Abstract
Environmental effects associated with the release of various metals even at maximum permissible concentrations (MPC) to the aquatic ecosystems are evident. In the present work, time-dependent increase in accumulated metals amount in gills of Anodonta cygnea after exposure to complex metal (Zn 0.1, Cu 0.01, Ni 0.01, Cr 0.01, Pb 0.005, and Cd 0.005 mg/L, MPC accepted for the inland waters in EU) mixture at various time points (1, 2, 4, 7, 14, and 28 days) was investigated. Statistically significant increase of Cu and Cd was determined in mussel’s gills after 7-day exposure, in comparison to control group; moreover, significantly elevated concentration of Cu was measured and after 14-day treatment (in comparison to control and pre-exposure group). Concentrations of five (Cu, Ni, Cr, Pb, and Cd) out of 6 investigated metals were statistically increased in gills tissue after 28-day treatment. Moreover, complex metal mixture has demonstrated tissue- and time-dependent genotoxicity (∑Gentox) and cytotoxicity (∑Cytox) responses in mussels. After 4-day exposure, there were found the highest ∑Gentox levels in gills cells and haemocytes. Two-day treatment of mussels resulted in the highest and statistically significant induction of ∑Cytox level (in gills). Furthermore, after short-term (4 days) exposure, statistically significant inhibition of AChE activity in hemolymph of metal mixture–exposed mussels, in comparison to control and pre-exposure group, was found. Comparison of investigated responses in different tissue of A. cygnea discloses new information about metal mixture (at MPC) impacts at different treatment time. According to the obtained geno- and cytotoxicity data, it is suggested that gills are more sensitive tissue. Environmentally relevant trace metal concentrations when existing in mixture are able to cause adverse effects in A. cygnea; therefore, biological effects at different levels of organism are expected as a realistic scenario.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal pollution from anthropogenic origin in the world’s water ecosystems is still continually rising up due to increasing urbanization, industrialization, agricultural expansion, and manipulation of mineral resources (Govind and Madhuri 2014; Jaishankar et al. 2014; Strungaru et al. 2018; Yahya et al. 2018). Concerning toxicity, bioaccumulation, and persistence in the aquatic environment, six metals such as Zn, Cu, Ni, Cr, Pb, and Cd are attributed to priority hazardous substances in many countries throughout the world (Directive 2008/105/EC; US EPA 2009). Toxic effects of these trace metals alone or in combination with other ones have been presented in multifarious aquatic species under laboratory and environmental conditions (Yahya et al. 2018). Usually in water environment, many of the metals exist in various mixtures. Due to the interactions between chemicals, even low metal concentrations in the mixture (at the Maximum Permissible Concentrations (MPC)) are able to induce DNA damage in aquatic organisms (Stankevičiūtė et al. 2017, 2018). Furthermore, trace metals can be accumulated in the tissues of organisms and may adversely affect functions of cell by interacting with the systemic enzymes. This can lead to disturbances of growth, reproduction, the immune system, and metabolism of the organisms. In order to achieve “good ecological and chemical status” in transitional and coastal waters, Water Framework Directive (WFD, 2000/60/EC) has defined a list of priority pollutants, which are deemed to have detrimental effects on the environment, with a requirement for substantial monitoring of these pollutants. Bivalve mussels are considered as reliable-sentinel species for the bioindication of aquatic pollution due to their sedentary nature, filter-feeding behavior, and ability of bioaccumulation (Torre et al. 2013; Pagano et al. 2017; Savorelli et al. 2017; Faggio et al. 2018). In freshwater food webs, Unionid mussels play an important role; they are able to control water quality. Moreover, they are widespread, abundant, large, and long-lived and are commonly used as bioindicators of freshwater ecosystem health (Guidi et al. 2010; Pourang et al. 2010; Kolarević et al. 2016), as biomonitors (Rzymski et al. 2014), and as model test organisms in various ecotoxicity (Bielen et al. 2016; Lopes-Lima et al. 2017) and genotoxicity (Baršienė et al. 2006) experimental investigations. Anodonta cygnea (belongs to Unionidae family) is a freshwater mussel, which can be found in rivers and lagoons all over the Europe and the Northern America (Lopes-Lima et al. 2017). In some countries, this species is listed as near threatened or threatened owing to severe declines in abundance of distribution (Lopes-Lima et al. 2017; Huber and Geist 2017). Most likely, this decline is mainly caused by anthropogenic activity (Dudgeon et al. 2006; Geist 2011).
Unionidae family is recognized as a suitable freshwater group for assessment of aneugenic and clastogenic damage in aquatic systems under controlled experimental (Sohail et al. 2017) and environmental conditions (Guidi et al. 2010; Kolarević et al. 2016).
In the hemolymph, circulating haemocytes play an important role not only in immune system, but also in detoxification, oxygen transport, and biomineralization (Hinzmann et al. 2013; Matozzo et al. 2016; Burgos-Aceves and Faggio 2017; Burgos-Aceves et al. 2018). As target tissue, haemocytes of Anodonta sp. were used in environment genotoxicity investigations (Falfushynska et al. 2016; Kolarević et al. 2016). Experimental studies of dose and/or time-related induction of micronuclei and/or other nuclear abnormalities in Anodonta sp. was found after treatment with different compounds: mitomicin C (Scarpato et al. 1990), B(a)P (Woźnicki et al. 2004), crude oil (Baršienė et al. 2006; Eskandari et al. 2012), nanoscaled polymeric complex (Falfushynska et al. 2012), metals Cu, Zn, and Cd (Falfushynska et al. 2013a), fungicide (Falfushynska et al. 2013b).
Bivalve’s gills have respiratory and feeding functions. Furthermore, significant potential for accumulation of pollutants is either noticed. Gill cells can filter great quantities of contaminant agents presented in water; therefore, they are used as target tissue for assessment of pollution in various water bodies. Constantly exposed to dissolve contaminants, gills are capable of metabolizing carcinogens and mutagens into reactive products (Zhang et al. 2017). In several investigations, gill cells of the mussels have been presented as more vulnerable tissue for genotoxic-related contaminant’s determination than even haemocytes (Bourgeault et al. 2010; Vincent-Hubert et al. 2011).
Evaluation of geno- and cytotoxicity endpoint responses in various aquatic organisms and their tissues is one of the most promising, sensitive, and fastest cytogenetic methods in detecting of DNA damage caused by toxic agents. Usually, micronuclei (MN) are formed during the process of cell division. Induction of MN depends on cell cycle kinetics; and their expression can occur at different times after the DNA damage. Other nuclear abnormalities such as nuclear buds, fragmented-apoptotic, binucleated, and 8-shaped cells can also present the changes at the DNA level. Formation of nuclear buds (NB) may either reflect the clastogenic action of the toxic agents and unequal capacity of the organisms to expel damaged, amplified, failed DNA replication or improperly condensed chromatin, chromosome fragments without telomeres and centromeres from the nucleus (Lindberg et al. 2007). MN and NB suggest a similar origin, and both can be applied as genotoxicity analogs (Crott et al. 2001; Serrano-García and Montero-Montoya 2001; Lindberg et al. 2007). Binucleated cells are formed in abnormal cell division due to blocking of cytokinesis (Cavas et al. 2005). Eight-shaped cells also reflect a failure of the cell division, and they are formed from a part of the mitotic spindle. Elimination of cytogenetic damage by the apoptosis and necrosis is a key process, which occurs at different rates in various organisms (Elmore 2007).
Acetylcholinesterase enzyme (AChE) is an essential enzyme in the prevention of acetylcholine accumulation and the termination of the nerve impulse transmission frequently used in marine and freshwater pollution monitoring as biomarker of exposure to neurotoxic compounds (Robillard et al. 2003; Falfushynska et al. 2009; Palais et al. 2012; Vidal-Liñán et al. 2014; Benali et al. 2015). Earlier studies have shown that lower AChE activity is associated with effects of phosphoroorganic and carbamate insecticides. However, the number of other investigations revealed that other compounds such as metals can also change AChE activity. Laboratory research studies with bivalves exposed to various metals (Fe, Cu, Cd, and Pb) (Bainy et al. 2006), pesticides (Rickwood and Galloway 2004; Khazri et al. 2017; Perić et al. 2017), polycyclic hydrocarbons PAH, BDE, and PCBS (Vidal-Liñán et al. 2015a, b, 2016) demonstrate inconsistent results in AChE activity.
The goal of this study was to assess the toxicity effects of six-metal (Zn, Cu, Ni, Cr, Pb, and Cd) mixture at the maximum permissible concentrations (MPC) after 1, 2, 4, 7, 14, and 28-day exposure on molecular and cellular stress responses of Anodonta cygnea (Unionidae) in hemolymph and gill tissues. Analysis of geno- and cytotoxicity (five different endpoints) in haemocytes and gill cells was used to evaluate tissue-specific responses over time after six-metal mixture treatment. Neurotoxicity was determined by measuring acetylcholinesterase activity (AChE) in hemolymph in pre-exposure group (from River Neris), control, and after short-term (4 days) and long-term (28 days) exposure to six-metal mixture.
Materials and methods
Chemicals
All chemicals were purchased from Sigma Aldrich (Germany), Merck (Germany), Roth (Germany), and Reachim (Russia) and were of the analytical grade or higher.
Preparation of test solutions
Reagent grade metal salts («REACHIM» Company, Russia) were used as the toxicants. For each metal, stock solutions 1000 mg/L were prepared by dissolving necessary amount of the salts in distilled water, the final concentration being recalculated according to the amount of metals ions. These dilutions were further diluted. For test concentration, 1000 mL complex solution was made up in a HDPE bottle. Solution was acidified with reagent-grade nitric acid (final concentration 0.5% v/v). Concentrated stock solution was prepared one day prior to the test. Mussels were transferred to tanks after concentrated stock solutions were diluted in the deep-well water.
Animal collection and experimental exposures
Adults of Swan Mussels (Anodonta cygnea Linnaeus, 1758) at approximately the same size (the average of shell length 92.46 ± 5.03 mm, width − 46.49 ± 4.56 mm, weight 50.86 ± 5 g) were collected at the depth of 1.5 m, about 0.8 m from the bank of River Neris (Lithuania) in Verkiai Regional Park (54° 44′ 47.3″ N and 25° 17′ 39.4″ E) in 2017 autumn. According to, visible annual growth lines on the shell, there were estimated age of mussels (~ 5–6 years) (according to method by Czechowski et al. 1994). Site of collection is expected to nonsource pollution from agricultural runoff, urban waste, or industrial pollution. Pre-exposure group of mussels (seven individuals) from River Neris was dissected straight off, and their tissues collected for further analyses. Other specimens were transported to the laboratory in cages with aerated native water. Mussels were kept for acclimation in holding tanks (1000-L volume) supplied with flow-through aerated deep-well water at least 2 weeks prior to testing. Mussels were kept under a natural light cycle and fed daily in the morning by commercial mussel’s feed (algal concentrate “Shellfish Diet 1800”), amount was given according to recommendations presented by producer. During the experiment, both water and diet were of the same type. Deep-well water was used as the dilution water. Its chemical characteristics are given in Table 1 (ISO 15586 2003; ISO 6332 1988; ISO 5814 1990; ISO 10523 2008; ISO 14911 1998; ISO 10304-1 2007; ISO 9963-1 1994). Mussels were randomly split into 10 groups (seven individuals per group, N = 70). A. cygnea specimens were put in polyethylene tanks of 35 L total volume filled to a level of 30 L with continuously aerated dilution water. The experiment was conducted under semi-static rotating water current conditions. In this experiment, four controls for exposure 4, 7, 14, and 28 days were selected for making sure that animals used in minimum numbers (according to the “3 Rs”—reduce the number of animals used to a minimum, to obtain information from a smaller number of animals) (Directive 2010/63/EU). Control groups (for 4, 7, 14, and 28-day exposure) were exposed to the same handling procedure, but not treated with six-metal mixture (since there were no differences found in geno- and cytotoxicity, they were expressed as one control group).
Test mussels were exposed for the 1-, 2-, 4-, 7-, 14-, and 28-day period to a six-metal (Zn, Cu, Ni, Cr, Pb, and Cd) mixture at a concentration corresponding to maximum permissible concentrations (MPC) accepted for the inland waters in EU (Directive 2008/105/EC) (Table 1). Test solutions and clean water were renewed every day, and mussels were transferred into freshly prepared solutions after they were fed. Water conditions (chemical and physical characteristics) are presented in Table 1. After, treatments with six-metal mixture at different time mussels were dissected, and their tissues (gills and hemolymph) collected for further (geno-cytotoxicity, neurotoxicity, and metal bioaccumulation) analyses. Only one individual after 28-day treatment with metal mixture was detected dead throughout all experiment. For each mussel physiological parameters, shell length, total weight, whole soft body, and gill weight were recorded. The condition index of the gills (GI) was calculated as the ratio: (drained mass of organ/total mass) × 100 (according to Falfushynska et al. 2010).
Analytical procedures
The main physico-chemical parameters of the water (temperature, dissolved O2, pH, and conductivity) were measured routinely with a hand-held multi-meter (Hanna Instruments HI98196, USA) (Table 1). Designed nominal metal concentrations in the tanks were checked during blank tests (without mussels) (N = 4) with an atomic absorption spectrophotometer (SHIMADZU AA-6800, Japan) by graphite furnace technique using proprietary software. Each water sample was acidified with reagent-grade nitric acid (final concentration 0.5% v/v) and analyzed in triplicate. Mean measured concentrations were within 5–20% of the target (Table 1).
Geno-cytotoxicity biomarkers
Hemolymph from A. cygnea was carefully extracted from the organisms using a 25 G needle (B. Braun, Germany) attached to a 1 mL sterile syringe (B. Braun) by insertion between the valves across the inner layer of the mantle into the interepithelial space. Three drops of hemolymph were smeared on clean microscopic slide and easily distributed with a spatula; another part was injected to a 1.5 mL microcentrifuge tube (Eppendorf, Germany) for enzyme activity analysis. Hemolymph was centrifuged at 3000 rcf for 5 min at 4 °C and collected supernatant was stored on ice until AChE assay.
A piece of mussel’s gills was placed on a slide and gently nipped with tweezers for 2–3 min until the cells spread within the drop. The cell suspension was then softly smeared on the whole surface of the slide; all other gills were frozen for metal accumulation analysis. Microscopic slides were air-dried, fixed in methanol for 10 min, and stained with 5% Giemsa solution in phosphate buffer (pH 6.8). Blind scoring of nuclear abnormalities was performed on coded slides under the light microscopes (Olympus BX51 (Tokyo, Japan) and Nikon Eclipse 50i (Tokyo, Japan)) at a final magnification of × 1000. The photos were taken with the microscope camera Olympus DP-70 (Tokyo, Japan). For each studied mussels’ specimen, all cells with intact cytoplasm were scored. Final results were expressed as the mean value (promiles, ‰) of sums of the analyzed individual lesions scored in 1000 cells per bivalve from each experiment group.
Haemocytes and gill cells with micronuclei (MN) and nuclear buds (NB) were registered as genotoxicity endpoints, fragmented-apoptotic (FA), binucleated (BN), and eight-shaped nuclei cells—as cytotoxicity endpoints. Sum of genotoxicity parameters (MN + NB) was expressed as total genotoxicity (∑Gentox), sum of cytotoxicity (FA + BN + 8-shaped)—as total cytotoxicity (∑Cytox). Registered nuclear abnormalities (NAs) were identified using criteria described in previous publications (Baršienė et al. 2012, 2015; Butrimavičienė et al. 2018; Valskienė et al. 2018). The morphological features of the studied NAs are presented in Fig. 1.
Neurotoxicity biomarker
The acetylcholinesterase activity was determined in hemolymph samples using the method of Ellman et al. (1961) modified for microplates. Briefly, samples or buffer blanks (50 μL) were incubated for 5 min in microplates at 20 °C with 270 μM 5,5’-dithio-bis-(2-nitrobenzoic acid), in 50 mM phosphate buffer, pH 7.4. Measurement of absorbance at 412 nm was started after addition of substrate acetylthiocholine iodide (ACI), 3 mM, and was finished after 5 min. Spontaneous ACI hydrolysis was determined in the absence of hemolymph. Measurements were conducted in triplicate. The enzyme activity was expressed as nmol substrate hydrolyzed min−1 mg−1 relative to the protein in the sample. Total protein concentration was determined according to the method of Bradford (1976) using Bradford’s reagent with bovine serum albumin as standard. Absorbance readings were taken in the 96-well flat-bottom microplates (BRAND, Wertheim, Germany) by using an absorbance plate reader Tecan Infinite M200 with computer program i-controlTM (Tecan Group Ltd, Männedorf, Switzeland).
Metals determination
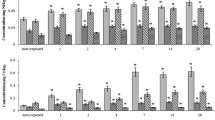
Concentrations of lead (Pb), nickel (Ni), copper (Cu), chromium (Cr), cadmium (Cd), and zinc (Zn) were assessed via inductively coupled argon plasma spectroscopy (ICP). For metal determination in mussels’ tissue samples, a procedure adapted from Sen and co-authors (Sen et al. 2011) was applied. For metal analysis, frozen A. cygnea mussels were dissected using stainless steel instruments. One to two grams w.w. of the mussels’ samples were used for the analysis. The samples were digested with 10 ml of ultra-pure nitric acid at 95 °C until the solution became clear (around 90 min). Subsequently, 3 ml of H2O2 were added and the solution was kept at 100 °C for 120 min. Afterwards, the volume of the solution was reduced to approx. 2 ml by evaporating the acid at 80 °C without boiling, the cooled digested solutions were filtered through a Whatman filter into 25 ml volumetric flask and made up to a 100 ml volume using deionized water. The as-prepared samples were analyzed for metals using inductively coupled argon plasma spectrometer Perkin Elmer Optima 7000 DV ICP-OES. For quality control of the results, the NIST Standard reference material 2702 was used. The precision of the analytical procedures, expressed as the standard deviation, was approximately up to 15%.
Statistical analysis
The most of geno- and cytotoxicity, acetylcholinesterase activity, and metal bioaccumulation data do not follow a normal distribution (Kolmogorov-Smirnov and Shapiro-Wilk normality test). Geno-, cytotoxicity, and acetylcholinesterase activity data were analyzed by the nonparametric Mann-Whitney U test (using GraphPad Prism® 5.01 (GraphPad Software Inc., San Diego, CA, USA)). Kruskal-Wallis test followed by Dunn’s multiple comparison test was applied to analyze metal bioaccumulation data. The results were expressed as mean ± standard error or standard deviation. The level of significance was established at p < 0.05.
A discriminant function analysis (DA) using STATISTICA 7.0 (StatSoft Inc., Tulsa, Oklahoma, USA) was performed to determine which of analyzed variable (metal concentration, GI, and nuclear abnormalities) coefficients distinguished exposure days from each other and to identify any statistical similarity among data due to overlapping of statistical ellipses. Functions 1 and 2 explain the highest proportion of the total variability in the data. Only functions that presented p < 0.05 and eigenvalues greater than 1 were considered statistically significant. Ellipses (round) show 95% confidence intervals and if they did not overlap indicate differences between exposure days.
Results
Geno- and cytotoxicity in haemocytes of mussels
The levels of total genotoxicity (∑Gentox) and total cytotoxicity ((∑Cytox) in A. cygnea haemocytes are given in Fig. 2. The level of ∑Gentox in haemocytes varied from 8.16‰ in pre-exposure to 34.76‰ in 4-day treatment group; the value of the ∑Gentox incidences in haemocytes of mussels after 4-day exposure was 4.3 times higher and statistically significant (p = 0.025) in comparison to pre-exposure level (8.16‰). The lower level of ∑Gentox, than in 4-day treatment group, was determined in 14 days group and was equal to 31.76‰. The lowest ∑Gentox after exposure to six-metal mixture was estimated in A. cygnea specimens after 28 days in comparison to other (1, 2, 4, 7, and 14 days) treatment groups. Statistical analysis revealed significant differences of ∑Gentox in the mussels exposed for 1, 2, 4, and 7 days in comparison to pre-exposure group. Moreover, after 7-day treatment ∑Gentox level was significantly higher in comparison to pre-exposure (p = 0.015) and control (p = 0.041) groups.
The levels of the ∑Cytox in haemocytes of mussels were lower (from 2 to 8 times) in comparison to the ∑Gentox. The lowest level of ∑Cytox was found in specimens from pre-exposure group (0.4‰), while the highest one (21.80‰) in 7-day treatment group. Moreover, after 7-day treatment, the level of ∑Cytox was statistically significant in comparison to pre-exposure (p = 0.006) and control (p = 0.011) groups. Statistically significant changes of ∑Cytox levels in comparison with pre-exposure and control groups were found in A. cygnea specimens after 1-day (p = 0.001 and p = 0.011, respectively), 2-day (p = 0.001 and p = 0.011), 4-day (p = 0.002 and p = 0.019), and 14-day (p = 0.002 and p = 0.037) treatment.
Likewise, in ∑Gentox, the lowest ∑Cytox level after treatment with six-metal mixture was estimated in specimens after 28-day exposure, in comparison to other treatment groups (Fig. 2).
Geno- and cytotoxicity in gill cells of mussels
The levels of total genotoxicity (∑Gentox) and total cytotoxicity (∑Cytox) in A. cygnea gill cells are shown in Fig. 3. The highest induction of ∑Gentox in gill cells of mussels was estimated in 4-day treatment group and was equal to 40.80‰. The lowest level of ∑Gentox was found in control group (6.47‰). Fewer incidences of genotoxicity (MN and NB) were found in A. cygnea gill cells after 14-day treatment with six-metal mixture.
Statistically significant changes of ∑Gentox were found in 2- (p = 0.003 and p = 0.002), 4- (p = 0.002 and p = 0.002), and 14-day (p = 0.007 and p = 0.002, respectively) treatment groups in comparison to pre-exposure and control groups. After 7-day exposure, the level of ∑Gentox differed significantly from control group (p = 0.003).
Induction of ∑Cytox was at the highest level (38.90‰) in mussels from 2-day treatment group. The lower level (34.34‰) of ∑Cytox was found in specimens from 4-day treatment group. The lowest value (4.29‰) of ∑Cytox was found in control group. Significant differences compared to pre-exposure and control groups were found in 2- (p = 0.004 and p = 0.002) and 4-day (p = 0.009 and p = 0.002, respectively) treatment groups. After 14- and 28-day exposure, the levels of ∑Cytox differed significantly from control (p = 0.008 and p = 0.040, respectively).
Interestingly, 2-day exposure to six-metal mixture caused much higher level of the ∑Cytox than ∑Gentox in comparison to long-term treatment groups.
Neurotoxicity (AChE) in hemolymph of mussels
After 4-day of exposure to six-metal mixture, mussels presented a statistically significant decrease in AChE activity (12.37 ± 1.462 nmol ACTC min−1 mg), when compared to the control (18.19 ± 1.58 nmol ACTC min−1 mg; p = 0.048) and pre-exposure (17.81 ± 1.977 nmol ACTC min−1 mg; p = 0.038) groups, which corresponds to approximately 32% of control and 31% of pre-exposure groups. Slight decrease (16.17 ± 1.748 nmol ACTC min−1 mg; p = 0.0381) in AChE activity was measured after long-term (28 days) metal mixture treatment and it was approximately 11% lower, in comparison to 28-day control (18.22 ± 2.189 nmol ACTC min−1 mg) and 9% lower in comparison to pre-exposure (17.81 ± 1.977 nmol ACTC min−1 mg) group, but did not reach statistical significance.
Determination of metals in gills of mussels
Analysis of six-metal concentrations in gills of the mussel’s revealed time-dependent accumulation. The highest and mostly statistically significant accumulation of all examined metals, except Zn, was determined in gills after 28-day treatment. After 7-day exposure, there found statistically significant accumulation of Cu and Cd in comparison to control group (Table 2). Moreover, significant amount of Cu was accumulated after 14-day treatment in comparison to pre-exposed and control groups.
Discriminant function analysis
The discriminant analysis using data of metal concentrations in mussel’s gills and nuclear lesions in gills and hemolymph cells after different metal mixture exposure days indicated three significant functions (Table 3). The first two discriminant functions have the highest eigenvalues and explain the largest proportion of the total variability in the data (Table 3). Discriminant function analysis indicated Cu, Cd, Cytox (in gills), Gentox (in gills), and CI as variables, which significantly (p < 0.049) contributed to separation between exposure days. Copper indicated the highest coefficient (− 1.042) in function 1. ∑Cytox and ∑Gentox (in gills) and Cd indicated the highest coefficients (0.583, 0.621, and − 0.537, respectively) in function 2, whereas ∑Cytox and ∑Gentox (in gills), Cu, Cd, and CI presented the highest coefficients (− 0.955, 0.572, 0.532, − 0.564, and 0.730) in function 3. Data of 28 days of exposure to metal mixture were separated from the data of other treatment days. The similarities were detected between data of pre-exposure, control, and 1-day treatment. Scatterplots of function 1 versus function 2 are presented in Fig. 4.
Discussion
In the present study, time-dependent increase in accumulated metal amount was found in investigated mussels exposed to six-metal mixture. For the first, statistically significant increase of metals, i.e., Cu and Cd after 7 days, and Cu after 14-day exposure, was determined in mussel’s gills. Moreover, concentrations of five (Cu, Ni, Cr, Pb, and Cd) out of six investigated metals were statistically increased after 28-day treatment. Most likely, determined metal concentrations, even after short exposure time, resulted in a significant DNA damage, especially after 4-day treatment. After 4-day exposure, there were found the highest ∑Gentox levels in both analyzed tissues. Moreover, statistically noticeable decrease in physiological response such as gill’s condition index (GI) was detected in this treatment group. It must be noted that after short-term (4 days) treatment, there was measured statistically significant inhibition of AChE activity in hemolymph of investigated mussels, in comparison to control and pre-exposure group. Decrease in AChE activity observed initially in the present study might be a response to metal mixture toxicity.
Two-day treatment of mussels resulted in the highest and statistically significant induction of ∑Cytox level. Comparison of geno- and cytotoxicity responses in hemolymph and gill tissue of A. cygnea discloses new information about six-metal mixture impacts at different treatment time. According to the obtained geno- and cytotoxicity data, it is suggested that gills are more sensitive and “first attack” tissue. Short-term (4 day) treatment with six-metal mixture initiated 32% decrease in AChE activity, while long-term (28 days) exposure had only 11% decrease compared to control group.
Measurement of AChE activity in hemolymph of bivalve mussels (Mytilus galloprovincialis, M. edulis, and Aulacomya ater) as biomarker was discussed in earlier investigations (Moreira et al. 2001; Rickwood and Galloway 2004; Führer et al. 2012). The main advantages of hemolymph usage for the toxicity analysis are quick, easy to use, and a nondestructive technique. It was shown that AChE activity in M. edulis hemolymph was not reduced by pesticide chlorfenvinphos after 24-, 48-, and 96-h exposure (Rickwood and Galloway 2004). According to Brown and co-authors (Brown et al. 2004), the AChE activity in M. edulis hemolymph was not inhibited after 7-day exposure to copper. Inhibition of AChE activity in M. edulis hemolymph was observed after 24-h treatment with 0.1 mg L−1 insecticide azamethiphos (Canty et al. 2007). There was detected inhibition of AChE in hemolymph of mussels Aulacomya ater after exposure with insecticide Lorsban 4E containing chlorpyrifos (Führer et al. 2012).
Results of enzyme’s activity in mussels can also depend on target tissue used for AChE assay (Franco-Martinez et al. 2016). Inhibition in AChE activity in various mussels and in their different tissues was presented previously in experimental and in situ studies. Exposure with 40 mg L−1 of Cu for 12, 24, 72, and 120 h has not affected AChE activity in digestive gland of Perna perna (Bainy et al. 2006). Very strong AChE inhibition in gills of M. trossulus was observed in response to an organophosphate dichlorvos treatment (Kopecka-Pilarczyk 2010). Inhibition of AChE activity in gills of M. galloprovincialis was measured after long-term (30 days) treatment to 4-nonylphenol (Vidal-Liñán et al. 2015a). Sublethal dose of B(a)P significantly inhibited AChE activity in M. galloprovincialis digestive gland (Kamel et al. 2012). AChE activity in freshwater mussels Unio ravoisieri gills significantly decreased with the increase of permethrin concentration (Khazri et al. 2017). Significant inhibition of AChE activity was noted in the gills (16.93 ± 3.1 nmol min−1 mg prot−1) and the digestive gland (7.69 ± 1.79 nmol min−1 mg prot−1) of the M. galloprovincialis after caging in harbor zone (Taleb et al. 2009). Significantly inhibited AChE activity in gills was related to the trace metals concentrations in transplanted M. galloprovincialis (Vidal-Liñán et al. 2014). The response of AChE activity (in the whole soft tissues) was significantly induced in mussels P. perna collected from various polluted beaches of the Big Casablanca, in comparison to control values (El Jourmi et al. 2014).
Our results indicate that even at the low concentrations (at MPC) in metal mixture, metals can induce significant AChE inhibition in Anodonta cygnea hemolymph after short-term (4 days) exposure. However, possible defense mechanisms exist in A. cygnea, which can reduce effects of metal mixture on AChE after prolonged exposure time (28 days). According to Jackin (1974), metals can stimulate or even depress enzymes activity. Concentration of certain metals in the mixture and exposure time can also affect these processes.
Genotoxic properties of heavy metals are related evidently to the accumulation of DNA damaging free radicals, clastogenic process, or simultaneously to clastogenic and aneugenic action in aquatic organisms (Nepomuceno et al. 1997). Metal related induction in micronuclei was assessed in haemocytes of A. anatina (Falfushynska et al. 2013a). Exposure with cadmium increased concentration-dependent DNA damage in haemocytes of mussel Sinanodonta woodiana (Kolarević et al. 2013). Metal (Pb + Cr + Cu) mixture induced DNA damage in gill cells of mussels Anodonta anatina (Sohail et al. 2017). Induction of MN incidences was higher after ternary metal (Cu + Cd + Pb) mixture treatment compared to single metal exposure (Zhang et al. 2017). Induction of genotoxic effects, caused by heavy metal contamination was assessed in haemocytes of freshwater mussel A. cygnea collected in River Kabul (Khan et al. 2018).
The long-term exposure with metals might make cells more susceptible to mitogenic stimulation and that alterations in mitogenic signalling proteins might contribute to the ability to damage the genome or to the disruption of cellular metabolic processes. The present study revealed that metals even at MPC concentrations are able to induce time- and tissue-dependent geno- and cytotoxicity effects in A. cygnea hemolymph and gills. According to literature data, time-related and statistically significant induction of genotoxicity (MN incidences were 20 times higher) was found after 7-day exposure to B(a)P in haemocytes of A. woodiana in comparison to control group (Woźnicki et al. 2004). After 7-day exposure to B(a)P and heat stress, there were assessed significant correlation between MN frequency and AChE activity in digestive gland of M. galloprovincialis (Kamel et al. 2012). Exposure for 14 days with Cu (10 mg L−1), Zn (130 mg L−1), and Cd (15 mg L−1) provoked an increase in MN and other nuclear abnormalities in haemocytes of A. anatina (Falfushynska et al. 2012). 10-day treatment with different concentrations (150, 350, 450 μg L−1) of Cu induced DNA damage in A. cygnea hemolymph (Arjmand et al. 2012). After 24-day exposure with Cr followed by 12 days of depuration in gill cells of A. woodiana, induced DNA damage was presented by Nugroho and co-authors (Nugroho et al. 2015). However, after 28-day treatment, there was observed reduction in geno- and cytotoxicity levels, in comparison to fourth day’s levels, it could be due to prolonged exposure to contaminants (28 days), which may result in acclimatization process or due to cells proliferation in tested organism tissues. Time-related decrease in the rate of nuclear abnormalities after exposure with metals was noticed in freshwater fish Labeo rohita exposed to CdCl2 (0.37 and 0.62 mg/L) for 100 days. Induction in erythrocytic nuclear abnormalities increased with the exposure period up to the tenth day, but after 15 days of the exposure, the rate of nuclear abnormalities began to decline (Jindal and Verma 2015). Treatment with B(a)P induced a significant increase in DNA strand breaks in mussel P. viridis hepatopancreas after 1 day of exposure, followed by a gradual decrease in strand breaks after 3–6 days, and after 12 days, the frequency of DNA strand breaks returned to the control level (Ching et al. 2001).
Tissue-specific genotoxicity has been assessed in M. galloprovincialis cells in vitro after exposure to heavy metals Zn, Cu, Cd, and Pb. Gill cells were more sensitive to Zn exposure among hemolymph and digestive gland cells (Zhang et al. 2017).
Correlation between DNA damage and hazardous priority substances was estimated in the haemocytes of freshwater mussels Unio pictorum, U. tumidus, and S. woodiana (Kolarević et al. 2016). DNA damage in haemocytes of S. woodiana, which were collected from polluted environment sites, significantly correlated with Zn concentration in water (Kolarević et al. 2013). Statistically significant differences of elevated DNA damage in soft tissues of zebra mussels (Dreissena polymorpha) from the harbor (Great Lakes tributaries) and the measured concentrations of heavy metals in comparison with the reference site were presented by Jaruga and co-authors (Jaruga et al. 2017). Accumulation of metals in organisms depend upon abiotic (metal solubility, metal speciation, and complexion) and biotic (growth, biochemical composition, reproductive condition, metabolism, and excretion) factors. Different metal bioconcentration factor for mussels from sediments and from water was demonstrated in bivalves earlier (Jitar et al. 2015).
After 4-day exposure, there were found the highest induction of ∑Gentox in both A. cygnea tissues, while gill’s condition index (GI) showed a noticeable decrease. Experimental studies have revealed a negative correlation between MN, NB, and biological parameters and gross morphometric indices in fish (Stankevičiūtė et al. 2016). Moreover, statistical discriminant function analysis (DA) indicates that Cu, Cd, GI, ∑Cytox (in gills), and ∑Gentox (in gills) significantly (p < 0.05) contribute to separation between exposure days. Determined cellular effects (geno-, cytotoxicity) demonstrate visible interfaces between applied biomarkers (metals accumulation and morphometric indices); the necessity to investigate them is evident. Genotoxic and cytotoxic effects caused by pollutants are leading to changes in biological parameters and may also affect health at individual and at population levels in time.
Conclusion
In conclusion, this study revealed that six-metal mixture at the maximum permissible concentrations was geno- and cytotoxic to A. cygnea mussels. Tissue- and time-specific changes in responses of nuclear abnormalities were assessed. Neurotoxicity studies have shown the metal mixture ability to reduce AChE activity in mussel’s hemolymph after short-term (4 days) and long-term (28 days) exposure. Time-dependent metal accumulation in A. cygnea gills and significant relations between Cu, Cd, ∑Gentox (in gills), and ∑Cytox (in gills) demonstrate bioavailability of used trace metals for the bioindicator and time-related DNA damage. Realistic scenario of induced environmental genotoxicity and cytotoxicity in A. cygnea mussels by environmentally relevant trace metal concentrations is obvious and must be investigated.
References
Arjmand F, Mostafavi PG, Fazaeli R, Rad MHG, Vosoughi G (2012) The in vitro and in vivo effect of clinoptilolite on decreasing of copper ion and DNA damage of Anodonta cygnea. JACR 6(4):37–45
Bainy ACD, Gennari de Medeiros MH, Di Mascio P, de Almeida EA (2006) In vivo effects of metals on the acetylcholinesterase activity of the Perna perna mussel’s digestive gland. Biotemas 19(1):35–39
Baršienė J, Andreikėnaitė L, Rybakovas A (2006) Cytogenetic damage in perch (Perca fluviatilis L.) and Duck mussel (Anodonta anatina L.) exposed to crude oil. Ekologija 1:25–31
Baršienė J, Rybakovas A, Garnaga G, Andreikėnaitė L (2012) Environmental genotoxicity and cytotoxicity studies in mussels before and after the oil spill in marine oil terminal (Baltic Sea). Environ Monit Assess 184:2067–2078. https://doi.org/10.1007/s10661-011-2100-0
Baršienė J, Butrimavičienė L, Michailovas A, Grygiel W (2015) Assessing the environmental genotoxicity risk in the Baltic Sea: frequencies of nuclear buds in blood erythrocytes of three native fish species. Environ Monit Assess 187:4078. https://doi.org/10.1007/s10661-014-4078-x
Benali I, Boutiba Z, Merabet A, Chèvre N (2015) Integrated use of biomarkers and condition indices in mussels (Mytilus galloprovincialis) for monitoring pollution and development of biomarker index to assess the potential toxic of coastal sites. Mar Pollut Bull 95:385–394. https://doi.org/10.1016/j.marpolbul.2015.03.041
Bielen A, Bošnjak I, Sepčić K, Jaklič M, Cvitanić M, Lušić J, Lajtner J, Simčič T, Hudina S (2016) Differences in tolerance to anthropogenic stress between invasive and native bivalves. Sci Total Environ 543:449–459. https://doi.org/10.1016/j.scitotenv.2015.11.049
Bourgeault A, Gourlay-France C, Vincent-Hubert F, Palais F, Geffard A, Biagianti Risbourg S, Pain-Devin S, Tusseau-Vuillemin MH (2010) Lessons from a transplantation of zebra mussels into a small urban river: an integrated ecotoxicological assessment. Environ Toxicol 25:468–478. https://doi.org/10.1002/tox.20591
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brown RJ, Galloway TS, Lowe D, Brown MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66(3):267–278
Burgos-Aceves MA, Faggio C (2017) An approach to the study of the immunity functions of bivalve haemocytes: physiology and molecular aspects. Fish Shellfish Immunol 67:513–517. https://doi.org/10.1016/j.fsi.2017.06.042
Burgos-Aceves MA, Cohen A, Paolella G, Lepretti M, Smith Y, Faggio C, Lionetti L (2018) Modulation of mitochondrial functions by xenobiotic-induced microRNA: from environmental sentinel organisms to mammals. Sci Total Environ 645:79–88. https://doi.org/10.1016/j.scitotenv.2018.07.109
Butrimavičienė L, Baršienė J, Greiciūnaitė J, Stankevičiūtė M, Valskienė R (2018) Environmental genotoxicity and risk assessment in the Gulf of Riga (Baltic Sea) using fish, bivalves, and crustaceans. Environ Sci Pollut Res 25:24818–24828. https://doi.org/10.1007/s11356-018-2516-y
Canty MN, Hagger JA, Moore RTB, Cooper L, Galloway TS (2007) Sublethal impact of short term exposure to the organophosphate pesticide azamethiphos in the marine mollusc Mytilus edulis. Mar Pollut Bull 54(4):396–402. https://doi.org/10.1016/j.marpolbul.2006.11.013
Cavas T, Garanko NN, Arkhipchuk VV (2005) Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem Toxicol 43(4):569–574. https://doi.org/10.1016/j.fct.2004.12.014
Ching EW, Siu WH, Lam PK, Xu L, Zhang Y, Richardson BJ, Wu RS (2001) DNA adduct formation and DNA strand breaks in green-lipped mussels (Perna viridis) exposed to benzo[a]pyrene: dose- and time-dependent relationships. Mar Pollut Bull 42:603–610
Crott JW, Mashiyama ST, Ames BN, Fenech M (2001) The effect offolic acid deficiency and MTHFR C677T polymorphism on chromosomedamage in human lymphocytes in vitro. Cancer Epidemiol Biomarkers Prev 10:1089–1096
Czechowski W, Gajewski W, Garbaczewska G, Nowakowski E (1994) Biology. Państw. Wyd. Roln. Leśn, Warszawa (in Polish)
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z, Knowler D, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny ML, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status, and conservation challenges. Biol Rev 81(2):163–182. https://doi.org/10.1017/S1464793105006950
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Faggio C, Tsarpali V, Dailianis S (2018) Mussel digestive gland as a model for assessing xenobiotics: an overview. Sci Total Environ 613:220–229. https://doi.org/10.1016/j.scitotenv.2018.04.264
Falfushynska HI, Delahaut L, Stoliar OB, Geffard A, Biagianti-Risbourg S (2009) Multi-biomarkers approach in different organs of Anodonta cygnea from the Dnister Basin (Ukraine). Arch Environ Contam Toxicol 57(1):86–95. https://doi.org/10.1007/s00244-008-9234-2
Falfushynska HI, Gnatyshyna LL, Farkas A, Vehovszky A, Gyori J, Stoliar OB (2010) Vulnerability of biomarkers in the indigenous mollusk Anodonta cygnea to spontaneous pollution in a transition country. Chemosphere 81:1342–1351. https://doi.org/10.1016/j.chemosphere.2010.08.016
Falfushynska H, Gnatyshyna L, Stoliar O, Mitina N, Skorokhoda T, Filyak Y, Zaichenko A, Stoika R (2012) Evaluation of biotargeting and ecotoxicity of Co2+-containing nanoscale polymeric complex by applying multi-marker approach in bivalve mollusk Anodonta cygnea. Chemosphere 88:925–936. https://doi.org/10.1016/j.chemosphere.2012.02.087
Falfushynska H, Gnatyshyna L, Stoliar O (2013a) Effect of in situ exposure history on the molecular responses of bivalve mollusks Anodonta anatina (Unionidae) to trace metals. Ecotoxicol Environ Saf 89:73–83. https://doi.org/10.1016/j.ecoenv.2012.11.024
Falfushynska H, Gnatyshyna L, Stoliar O (2013b) In situ exposure history modulates the molecular responses to carbamate fungicide Tattoo in bivalve mollusk. Ecotoxicology 22:433–445. https://doi.org/10.1007/s10646-012-1037-6
Falfushynska GI, Gnatyshyna LL, Yurchak IV, Stoliar OB, Sokolova I (2016) Interpopulational variability of molecular responses to ionizing radiation. Sci Total Environ 568:444–456. https://doi.org/10.1016/j.scitotenv.2016.05.175
Franco-Martinez L, Romero D, García-Navarro JA, Tecles F, Teles M, Tvarijonaviciute A (2016) Measurement of p-nitrophenyl acetate esterase activity (EA), total antioxidant capacity (TAC), total oxidant status (TOS) and acetylcholinesterase (AChE) in gills and digestive gland of Mytilus galloprovincialis exposed to binary mixtures of Pb, Cd and Cu. Environ Sci Pollut Res 23(24):25385–25392. https://doi.org/10.1007/s11356-016-7677-y
Führer E, Rudolph A, Espinoza C, Díaz R, Gajardo M, Camaño N (2012) Integrated use of biomarkers (O: N ratio and acetylcholinesterase inhibition) on Aulacomya ater (Molina, 1782) (Bivalvia: Mytilidae) as a criteria for effects of organophosphate pesticide exposition. J Toxicol 2012:1–6. https://doi.org/10.1155/2012/951568
Geist J (2011) Integrative freshwater ecology and biodiversity conservation. Ecol Indic 11:1507–1516. https://doi.org/10.1016/j.ecolind.2011.04.002
Govind P, Madhuri S (2014) Heavy metals causing toxicity in animals and fishes. Research Journal of Animal, Veterinary and Fishery. Sciences 2(2):17–23
Guidi P, Frenzilli G, Benedetti M, Bernardeschi M, Falleni A, Fattorini D, Regoli F, Scarcelli V, Nigro M (2010) Antioxidant, genotoxic and lysosomal biomarkers in the freshwater bivalve (Unio pictorum) transplanted in a metal polluted river basin. Aquat Toxicol 100:75–83. https://doi.org/10.1016/j.aquatox.2010.07.009
Hinzmann MF, Lopes-Lima M, Gonçalves J, Machado J (2013) Antiaggregant and toxic properties of different solutions on haemocytes of three freshwater bivalves. Toxicol Environ Chem 95(5):790–805. https://doi.org/10.1080/02772248.2013.818149
Jackin E (1974) Enzyme responses to metals in fish. In: Vemberg EG, Vemberg WB (eds) Pollution and Physiology of Marine Organisms. Academic Press, New York, USA, pp 59–65
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Jaruga P, Coskun E, Kimbrough K, Jacob A, Johnson WE, Dizdaroglu M (2017) Biomarkers of oxidatively induced DNA damage in dreissenid mussels: a genotoxicity assessment tool for the Laurentian Great Lakes. Environ Toxicol 00:1–10. https://doi.org/10.1002/tox.22427
Jindal R, Verma S (2015) In vivo genotoxicity and cytotoxicity assessment of cadmium chloride in peripheral erythrocytes of Labeo rohita (Hamilton). Ecotoxicol Environ Saf 118:1–10. https://doi.org/10.1016/j.ecoenv.2015.04.005.
Jitar O, Teodosiu C, Oros A, Plavan G, Nicoara M (2015) Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol 32(3):369–378. https://doi.org/10.1016/j.nbt.2014.11.004
El Jourmi L, Amine A, Boutaleb N, Abouakil N, Lazar S, El Antri S (2014) Multimarker approach analysis in the brown mussel to evaluate the anthropogenic stress: a preliminary study. JMES 5(5):1326–1331
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Eskandari S, Mozdarani H, Mashinchian Moradi A, Shahhosseiny MH (2012) Cytogenetic damage induced by crude oil in Anodonta cygnea (mollusca, bivalvia) assessed by the comet assay and micronucleus test. IJMASE 2(4):215–224
Kamel N, Attig H, Dagnino A, Boussetta H, Banni M (2012) Increased temperatures affect oxidative stress markers and detoxification response to benzo[a]pyrene exposure in mussel Mytilus galloprovincialis. Arch Environ Contam Toxicol 63:534–543. https://doi.org/10.1007/s00244-012-9790-3
Khan MI, Zahoo M, Khan A, Gulfam N, Khisroon M (2018) Bioaccumulation of heavy metals and their genotoxic effect on freshwater mussel. Bull Environ Contam Toxicol 102:52–58. https://doi.org/10.1007/s00128-018-2492-4
Khazri A, Sellami B, Hanachi A, Dellali M, Eljarrat E, Beyrem H, Mahmoudi E (2017) Neurotoxicity and oxidative stress induced by permethrin in gills of the freshwater mussel Unio ravoisieri. Chem Ecol 33(1):88–101. https://doi.org/10.1080/02757540.2016.1248948
Kolarević S, Knežević-Vukčević J, Paunović M, Kračun M, Vasiljević B, Tomović J, Vuković-Gačić B, Gačić Z (2013) Monitoring of DNA damage in haemocytes of freshwater mussel Sinanodonta woodiana sampled from the Velika Morava River in Serbia with the comet assay. Chemosphere 93:243–251. https://doi.org/10.1016/j.chemosphere.2013.04.073
Kolarević S, Kračun-Kolarević M, Kostić J, Slobodnik J, Liška I, Gačić Z, Paunović M, Knežević-Vukčević J, Vuković-Gačić B (2016) Assessment of the genotoxic potential along the Danube River by application of the comet assay on haemocytes of freshwater mussels: the Joint Danube Survey 3. Sci Total Environ 540:377–385. https://doi.org/10.1016/j.scitotenv.2015.06.061
Kopecka-Pilarczyk J (2010) The effect of pesticides and metals on acetylcholinesterase (AChE) in various tissues of blue mussel (Mytilus trossulus L.) in short-term in vivo exposures at different temperatures. 2010. J Environ Sci Health B 45:336–346. https://doi.org/10.1080/03601231003704390
Lindberg HK, Wang X, Järventaus H, Falck GC, Norppa H, Fenech M (2007) Origin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytes. Mutat Res 617:33–45. https://doi.org/10.1016/j.mrfmmm.2006.12.002
Lopes-Lima M, Sousa R, Geist J, Aldridge DC, Araujo R, Bergengren J, Bespalaya J et al (2017) Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biol Rev 92:572–607. https://doi.org/10.1111/brv.12244
Matozzo V, Pagano M, Spinelli A, Caicci F, Faggio C (2016) Pinna nobilis: a big bivalve with big haemocytes? Fish Shellfish Immunol 55:529–534. https://doi.org/10.1016/j.fsi.2016.06.039
Moreira SM, Coimbra J, Guilhermino L (2001) Acetylcholinesterase of Mytilus galloprovincialis LmK. hemolymph: a suitable environmental biomarker. Bull Environ Contam Toxicol 67(4):470–475
Nepomuceno JC, Ferrari I, Spano MA, Centeno AC (1997) Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic mercury. Environ Mol Mutagen 30:293–297
Nugroho, A.P., Handayani, N.S.N., Pramudita, I.G.A., 2015. Effects of chromium (Cr) on freshwater mussel Anodonta woodiana (Lea, 1834): distribution, bioaccumulation, and genomic DNA damage. The 3rd International Conference on Biological Science 2013 (The 3rd ICBS-2013) Volume 2: 180. https://doi.org/10.18502/kls.v2i1.139
Pagano M, Porcino C, Briglia M, Fiorino E, Vazzana M, Silvestro S, Faggio C (2017) The influence of exposure of cadmium chloride and zinc chloride on haemolymph and digestive gland cells from Mytilus galloprovincialis. Int J Environ Res 11(2):207–216. https://doi.org/10.1007/s41742-017-0020-8
Palais F, Dedourge-Geffard O, Beaudon A, Pain-Devin S, Trapp J, Geffard O, Noury P, Gourlay-Francé C, Uher E, Mouneyrac C, Biagianti-Risbourg S, Geffard A (2012) One-year monitoring of core biomarker and digestive enzymeresponses in transplanted zebra mussels (Dreissena polymorpha). Ecotoxicology 21(3):888–905. https://doi.org/10.1007/s10646-012-0851-1.
Perić L, Nerlović V, Žurga P, Žilić L, Ramšak A (2017) Variations of biomarkers response in mussels Mytilus galloprovincialis to low, moderate and high concentrations of organic chemicals and metals. Chemosphere 174:554–562. https://doi.org/10.1016/j.chemosphere.2017.01.138
Pourang N, Richardson CA, Mortazavi MS (2010) Heavy metal concentrations in the soft tissues of swan mussel (Anodonta cygnea) and surficial sediments from Anzali wetland, Iran. Environ Monit Assess 163(1-4):195–213. https://doi.org/10.1007/s10661-009-0827-7
Rickwood CJ, Galloway TS (2004) Acetylcholinesterase inhibition as a biomarker of adverse effect A study of Mytilus edulis exposed to the priority pollutant chlorfenvinphos. Aquat Toxicol 67:45–56. https://doi.org/10.1016/j.aquatox.2003.11.004.
Robillard S, Beauchamp G, Laulier M (2003) The role of abiotic factors and pesticide levels on enzymatic activity in the freshwater mussel Anodonta cygnea at three different exposure sites. Comp Biochem Physiol Part C: Toxicol Pharmacol 135(1):49–59. https://doi.org/10.1016/S1532-0456(03)00049-8
Rzymski P, Niedzielski P, Klimaszyk P, Poniedziałek B (2014) Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ Monit Assess 186(5):3199–3212. https://doi.org/10.1007/s10661-013-3610-8.
Savorelli F, Manfra L, Croppo M, Tornambè A, Palazzi D, Canepa S, Trentini PL, Cicero AM, Faggio C (2017) Fitness evaluation of Ruditapes philippinarum exposed to nickel. Biol Trace Elem Res 177(2):384–393. https://doi.org/10.1007/s12011-016-0885-y
Scarpato R, Migliore L, Barale R (1990) The micronucleus assay in Anodonta cygnea for the detection of drinking water mutagenicity. Mutat Res 245(4):231–237
Sen I, Shan dil A, Shrivastava VS (2011) Study for determination of heavy metals in fish species of River Yamuna (Delhi) by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Adv Appl Sci Res 2(2):161–166
Serrano-García L, Montero-Montoya R (2001) Micronuclei and chromatid buds are the result of related genotoxic events. Environ Mol Mutagen 38(1):38–45. https://doi.org/10.1002/em.1048
Sohail M, Khan MN, Qureshi NA, Chaudhry AS (2017) Monitoring DNA damage in gills of freshwater mussels (Anodonta anatina) exposed to heavy metals. Pak J Zoo 49(1):305–311. https://doi.org/10.17582/journal.pjz/2017.49.1.305.311
Stankevičiūtė M, Butrimavičienė L, Valskienė R, Greiciūnaitė J, Baršienė J, Vosylienė MZ, Svecevičius G (2016) Analysis of nuclear abnormalities in erythrocytes of rainbow trout (Oncorhynchus mykiss) treated with Cu and Zn and after 4-, 8-, and 12-day depuration (post-treatment recovery). Mutat Res Genet Toxicol Environ Mutagen 797:26–35. https://doi.org/10.1016/j.mrgentox.2016.01.003
Stankevičiūtė M, Sauliutė G, Svecevičius G, Kazlauskienė N, Baršienė J (2017) Genotoxicity and cytotoxicity response to environmentally relevant complex metal mixture (Zn, Cu, Ni, Cr, Pb, Cd) accumulated in Atlantic salmon (Salmo salar). Part I: importance of exposure time and tissue dependence. Ecotoxicology 26:1051–1064. https://doi.org/10.1007/s10646-017-1833-0
Stankevičiūtė M, Sauliutė G, Makaras T, Markuckas A, Virbickas T, Baršienė J (2018) Responses of biomarkers in Atlantic salmon (Salmo salar) following exposure to environmentally relevant concentrations of complex metal mixture (Zn, Cu, Ni, Cr, Pb, Cd). Part II. Ecotoxicology 27:1069–1086. https://doi.org/10.1007/s10646-018-1960-2
Strungaru SA, Jijie R, Nicoara M, Plavan G, Faggio C (2018) Micro (nano) plastics in freshwater ecosystems: abundance, toxicological impact and quantification methodology. Trends Anal Chem 110:116–128. https://doi.org/10.1016/j.trac.2018.10.025
Taleb ZM, Benali I, Gherras H, Ykhlef-Allal A, Bachir-Bouiadjra B, Amiard JC, Boutiba Z (2009) Biomonitoring of environmental pollution on the Algerian west coast using caged mussels Mytilus galloprovincialis. Oceanologia 51(1):63–84
Valskienė R, Baršienė J, Butrimavičienė L, Grygiel W, Stunžėnas V, Jokšas K, Stankevičiūtė M (2018) Environmental genotoxicity and cytotoxicity levels in herring (Clupea harengus), flounder (Platichthys flesus) and cod (Gadus morhua) inhabiting the Gdansk Basin of the Baltic Sea. Mar Pollut Bull 133:65–76. https://doi.org/10.1016/j.marpolbul.2018.05.023
Huber V, Geist J (2017) Glochidial development of the freshwater swan mussel (Anodonta cygnea, Linnaeus 1758) on native and invasive fish species. Biol Conserv 209:230–238. https://doi.org/10.1016/j.biocon.2017.02.030
Torre A, Trischitta F, Faggio C (2013) Effect of CdCl2 on regulatory volume decrease (RVD) in Mytilus galloprovincialis digestive cells. Toxicol in Vitro 27:1260–1266. https://doi.org/10.1016/j.tiv.2013.02.017
Vidal-Liñán L, Bellas J, Etxebarria N, Nieto O, Beiras R (2014) Glutathione S-transferase, glutathione peroxidase and acetylcholinesterase activities in mussels transplanted to harbour areas. Sci Total Environ 470–471:107–116. https://doi.org/10.1016/j.scitotenv.2013.09.073
Vidal-Liñán L, Bellas J, Salgueiro-Gonzalez N, Muniategui S, Beiras R (2015a) Bioaccumulation of 4-nonylphenol and effects on biomarkers, acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase, in Mytilus galloprovincialis mussel gill. Environ Pollut 200:133–139. https://doi.org/10.1016/j.envpol.2015.02.012
Vidal-Liñán L, Bellas J, Fumega J, Beiras R (2015b) Bioaccumulation of BDE-47 and effects on molecular biomarkers acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase in Mytilus galloprovincialis mussels. Ecotoxicology 24:292–300. https://doi.org/10.1007/s10646-014-1377-5
Vidal-Liñán L, Bellas J, Soriano JA, Concha-Graña E, Muniategui S, Beiras R (2016) Bioaccumulation of PCB-153 and effects on molecular biomarkers acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase in Mytilus galloprovincialis mussels. Environ Pollut 214:885–891. https://doi.org/10.1016/j.envpol.2016.04.083
Vincent-Hubert F, Arini A, Gourlay-Francé C (2011) Early genotoxic effects in gill cells and haemocytes of Dreissena polymorpha exposed to cadmium, B[a]P and a combination of B[a]P and Cd. Mutat Res 723(1):26–35. https://doi.org/10.1016/j.mrgentox.2011.03.008
Woźnicki P, Lewandowska R, Brzuzan P, Ziomek E, Bardega R (2004) The level of DNA damage and the frequency of micronuclei in haemolymph of freshwater mussels Anodonta woodiana exposed to benzo(a)pyrene. Acta Toxicologica 12(1):41–45
Yahya AN, Mohamed SK, Mohamed AG (2018) Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. OAJT 3(1):001–009. https://doi.org/10.19080/OAJT.2018.03.555603
Zhang YF, Chen SY, Qu MJ, Adeleye AO, Di YN (2017) Utilization of isolated marine mussel cells as an in vitro model to assess xenobiotics induced genotoxicity. Toxicol in Vitro 44:219–229. https://doi.org/10.1016/j.tiv.2017.05.018
Acknowledgements
We are thankful to Gerda Petkutė and Greta Ašmenaitė for the help during this experiment.
Funding
This study was funded by the Research Council of Lithuania through the project ACTIS S-MIP-17-10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Responsible editor: Cinta Porte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights:
•Six-metal mixture at the maximum permissible concentrations is geno- and cytotoxic to A. cygnea.
•The highest ∑Gentox levels in gills and haemocytes was determined after 4-day exposure.
•Significantly highest induction of ∑Cytox level was found in gills after 2-day treatment.
•Metal mixture possesses an inhibitory effect on the acetylcholinesterase activity in mussels.
•Significant decrease in AChE activity in A. cygnea was found after short-term (4 days) exposure.
•Significantly highest accumulation of trace metals has been determined after 28-day treatment.
Rights and permissions
About this article
Cite this article
Butrimavičienė, L., Stankevičiūtė, M., Kalcienė, V. et al. Genotoxic, cytotoxic, and neurotoxic responses in Anodonta cygnea after complex metal mixture treatment. Environ Sci Pollut Res 26, 7627–7639 (2019). https://doi.org/10.1007/s11356-019-04206-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04206-1