Abstract
Selected physiological responses of Tillandsia albida (Bromeliaceae) and two lichens (Hypogymnia physodes and Xanthoria parietina) exposed to simulated acid rain (AR) over 3 months were studied. Pigments were depressed in all species being affected the most in Tillandsia. Amounts of hydrogen peroxide and superoxide were elevated and soluble proteins decreased only in AR-exposed Hypogymnia. Free amino acids were slightly affected among species and only glutamate sharply decreased in AR-exposed Xanthoria. Slight increase in soluble phenols but decrease in flavonoids in almost all species suggests that the latter are not essential for tolerance to AR. Almost all phenolic acids in Tillandsia leaves decreased in response to AR and activities of selected enzymes (phenylalanine ammonia-lyase, polyphenol oxidase, ascorbate- and guaiacol-peroxidase) were enhanced by AR. In lichens, considerable increase in metabolites (physodalic acid, atranorin and parietin) in response to AR was found but amount of ergosterol was unchanged. Macronutrients (K, Ca, Mg) decreased more pronouncedly in comparison with micronutrients in all species. Xanthoria showed higher tolerance in comparison with Hypogymnia, suggesting that could be useful for long-term biomonitoring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing industrial activities lead to elevated release of by-products which may negatively affect environment. Production of carbon, nitrogen and sulphur oxides is one of the most adverse consequences of such activities resulting to potential injuries of all aerobic organisms. Species of Bromeliaceae family represent considerable part of epiphytic vegetation in tropical America (Monteiro et al. 2009) and the genus Tillandsia includes epiphytic plants with extraordinary capacity to obtain water and nutrients from the atmosphere where pollutants are present (Pignata et al. 2002). Lichens are symbiotic entities formed by the algal photobiont (usually green alga or cyanobacteria) and fungal mycobiont. This unique symbiotic nature of lichens is responsible for the production of more than 800 specific metabolites produced by mycobiont hyphae (Hauck and Huneck 2007). A wide range of functions has been suggested for these metabolites including filtration of UV light, regulation of heavy metal uptake or antifeedant effect (Edwards et al. 2003a, b).
In most countries, pH of acid rain (AR) ranges from 4.4 to 2.7 and values above 3.5 did not act as primary stress but below 2.0 it may induce visible injuries (Gabara et al. 2003 and the references therein). Acid rain, in addition to other environmental stresses, stimulates increase in reactive oxygen species (ROS) production as judged from enhancement of antioxidative enzyme activities (Gabara et al. 2003; Wyrwicka and Skłodowska 2006) but little is known about non-enzymatic antioxidants such as phenolic metabolites (Suomela et al. 1998) or proline. These data are even absent in epiphytic plants and lichens. Membrane permeability and K+ leakage in AR-treated lichens were not considerably affected in lichen Bryoria in two-year study (Tarhanen et al. 1999).
With respect to growth adaptations of epiphytic plants, they are potentially sensitive to atmospheric pollution. Several studies have presented use of epiphytic plants such as Tillandsia species in biomonitoring of atmospheric quality (Pignata et al. 2002 and the references therein) but deeper data about basic metabolic parameters and physiological responses to AR are not available. Lichens are also suitable objects for biomonitoring since adverse effects of industrial emissions on lichens are frequently ascribed to elevated SO2 level (Tarhanen et al. 1999). Lichens which we used are moderately tolerant to SO2 and heavy metals (Hauck and Huneck 2007) but Hypogymnia occurs mainly on the periphery of Košice city, while Xanthoria parietina is common lichen even in the city centre (Bačkor et al. 2003).
Root-less epiphytic plants are therefore good indicator of air pollution since they gain water and nutrients directly from rain/air. However, physiological data related to effects of AR on such species are almost absent. It was therefore the main aim of the present research to study quantitative changes of selected physiological parameters in three epiphytic plants, Tillandsia albida Mez and Purpus (Bromeliaceae) and two lichens (Hypogymnia physodes (L.) Nyl. and Xanthoria parietina (L.) Th. Fr.), exposed to simulated acid rain (pH 3.0) over 3 months. Selected basic physiological parameters (chlorophylls, soluble proteins, free amino acids, mineral nutrients) and specific metabolites/parameters (lichen’s metabolites, phenolic acids and enzymatic activities in Tillandsia, soluble phenols and flavonoids in all species) were assessed in order to compare resulting responses. Many of these parameters are reported here for the first time. Selected biochemical data are compared with vascular plant Matricaria chamomilla L. (chamomile), our standard experimental object.
Materials and methods
Plant material and simulated acid rain
Plants of Tillandsia albida were kindly provided by Dr. Klára Repčáková (Botanical Garden of P. J. Šafárik University in Košice); they were ca. 5 years old ones placed on branches of Vitis vinifera. Lichens Xanthoria parietina and Hypogymnia physodes were collected on the locality “Harmanec–jaskyňa” (central Slovakia) and grew on branches of Larix decidua (Fig. 1). We note absence of differences in mineral nutrients composition of lichens thalli growing on different trees and the same was found for Tillandsia (data not shown). Five individual branches for lichens and Tillandsia were used as control and five as acid rain-treated (AR). Cultivation was realised in the Laboratory of Plant Stress Physiology (Department of Botany, Faculty of Science, P. J. Šafárik University in Košice) during May–August 2009 (day/night temperature 25–30/18–23°C). Branches were placed on the window where they received natural full sun light (ca. 7:00–11:00 a.m.) with average daily irradiation ~650 μmol m−2 s−1 PAR. We have decided to culture plants in these semi-controlled conditions since artificial light with strict photoperiod in cultivation room is strongly different in comparison with conditions where plants previously grew. Control and AR-treated samples were watered using distilled water with pH 5.5 and 3.0, respectively. These values have been selected based on the literature survey and pH was corrected using 1 M H2SO4 (Tarhanen et al. 1999). Note that pH 5.5 is considered as natural rain. Since these plants are root-less, they were irrigated daily (4 L from each pH to 15 branches for either control or AR-treated samples) and solutions were applied in two doses within 1 h in order to maintain plants and branches wet for at least 3 h each day. Solutions were applied using hand-made sprayer and water from branches flowed into the washbasin. No artificial mineral nutrients were applied in order to prevent side effects on the metabolic parameters and composition of mineral nutrients. After 3 months, plants of Tillandsia were powdered in liquid N2 and aliquots were taken for assay of fresh mass-requiring parameters mentioned below; the residue was dried at 70°C in order to determine tissue water content allowing recalculation of parameters measured in fresh samples. Free amino acids, phenolic acids and mineral nutrients were measured in dried samples. Lichens were carefully removed from branches using forceps and since they are poikilohydric, a known amount of biomass was immersed in several drops of HEPES buffer in closed plastic tubes 24 h before measurements (Bačkor et al. 2009a). Spectrophotometry has been carried out with Uvi Light XTD 2 (Secomam, ALES Cedex, France).
Assay of pigments and ROS
Chlorophylls and total carotenoids were extracted from Tillandsia using pure methanol (Kováčik and Bačkor 2007a) and from lichens using DMSO (Bačkor et al. 2009a). Measurement and calculation was done according to equations for specific extraction solvent proposed by Wellburn (1994). We also verified efficiency of pigment extraction from Tillandsia using different solvents (80% acetone, methanol and DMSO) and non-significant differences were found (data not shown).
Hydrogen peroxide and superoxide were measured in 50 mM potassium phosphate buffer homogenates containing 5 mM polyvinyl polypyrrolidone (pH 7.0). H2O2 was measured using TiCl4 (Jana and Choudhuri 1981): to 0.50 ml of supernatant, 0.25 ml of 0.1% titanium chloride (Sigma–Aldrich, Germany) in 20% H2SO4 (v/v) was added and the mixture was centrifuged at 15,000 g for 20 min at 4°C. Absorbance was measured at 410 nm. The amount of H2O2 was calculated from standardized curve with known H2O2 concentrations. Superoxide was estimated according to Elstner and Heupel (1976) by monitoring formation of nitrite from hydroxylamine at 530 nm. Reaction mixture contained 0.27 ml of potassium phosphate buffer, 0.03 ml of 10 mM hydroxylamine, 0.3 ml of supernatant, 0.3 ml of 17 mM sulphanilamide, 0.3 ml of 7 mM α-naphthylamine and 0.3 ml of diethyl ether. The amount of nitrite was calculated from standardized curve with known NaNO2 concentrations.
Quantification of phenolic and lichen’s metabolites
Total soluble phenols and flavonoids were extracted with 80% methanol from fresh material. Phenols were quantified using Folin-Ciocalteu method (Singleton and Rossi 1965) with gallic acid as standard according to slightly modified protocol (Kováčik and Bačkor 2007b). Flavonoids were estimated using AlCl3 method (Ordoñez et al. 2006) with quercetin as standard: to 0.5 ml of methanol supernatants, 1 ml of 2% AlCl3 in methanol (w/v) was added. Samples were mixed and absorbance was measured 1 h later at 420 nm.
The selected cinnamic and benzoic acid derivatives in the Tillandsia plants were measured in 80% methanol extracts. Extraction and re-purification by solid phase extraction procedure at computer controlled robot Aspec XL, Gilson (USA), HPLC conditions and detection by a mass selective HP MSD quadrupole detector (G1946A, Hewlett-Packard, Palo Alto, USA) was done as described earlier (Kováčik et al. 2007; Kováčik et al. 2009c).
Lichen’s metabolites were extracted from dry material using pure acetone. Measurement and calculation using gradient HPLC system with UV-detection were done as described in detail previously (Pöykkö et al. 2005). Ergosterol has been extracted by 99% ethanol and analysed by HPLC as described earlier (Bačkor et al. 2009a).
Measurement of soluble proteins and free amino acids
Proteins were quantified in 50 mM potassium phosphate buffer homogenates (pH 7.0) according to Bradford (1976) using 20 μl of supernatants and bovine serum albumin as standard (595 nm). For calculation of PAL activity, proteins were quantified in respective homogenates (see below).
Free amino acids were extracted with 80% aqueous ethanol and analyses were performed on an HP 1100 liquid chromatograph (Hewlett Packard, Waldbronn, Germany) with fluorometric detector FLD HP 1100 and using precolumn derivatization with o-phtalaldehyde and 9-fluorenylmethyl chloroformate as described earlier (Kováčik et al. 2009a).
Assay of enzymatic activities in Tillandsia
Polyphenol oxidase (PPO, EC 1.10.3.2), ascorbate peroxidase (APX, EC 1.11.1.11) and guaiacol peroxidase (GPX, EC 1.11.1.7) activities were assayed in supernatants prepared using 50 mM potassium phosphate buffer containing 5 mM polyvinyl polypyrrolidone (1 g FW/5 ml of buffer) and assayed as the oxidation of catechol, ascorbate and guaiacol at 420, 290 and 470 nm, respectively (Kováčik and Bačkor 2007a; Kováčik et al. 2009c).
Activity of phenylalanine ammonia-lyase (PAL, EC 4.3.1.5) was determined as the production of trans-cinnamic acid from phenylalanine using the HPLC method with homogenates prepared using sodium borate buffer (pH 8.7) with UV detection at 275 nm (dos Santos et al., 2004; Kováčik et al. 2007). For all enzymes, randomly selected supernatants were boiled to destroy enzymes and to check that the observed reactions were enzymatic.
Quantification of mineral nutrients
Samples for quantification of mineral nutrients were prepared as described elsewhere (Kováčik et al. 2009c, d). Briefly, dry material was kept overnight in HNO3 and H2O2 mixture (10 ml + 10 ml, Suprapur, Merck) at laboratory temperature and next day evaporated to dryness at 90°C in a water bath (4–5 h). Dry residue was dissolved in 5% HNO3. Measurements were carried out using an atomic absorption spectrometer AA30 (Varian Ltd., Mulgrave, Australia) and an air-acetylene flame.
Statistical analyses
Data were evaluated using either ANOVA followed by a Tukey’s test (MINITAB Release 11, Minitab Inc., State College, Pennsylvania) or Student’s t-test. Number of replications (n) in tables/figures denotes number of samples = number of individual branches (then n = 5 as described at the beginning of Materials and Methods section). Two independent repetitions of whole experiment were performed in order to check reproducibility.
Results
Effect of AR on pigments and ROS
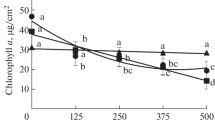
Content of chlorophyll a decreased in all AR-treated plants, being expressed the most in Tillandsia (−90%) while content of chlorophyll b showed both negative and positive changes in AR-exposed plants (Table 1). Accumulation of carotenoids decreased in Tillandsia and Hypogymnia in response to acid rain. Chlorophyll a pheophytinization (ratio of absorbance 435/415) increased in AR-treated Tillandsia and Xanthoria (expressed as decrease of chlorophyll a integrity in this value, Table 1).
Within reactive oxygen species (ROS), accumulation of hydrogen peroxide and superoxide was significantly elevated in AR-exposed Hypogymnia only (+133 and +47%, respectively).
Quantitative changes of proteins and free amino acids
Content of soluble proteins was the highest in Tillandsia and AR caused significant decrease only in Hypogymnia (−21%, Table 2).
Among 17 detected free amino acids, accumulation of glutamate and alanine was exceptionally high in Xanthoria control samples contributing to the highest sum of free amino acid in this taxon (Table 2). Both these amino acids significantly decreased in AR-treated samples (−45 and −64% for glutamate and alanine, respectively). Hypogymnia showed increase in the sum of amino acids after AR treatment and mainly serine (2.2-fold) and arginine (2.2-fold) contributed to this increase. Tillandsia showed the lowest sum of free amino acids and AR had no effect on this sum. Notwithstanding this, methione strongly increased (Table 2). Accumulation of proline, a stress-induced amino acid, has only slightly increased in Xanthoria but decreased in Hypogymnia (Table 2).
Effect of AR on phenolic metabolites and lichen’s specific metabolites
Total soluble phenols were found to be the most accumulated in Hypogymnia control samples, followed by Tillandsia. Xanthoria showed the lowest accumulation of total soluble phenols (Fig. 2). Acid rain stimulated increase in soluble phenols in Tillandsia (+27%) and Hypogymnia (+20%) but had no effect in Xanthoria. In contrast, flavonoids have decreased in Hypogymnia (−42%) and Xanthoria (−24%).
Among 12 derivatives of benzoic and cinnamic acid (so-called phenolic acids) detected in Tillandsia leaves, only vanillin increased in response to AR. Accumulation of chlorogenic acid showed the most pronounced decrease (Table 3).
Lichen’s specific aromatic metabolites showed increase in response to AR (parietin, atranorin, physodalic acid). Ergosterol, principal sterol of mycobiont (fungal) plasma membranes, showed tendency to increase but differences were not strong enough to be significant (Table 3).
Enzymatic activities in Tillandsia
Activities of two common phenolic metabolism-related enzymes were enhanced in AR-exposed samples: PAL +48% and PPO +89%. Two common peroxidases also showed increased activities in response to AR (APX +34% and GPX +146%, Fig. 3).
Activities of selected enzymes (PAL phenylalanine ammonia-lyase, PPO polyphenol oxidase, APX ascorbate peroxidase, GPX guaiacol peroxidase) in Tillandsia plants after 3 months of exposure to simulated acid rain. *** indicate significant difference at 0.001 level of Student’s t-test in comparison with respective control. Data are means ± SDs (n = 5)
Effect of AR on mineral nutrients
At the level of mineral nutrients, accumulation of potassium and magnesium decreased in AR-treated samples from each species (−34/−43/−26% for K+ and −14/−83/−37% for Mg2+ in Tillandsia/Hypogymnia/Xanthoria, Table 4). Content of calcium decreased in the lichens (−38 and −50% in Hypogymnia and Xanthoria, respectively) and Zn in Tillandsia. Amounts of Na, Fe and Cu were not affected by AR in any of species (Table 4).
Discussion
Negative effects of environmental stress are usually obvious at the level of chlorophyll which was also depleted in the present study (Table 1). This was the most pronounced in Tillandsia samples as the accumulation of chlorophyll a decreased ca. 10-times in response to AR. Since no direct damage of leaves were visible and chlorophyll a pheophytinization increased only slightly we assume that its biosynthesis was affected. This may also be judged from decrease of chlorophyll b and its concentration was extremely low in AR samples. Chlorophyll a/b ratio was ca. 3.0 in control samples, similarly to value found in vascular plants such chamomile measured by the same method in methanol extracts (Kováčik and Bačkor 2007a). In the literature, there are similar results presented on this ratio in the genus Tillandsia, e.g. 1.1 in T. fasciculata (Koh and Davies Jr 2001) or 3.0 in T. usneoides (Haslam et al. 2003) and other Bromeliaceae such as Aechmea and Guzmania have these ratios also ca. 3.0 (Croonenborghs et al. 2009). Sum of chlorophyll was found to be 1–2 mg g−1 DW in different Tillandsia species (Koh and Davies Jr 2001; Haslam et al. 2003) including our present data (Table 1). Xanthoria showed similar decrease of both chlorophyll a and b, indicating effect of AR on biosynthesis. We note that this decrease was very small in relation to duration of treatment and other kinds of stresses applied on lichens, such as excess of copper, caused more dramatic decrease (Bačkor et al. 2009a). This is good evidence that this species is well adapted to polluted environment and its populations really occur on the urban localities where more sensitive lichens are absent (Bačkor et al. 2003). In contrast, decrease of chlorophyll a but increase in chlorophyll b which we observed in AR-treated Hypogymnia samples confirms conversion of chlorophyll a to b which is mediated by the oxidation of methyl group on ring II to the aldehyde (Bačkor and Váczi 2002). However, decrease in chlorophylls alone in response to AR treatments was the most visible (in comparison with other parameters measured in this study) but it can only hardly be considered as the only evidence of AR toxicity and further studies are needed.
We have also measured tissue water content in Tillandsia and it was considerably high, being 87.4 and 86.9% in control and AR-treated ones, respectively. These data are similar to hydroponically cultured chamomile (ca. 92%) and suggest high water potential of leaf tissue (close to zero; see Martin et al. 2004 for details). It was really found that many epiphytic plants have high osmotic potential (slightly negative) which was explained by existence of “hydrenchyma” tissue allowing them to overcome drought periods (Martin et al. 2004), agreeing with high water content what we observed in the present study. This high osmotic potential also indicates that concentration of solutes will be low and more data were urged (Martin et al. 2004). Our results fit well with the mentioned facts since many compounds such as amino acids, proteins and mineral nutrients were less-accumulated in Tillandsia samples in comparison with C3 plants such as chamomile (Kováčik et al. 2009c, d).
Free amino acids represent pool for different metabolic processes therefore their damage by stress effects may have extensive impact on overall metabolism. We found relatively negligible effect of AR on amino acid composition and no similar study is available for deeper comparison. High amount of free amino acids in Xanthoria is mainly caused by abundance of glutamate, serving as primary acceptor for glutamine synthetase. Our earlier study showed that sum of free amino acids in terrestrial lichen Peltigera rufescens was also high (close to Xanthoria in the present study) but Cladina mitis had the lowest amount presently known (Bačkor et al. 2009b) suggesting genetically controlled inter-specific differences since lichens in the present study are both epiphytic, foliose and contain the same photobiont (from the green algal genus Trebouxia sensu lato). However, abundance of free amino acids in Xanthoria confirms that it is nitrophytic lichen (Pirintsos et al. 2009) and it also contains higher amount of total nitrogen in comparison with Hypogymnia (data not shown). Very low content of proline (in comparison with chamomile, Kováčik et al. 2009a, c) and its slight change in response to AR in all species is another indication of low impact of acid rain-evoked acidification on nitrogen metabolism. In accordance, no extreme decrease in soluble proteins has been found (Table 2). Low proline content in Tillandsia is also in accordance with high osmotic potential of epiphytic plants mentioned above since this compound is a well-known stress-induced amino acid being involved in the mechanisms of osmoregulation (Gajewska and Skłodowska 2005). We also observed strong increase in methionine in Tillandsia samples and significance of this observation remains unclear. To our knowledge, these are first data reporting profile of free amino acids in any Bromeliaceae species.
Phenolic metabolites are one of the most abundant organic compounds in plants being involved in many stress responses. At first sight, the highest content of soluble phenols in Hypogymnia (Fig. 2) could be evoked by an increase in atranorin and physodalic acid accumulation (Table 3) but we found that only atranorin reacts with Folin-Ciocalteu reagent thus can contribute to increase in soluble phenols in AR-treated samples. In combination with the highest content of flavonoids just in Hypogymnia (Fig. 2), this species seems to be potential source of new phenolic metabolites. We note that we measured flavonoids as AlCl3-reactive compounds and in this assay, only flavonoids possessing 4-keto and 3- and/or 5-hydroxy group have been suggested to react with AlCl3 (Deng and van Berel 1998) indicating presence of flavonols. Composition of flavonoids in lichens is not well known and their presence has only occasionally been mentioned (Momoh and Adikwu 2008). Our quantitative data indicate that flavonoids form small part of total soluble phenols and this could be explained by low volume of photobiont cells in lichen’s thalli (up to 10% in average). Increase in soluble phenols but decrease in flavonoid accumulation in almost all species suggests that flavonoids do not play important role in tolerance to acid rain. Our data are in accordance with results found in non-epiphytic plants such as Betula pubescens where individual phenolics were not considerably affected (Suomela et al. 1998).
In lichens, their specific metabolites could be more essential for responses to AR. For example, increase in parietin in Xanthoria (Table 3) was macroscopically visible as intensification in yellow-orange colour and since we cultured samples in the most natural environment (full sun light, see Materials and Methods section), this indicates AR-evoked increase in sensitivity to light. Hypogymnia is a moderately sensitive to SO2 and heavy metals (Hauck and Huneck 2007) and just physodalic acid was found to increase in thalli transplanted to areas with elevated pollution (Białońska and Dayan 2005). Our data fit well with this finding and strong induction of physodalic acid accumulation was observed in AR-treated Hypogymnia thalli (Table 3), representing first direct evidence of this process. Substances in Hypogymnia including physodalic acid have also other important functions such as the regulation of metal uptake (Hauck and Huneck 2007) therefore their role in metal homeostasis in the present study may be plausible (Table 4). Ergosterol is a main component of fungal plasma membrane (Tarhanen et al. 1999) and decrease in its concentration indicates damage to overall lichen’s vitality (Bačkor et al. 2009a). Our data showed that despite decrease in K+ content in lichens (Table 4) suggesting damage to membrane integrity, amounts of ergosterol did not decrease but showed tendency to increase (although differences were not strong enough to be significant, Table 3). Similar discrepancy has been found in lichen Bryoria fuscescens exposed to acidity (pH 3, H2SO4, Tarhanen et al. 1999) therefore AR-evoked K+ decrease is not related to changes in ergosterol accumulation and further studies are needed.
It was surprising to find that soluble phenols and flavonoids were present in low quantities in Tillandsia samples since it would be expected that such sun-loving plant will contain higher amount of UV-absorbing compounds (such as flavonoids) in comparison with vascular plant (Kováčik et al. 2009b). This paradox may be explained by its morphology and xerophytic leaves with almost white surface (highlighted in its species name—albida, Fig. 1) thus, increasing reflectance of sun rays. Decrease in individual phenols is also visible at the level of detected phenolic acids (we again note their substantially lower accumulation in comparison with chamomile) and only vanillin has increased in response to AR (Table 3). This is a well-known antioxidative compound (Kováčik et al. 2009a) then different responses even within the same group of phenols are presumed thus contributing to slight increase in total soluble phenols. This increase correlates with enhancement of phenylalanine ammonia-lyase (PAL) activity which we recoded in AR-exposed Tillandsia samples (Fig. 3). Increase in polyphenol oxidase activity could also contribute to modulation of soluble phenols. Despite prolonged exposure to AR, Tillandsia plants also showed elevated activities of peroxidases (APX and GPX), which are important components in removal of excess H2O2, thus leading to unchanged level of ROS (Table 1). We note that APX activities were even higher in comparison with chamomile leaves (Kováčik et al. 2009c) indicating significance of this enzyme at least in this Tillandsia species. Other enzymes showed similar or slightly lower activities in comparison with chamomile leaves, indicating that despite considerably lower amount of proteins (ca. 20-times in comparison with chamomile), enzymatic machinery has similar effectiveness.
Macronutrients were more depleted by AR in comparison with micronutrients (Table 4). Especially K+ was abundant in Tillandsia in comparison with lichens and it decreased in all three species. More expressive decrease in Ca and Mg in lichens (if expressed as % of control) could be ascribed to different morphology since minerals may be more readily eluted from damaged thalli in comparison with tissue structure of Tillandsia leaves. A study by Pyatt et al. (1999) has shown that in naturally growing Tillandsia usneoides and lichen Parmotrema praesorediosum, selected metals and sulphur were bio-accumulated and the accumulation of certain heavy metals increases with age of the T. usneoides.
In conclusion, comparison of vascular (Tillandsia) and non-vascular (lichens) epiphytic taxa showed that sensitivity to AR differed depending on evaluated parameter. Extensive decrease in chlorophyll amount in Tillandsia albida does not match well with absence of visible damage and slight decrease in Mg content suggesting specificity of this assay and further ecotoxicological studies are needed. Among lichens, Xanthoria showed higher tolerance in comparison with Hypogymnia (at the level of pigments, ROS, mineral nutrients), suggesting that it could be useful for long-term biomonitoring. Accumulation of free amino acids and proteins were not considerably damaged and there exist no data in these or similar species which we could discuss in details. Overall, lichens seem to respond to AR by synthesis of specific metabolites while in Tillandsia, enhanced enzymatic activities (including peroxidases we measured) may contribute to low oxidative stress as visible from unaffected ROS accumulation.
References
Bačkor M, Váczi P (2002) Copper tolerance in the lichen photobiont Trebouxia erici (Chlorophyta). Environ Exp Bot 47:11–20
Bačkor M, Paulíková K, Geralská A, Davidson R (2003) Monitoring of air pollution in Košice (eastern Slovakia) using lichens. Polish J Environ Stud 12:141–150
Bačkor M, Kováčik J, Dzubaj A, Bačkorová M (2009a) Physiological comparison of copper toxicity in the lichens Peltigera rufescens (Weis) Humb. and Cladina arbuscula subsp. mitis (Sandst.) Ruoss. Plant Growth Regul 58:279–286
Bačkor M, Klejdus B, Vantová I, Kováčik J (2009b) Physiological adaptations in the lichens Peltigera rufescens and Cladina arbuscula var. mitis, and the moss Racomitrium lanuginosum to copper-rich substrate. Chemosphere 76:1340–1343
Białońska D, Dayan FE (2005) Chemistry of the lichen Hypogymnia physodes transplanted to an industrial region. J Chem Ecol 31:2975–2991
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Croonenborghs S, Ceusters J, Londers E, De Proft MP (2009) Effects of elevated CO2 on growth and morphological characteristics of ornamental bromeliads. Sci Hortic 121:192–198
Deng HT, van Berel GJ (1998) Electrospray mass spectrometry and UV/visible spectrophotometry studies of Aluminum(III)-flavonoid complexes. J Mass Spectrom 33:1080–1087
dos Santos WD, Ferrarese MLL, Finger A, Teixeira CAN, Ferrarese-Filho O (2004) Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J Chem Ecol 30:1203–1212
Edwards HGM, Newton EM, Wynn-Williams DD, Coombes SR (2003a) Molecular spectroscopic studies of lichen substances 1: parietin and emodin. J Mol Struct 648:49–59
Edwards HGM, Newton EM, Wynn-Williams DD (2003b) Molecular structural studies of lichen substances II: atranorin, gyrophoric acid, fumarprotocetraric acid, rhizocarpic acid, calycin, pulvinic dilactone and usnic acid. J Mol Struct 651–653:27–37
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium-chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
Gabara B, Skłodowska M, Wyrwicka A, Glińska S, Gapińska M (2003) Changes in the ultrastructure of chloroplasts and mitochondria and antioxidant enzyme activity in Lycopersicon esculentum Mill. leaves sprayed with acid rain. Plant Sci 164:507–516
Gajewska E, Skłodowska M (2005) Antioxidative responses and proline level in leaves and roots of pea plants subjected to nickel stress. Acta Physiol Plant 27:329–339
Haslam R, Borland A, Maxwell K, Griffiths H (2003) Physiological responses of the CAM epiphyte Tillandsia usneoides L, (Bromeliaceae) to variations in light and water supply. J.Plant Physiol 160:627–634
Hauck M, Huneck S (2007) Lichen substances affect metal adsorption in Hypogymnia physodes. J Chem Ecol 33:219–223
Jana S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperm during aging. Aquat Bot 12:345–354
Koh YC, Davies FT Jr (2001) Mutagenesis and in vitro culture of Tillandsia fasciculata Swartz var. fasciculata (Bromeliaceae). Sci Hortic 87:225–240
Kováčik J, Bačkor M (2007a) Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant Soil 297:255–265
Kováčik J, Bačkor M (2007b) Phenylalanine ammonia-lyase and phenolic compounds in chamomile tolerance to cadmium and copper excess. Water Air Soil Pollut 185:185–193
Kováčik J, Klejdus B, Bačkor M, Repčák M (2007) Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci 172:393–399
Kováčik J, Klejdus B, Bačkor M (2009a) Nitric oxide signals ROS scavenger-mediated enhancement of PAL activity in nitrogen-deficient Matricaria chamomilla roots: side effects of scavengers. Free Radical Biol Med 46:1686–1693
Kováčik J, Klejdus B, Bačkor M (2009b) Phenolic metabolism of Matricaria chamomilla plants exposed to nickel. J Plant Physiol 166:1460–1464
Kováčik J, Klejdus B, Hedbavny J, Bačkor M (2009c) Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18:544–554
Kováčik J, Klejdus B, Hedbavny J, Štork F, Bačkor M (2009d) Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 320:231–242
Martin CE, Lin TC, Lin KC, Hsu CC, Chiou WL (2004) Causes and consequences of high osmotic potentials in epiphytic higher plants. J Plant Physiol 161:1119–1124
Momoh MA, Adikwu MU (2008) Evaluation of the effect of colloidal silver on the antibacterial activity of ethanolic extract of the lichen Parmelia perlata. Afr J Pharm Pharmaco 2:106–109
Monteiro JAF, Zotz G, Körner C (2009) Tropical epiphytes in a CO2-rich atmosphere. Acta Oecol 35:60–68
Ordoñez AAL, Gomez JD, Vattuone MA, Isla MI (2006) Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem 97:452–458
Pignata ML, Gudino GL, Wannaz ED, Plá RR, González CM, Carreras HA, Orellana L (2002) Atmospheric quality and distribution of heavy metals in Argentina employing Tillandsia capillaris as a biomonitor. Environ Pollut 120:59–68
Pirintsos SA, Munzi S, Loppi S, Kotzabasis K (2009) Do polyamines alter the sensitivity of lichens to nitrogen stress? Ecotox Environ Safe 72:1331–1336
Pöykkö H, Hyvärinen M, Bačkor M (2005) Removal of lichen secondary metabolites affects food preference and survival of lichenivorous moth larvae. Ecology 86:2623–2632
Pyatt FB, Grattan JP, Lacy D, Pyatt AJ, Seaward MRD (1999) Comparative effectiveness of Tillandsia usneoides L. and Parmotrema praesorediosum (Nyl.) Hale as bio-indicators of atmospheric pollution in Louisiana (USA). Water Air Soil Pollut 111:317–326
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158
Suomela J, Neuvonen S, Ossipova S, Ossipov V, Pihlaja K (1998) A long-term study of the effects of simulated acid rain on birch leaf phenolics. Chemosphere 36:639–644
Tarhanen S, Metsärinne S, Holopainen T, Oksanen J (1999) Membrane permeability response of lichen Bryoria fuscescens to wet deposited heavy metals and acid rain. Environ Pollut 104:121–129
Wellburn AR (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolutions. J Plant Physiol 144:307–313
Wyrwicka A, Skłodowska M (2006) Influence of repeated acid rain treatment on antioxidative enzyme activities and on lipid peroxidation in cucumber leaves. Environ Exp Bot 56:198–204
Acknowledgments
This work was supported by the Slovak grant agency VEGA (1/0521/10) and the grant agency of the Czech Republic (GA CR 525/07/0338). We thank Prof. Dianne Fahselt (University of Western Ontario, Canada) for proofreading the manuscript, Mrs. Anna Michalčová for excellent technical assistance and MSc. Silvia Malčovská and BSc. Lukáš Broda for Fig. 1 processing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kováčik, J., Klejdus, B., Bačkor, M. et al. Physiological responses of root-less epiphytic plants to acid rain. Ecotoxicology 20, 348–357 (2011). https://doi.org/10.1007/s10646-010-0585-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0585-x