is a worldwide problem (Paerl et al. 2001). MCs produced during blooms can be toxic to aquatic animals and can accumulate in the food chain and become hazardous to wildlife and humans. Small concentrations of MC-LR can cause oxidative DNA damage and may be the mechanism through which chronic exposure to MCs contributes to increased cancer incidence (Zegura et al. 2003). A positive association has been reported between the incidence of primary liver cancer and exposure to cyanobacteria-contaminated drinking water (Yu 1995).
To our knowledge, in vitro studies using mammalian cells (Zhan et al. 2004; Lakshmana Rao et al. 1998; Humpage et al. 2000; Zegura et al. 2003; Lankoff et al. 2003, 2004, 2006), bacteria cells (Mankiewicz et al. 2002; Ding et al. 1999), as well as in vivo experiments using mice and brown rats (Lakshmana Rao et al. 1998; Rao and Bhattacharya 1996; Shen et al. 2002; Bouaicha et al. 2005) have reported that MCs are genotoxic. However, most previous studies have used commercially available MCs rather than MCs extracted from cyanobacteria blooms, and these commercially available MCs were less genotoxic than CBE (Mankiewicz et al. 2002). As far as we know, the current study is the first report of in vivo genotoxicity of crude MCs products extracted from a cyanobacteria bloom on fish, especially regarding on organs other than fish liver.
Conclusions
CBE from Taihu Lake are genotoxic to carp and can lead to formation of micronuclei and cause DNA strand breaks in a time- and dose-depended manner. The ultrastructure of carp liver and gill cells organelles including mitochondria, ER, nuclear membranes of organelles were changed by exposure to CBE. These changes on cell structures are supposed to result in changes in cell functions. More studies are needed to find the mechanism and pathways of the genotoxic damages. Eutrophication, cyanobacteria blooms are severe problems in Taihu Lake, and this highlights the need to investigate the corresponding toxicity of cyanobacteria, especially considering the long term toxicity effects.
References
Al-Sabti K, Metcalfe CD (1995) Fish micronuclei for assessing genotoxicity in water. Mutat Res 343:121–135
Aranda-Rodriguez R, Tillmanns A, Benoit FM, Pick FR, Harvie J, Solenaia L (2005) Pressurized liquid extraction of toxins from cyanobacterial cells. Environ Toxicol 20:390–396
Berg K, Wyman J, Carmichael W, Dabholkar A (1988) Isolated rat liver perfusion studies with cyclic heptapeptide toxins of microcystins and oscillatoria (freshwater cyanobacteria). Toxicon 26:827–837
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009) Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp Biochem Physiol C 150(1):50–56
Bolognesi C, Perrone E, Roggieri P, Pampanin DM, Sciutto A (2006) Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat Toxicol 78S:S93–S98
Bouaicha N, Maatouk I, Plesis MJ, Périn F (2005) Genotoxic potential of microcystin-LR and nodularin in vitro in primary cultured rat hepatocytes and in vivo in rat liver. Environ Toxicol 20:341–347
Buschini A, Martino A, Gustavino B, Monfrinotti M, Poli P, Santoro M, Dorr AJM, Rizzoni M (2004) Comet assay and micronucleus test in circulating erythrocytes of Cyprinus carpio specimens exposed in situ to lake waters treated with disinfectants for potabilization. Mutat Res 557:119–129
Chen HG, Li ZL, Xu Y, Kong ZM, Liu ZT (2006) DNA damage induced by sodium pentachlorophenate in crucian kidney cells in vivo and in vitro. J Environ Health 23:515–517 (in Chinese)
Chorus I, Bartram J (eds) (1999) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. E and FN Spon; World Health Organization, London; Geneva
Collins A, Dusinska M, Franklin M, Somorovska M, Petrovska H, Duthie S, Fillion L, Panayiotidis M, Raslova K, Vaughan N (1997) Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen 30:139–146
Datta S, Saha DR, Ghosh D, Majumdar T, Bhattacharya S, Mazumder S (2007) Sub-lethal concentration of arsenic interferes with the proliferation of hepatocytes and induces in vivo apoptosis in Clarias batrachus L. Comp Biochem Physiol C 145:339–349
Davidson HW, McGowan CH, Balch WE (1992) Evidence for the regulation of exocytic transport by protein phosphorylation. J Cell Biol 116:1343–1355
Dietrich DR, Hoeger SJ (2005) Guidance values for microcystin in water and cyanobacterial supplement products (blue-green algae supplements): a reasonable or misguided approach? Toxicol Appl Pharmacol 203:273–289
Ding WX, Shen HM, Shen Y, Zhu HG, Ong ChN (1998) Microcystic cyanobacteria causes mitochondrial membrane potential alteration and reactive oxygen species formation in primary cultured rat hepatocytes. Environ Health Perspect 106:409–413
Ding WX, Shen HM, Zhu HG, Le BL, Ong ChN (1999) Genotoxicity of microcystic cyanobacteria extract of a water source in China. Mutat Res 442:69–77
Djediat C, Malecot M, Luze A, Bernard C, Dao SP, Edery M (2010) Localization of microcystin-LR in medaka fish tissues after cyanotoxin gavage. Toxicon 55:531–535
Fawell JK, Mitchell RE, Everett DJ, Hill RE (1999) The toxicity of cyanobacterial toxins in the mouse: I microcystin-LR. Hum Exp Toxicol 18:162–167
Fenech M, Chang WP, Kirsch VM, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Fischer WJ, Altheimer S, Cattori V, Meier PJ, Dietrich DR, Hagenbuch B (2005) Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol Appl Pharmacol 203:257–263
Gaudin J, Huet S, Jarry G, Fessard V (2008) In vivo DNA damage induced by the cyanotoxin microcystin-LR: comparison of intra-peritoneal and oral administrations by use of the comet assay. Mutat Res 652:65–71
Gérard C, Poullain V, Lance E, Acou A, Brient L, Carpentier A (2009) Influence of toxic cyanobacteria on community structure and microcystin accumulation of freshwater mollusks. Environ Pollut 157:609–617
Gulland FM, Fauquier D, Langlois G, Lander ME, Zabka T, Duerr R (2002) Domoic acid toxicity in Californian sea lions (Zalophus californianus): clinical signs, treatment and survival. Vet Rec 150:475–480
Harada KI, Suzuki M, Dhalem AM, Beasly VR, Carmichael WW, Rinehart KL (1988) Improved method for purification of toxic peptides produced by cyanobacteria. Toxicon 26:433–439
Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys P, MacGregor JT (1991) Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen 18:277–291
Hicks SW, Machamer CE (2005) Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta 1744:406–414
Hoeger SJ, Hitzfeld BC, Dietrich DR (2005) Occurrence and elimination of cyanobacterial toxins in drinking water treatment plants. Toxicol Appl Pharmacol 203:231–242
Humpage AR, Fenech M, Thomas P, Falconer IR (2000) Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat Res 472:155–161
Lakshmana Rao PV, Bhattacharya R, Parida MM, Jana AM, Bhaskar ASB (1998) Freshwater cyanobacterium Microcystis aeruginosa (UTEX 2385) induced DNA damage in vivo and in vitro. Environ Toxicol Pharmacol 5:1–6
Lance E, Josso C, Dietrich D, Ernst B, Paty C, Senger F, Bormans M, Gérard C (2010) Histopathology and microcystin distribution in Lymnaea stagnalis (Gastropoda) following toxic cyanobacterial or dissolved microcystin-LR exposure. Aquat Toxicol 98:211–220
Lankoff A, Banasik A, Obe G, Deperas M, Kuzminski K, Tarzynska M, Jurczak T, Wojcik A (2003) Effect of microcystin-LR and cyanobacterial extract from Polish reservoir of drinking water on cell cycle progression, mitotic spindle, and apoptosis in CHO-K1 cells. Toxicol Appl Pharmacol 189:204–213
Lankoff A, Krzowski L, Glab J, Banasik A, Lisowska H, Kuszewski T, Gozdz S, Wojcik A (2004) DNA damage and repair in human peripheral blood lymphocytes following treatment with microcystin-LR. Mutat Res 559:131–142
Lankoff A, Wojcik A, Fessard V, Meriluoto J (2006) Nodularin-induced genotoxicity following oxidative DNA damage and aneuploidy in HepG2 cells. Toxicol Lett 164:239–248
Li XY, Liu YD, Song LR (2001) Cytological alterations in isolated hepatocytes from common carp (Cyprinus carpio L.) exposed to microcystin-LR. Environ Toxicol 16:517–522
Li YQ, Sun LW, Qu MM, Kong ZM, Liu ZT (2004) In vivo genotoxic effects of aminophenols in carp (Cyprinus caprio) kidney cells. Res Environ Sci 17:35–37 (in Chinese)
Lovell DP, Omori T (2008) Statistical issues in the use of the comet assay. Mutagenesis 23:171–182
Maag RS, Hicks SW, Machamer CE (2003) Death from within: apoptosis and the secretory pathway. Curr Opin Cell Biol 15:456–461
Maatouk I, Bouaicha N, Plessis MJ, Perin F (2004) Detection by 32P-postlabelling of 8-oxo-7,8-dihydro-2-deoxyguanosine in DNA as biomarker of microcystin-LR- and nodularin-induced DNA damage in vitro in primary cultured rat hepatocytes and in vivo in rat liver. Mutat Res 564:9–20
Mankiewicz J, Walter Z, Tarczynska Z, Palyvoda O, Wojtysiak SM, Zalewski M (2002) Genotoxicity of cyanobacterial extracts containing microcystins from Polish water reservoirs as determined by SOS chromotest and comet assay. Environ Toxicol 17:341–350
Mitchelmore CL, Chipman JK (1998) DNA strand breakage in aquatic organisms and the potential values of the comet assay in environmental monitoring. Mutat Res 399:135–147
Morel FMM, Price NM (2003) The biogeochemical cycles of trace metals in the oceans. Science 300:944–947
Paerl HW, Fulton R, Moisander PH, Dyble J (2001) Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci World 1:76–113
Puschner B, Galey FD, Johnson B, Dickie CW, Vondy M, Francis T, Holstege DM (1998) Blue-green algae toxicosis in cattle. J Am Vet Med Assoc 213(11):1605–1607
Qi LL, Chen ZB, Zou H, Ruan WQ (2009) HPLC determination of microcystin in Taihu Lake. J Food Sci Biotechnol 28(1):97–101 (in Chinese)
Rao PVL, Bhattacharya R (1996) The cyanobacterial toxin microcystin-LR induced DNA damage in mouse liver in vivo. Toxicology 114:29–36
Ross C, Santiago VL, Paul V (2006) Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquat Toxicol 78:66–73
Runnegar M, Berndt N, Kong SM, Lee EYC, Zhang LF (1995) In vivo and in vitro binding of microcystin to protein phosphatase 1 and 2A. Biochem Biophys Res Commun 216:162–169
Schnetzer A, Miller PE, Schaffner RA, Stauffer BA, Jones BH, Weisberg SB, DiGiacomo PM, Berelson WM, Caron DA (2007) Blooms of Pseudo-nitzschia and domoic acid in the San Pedro Channel and Los Angeles harbor areas of the Southern California Bight, 2003–2004. Harmful Algae 6:372–387
Shen X, Lam PKS, Shaw GR, Wickramasinghe W (2002) Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Short Commun Toxicon 40:1499–1501
Shumway SE, Allen SM, Boersma PD (2003) Marine birds and harmful algal blooms: sporadic victims or under-reported events? Harmful Algae 2:1–17
Sournia A, Chrdtiennot-Dinet MJ, Ricard M (1991) Marine phytoplankton: how many species in the world ocean? J Plankton Res 13:1093–1099
Steinert SA (1999) DNA damage as bivalve biomarker and as an environmental assessment tool. Biomarkers 4:492–496
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Rojas E, Miyamae Y, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Xu QJ, Chen WM, Gao G (2008) Seasonal variations in microcystin concentrations in Lake Taihu, China. Environ Monit Assess 145:75–79
Yu SJ (1995) Primary prevention of hepatocellular carcinoma. J Gastroenterol Hepatol 10:674–682
Zegura B, Sedmak B, Filipic M (2003) Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 41:41–48
Zhan L, Sakamoto H, Sakuraba M, Wu DS, Zhang LS, Suzuki T, Hayashi M, Honma M (2004) Genotoxicity of microcystin-LR in human lymphoblastoid TK6 cells. Mutat Res 557:1–6
Zhu Y, Wang J, Zhang R (2004) Cadmium, chromium, and copper induce polychromatocyte micronuclei in Carp (Cyprinus carpio L.). Bull Environ Contam Toxicol 72:78–86
Acknowledgments
This work was supported by the National Basic Research Program of China (973 program) (No. 2008CB418102 and 2008CB418103) and the National Major Project of Science & Technology Ministry of China ((2008ZX07101-004, 2008ZX07526-003). The research was supported, in part, by a Discovery Grant from the National Science and Engineering Research Council of Canada (Project # 326415-07) and a grant from the Western Economic Diversification Canada (Project # 6578 and 6807). The authors wish to acknowledge the support of an instrumentation grant from the Canada Foundation for Infrastructure. Prof. Giesy was supported by the Canada Research Chair program and an at large Chair Professorship at the Department of Biology and Chemistry and State Key Laboratory of Marine Pollution, City University of Hong Kong. We also want to express gratefulness to Prof. Xiaorong Wang and Dr. Jinlin Jiang of Nanjing University for their assistance in the HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Li, M., Gao, X. et al. Genotoxicity of crude extracts of cyanobacteria from Taihu Lake on carp (Cyprinus carpio). Ecotoxicology 20, 1010–1017 (2011). https://doi.org/10.1007/s10646-011-0670-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0670-9