Abstract
Atractylis serratuloides is an abundant native spiny species that grows in the surroundings of superphosphate factories in Tunisia. This plant species is adapted to arid environments and tolerates a high level of fluoride pollution in soils. The aim of this study was to better understand the physiological mechanisms of fluoride tolerance of this species, comparing the fluoride-contaminated sites of Gabes and Skhira with the reference site of Smara. Results demonstrated the involvement of leaf element and phytometabolite balances in the in situ response of A. serrulatoides to fluoride. Calcium, sulphur and magnesium were differently distributed between the sites of Gabes and Smara in all plant organs. No specific tissue fluorine accumulation in root, stem and leaf, even in the most contaminated site at Gabes, was detected by EDAX mapping. Lower anthocyan and flavonol levels but enhanced nitrogen balance index were found in A. serrulatoides leaves from Gabes compared to the two other sites. A. serratuloides appeared as a fluoride excluder and its tolerance involved calcium interactions with fluoride. Moreover, an occurrence of dark septate endophytes and arbuscular mycorhizal fungi in root systems of A. serratuloides was reported for the first time, and these symbioses were present but low at all sites. We suggest the use of this plant species for fluoride-polluted soil stabilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has been well documented that industrial activities such as phosphate fertilizer production are causing increasing fluoride pollution in the environment (Rouis and Bensalah 1990; Rutherford et al. 1994; Weinstein and Davison 2004). It has been generally observed that in plant species growing in the vicinity of a source of atmospheric fluoride, foliar fluoride concentrations will be dominated by direct uptake from the air and the contribution from the soil will be minimal (Braen and Weinstein 1985), except for very acidic soils where fluoride uptake from soil may play a major role (Treshow and Anderson 1989). Plant fluorine uptake from areas receiving fluorine inputs has attracted increasing attention due to the risk of plant fluorine accumulation and further transfer to the higher trophic levels of food web and thus health risks to humans and other mammals (Valdez-Jiménez et al. 2011).
Soils act as a sink of fluoride for atmospheric deposition (Davison and Weinstein 2006). Therefore, long-term extensive use of phosphorus fertilizers may also lead to fluoride contamination of soils, water and also vegetation (Loganathan et al. 2001). Arid conditions and the gypsum content of soils represent an additional source of stressful physical and chemical properties for plant life (Guerrero-Campo et al. 1999).
Since the 1960s, in Tunisia, the historical deposition of phosphogypsum in the vicinity of phosphate fertilizer factories has resulted in environmental pollution (Mezghani et al. 2005). Fluoride contamination of the neighbouring spontaneous flora has been recently reported following the gradient of soil fluoride pollution, and some ubiquitous but non-invasive plant species tolerant to high-level fluoride contamination were identified (Boukhris et al. 2015).
Plant sensitivity to fluoride varies with the vegetation communities, plant species and environmental conditions (Davison and Weinstein 2006; Koblar et al. 2011; Weinstein and Davison 2004). Plant responses to fluoride are based on biochemical and physical mechanisms (Fornasiero 2001; Brougham et al. 2013) such as lower chlorophyll and higher anthocyan content, lower stomatal conductance, etc. In situ measurements of chlorophyll and anthocyan content may reveal the plant stress status, as previously reported for Atriplex halimus in heavy metal-contaminated soils (Rabier et al. 2014).
Plant physiological mechanisms in response to fluoride-contaminated environments may lead to fluoride exclusion or resistance or accumulation. Fluoride localization in tissue may provide information about the plant’s tolerance strategy. However, the distribution of other elements in the different plant organs may also help us to understand the tolerance mechanism of plant species to fluoride. Electron microscopy equipped with EDXS microanalysis is an efficient tool to identify preferential accumulation of elements in plant tissues (Rabier et al. 2008) and compare element balance in plant organs.
Furthermore, when faced with harsh conditions, the majority of plant species form symbiotic associations to improve their ability to uptake nutrients and cope with water stress (Leung et al. 2010), which have been suggested as important factors for plant edaphic adaptation (Schechter and Bruns 2008). It is well known that arbuscular mycorrhizal fungi (AMF) have beneficial effects on host plants growing on contaminated soils (Leung et al. 2007; Wu et al. 2010). However, the literature on the occurrence of fungi in fluoride-contaminated environments is scarce and most studies deal with the ability of soil fungi to solubilize insoluble fluorides (Wainwright and Supharungsun 1984; Singh et al. 2014). Choi et al. (2006) thought of assessing the ectomyccorhizal status of two pine species in an industrial environment exposed to fluorine pollution but both pines were not found to be ectomycorrhized. There is therefore a need for knowledge on the occurrence of root symbioses under fluorine pollution.
Amongst the plant species encountered in the surroundings of the Tunisian phosphate factories, Atractylis serratuloides is an abundant native spiny species adapted to degraded soils (Chaieb et al. 1992; Slimani et al. 2010). A. serratuloides is also widespread in severe drought areas of the Mediterranean Basin, e.g. Tunisia, Algeria, Morocco, Spain (Le Houérou 1995; Rodríguez et al. 2005; Navarro et al. 2009; Slimani et al. 2010). This species has been identified as stress tolerant (Benaradj et al. 2013; Jauffret and Visser 2003) and, recently, as a potential candidate for fluoride stabilization (Boukhris et al. 2015). This spiny species has a different life cycle from other non-spiny species, characterized by later flowering in summer leading to low herbivory pressure (Ronel et al. 2010) due to its xeromorphic spiny characterics, which shield it against herbivores and low palatability, reducing potential fluoride transfer into the food web. However, there is a lack of information regarding the ecophysiological traits of this species and more specifically its mechanisms of fluoride tolerance. Moreover, to the best of our knowledge, root symbiont occurrence has never been previously investigated in this under-documented plant species.
The aim of this study was to assess the ecophysiological responses of this native plant species along a gradient of fluoride pollution in Tunisia. First, fluorine concentrations in both soil and plant samples were assessed at the three sites. Secondly, root symbioses were estimated with a particular focus on arbuscular mycorrhizal fungi (AMF) and dark septate endophytes (DSE). Third, transversal sections of plant organ were observed and analysed with scanning electronic microscopy (SEM) coupled with X analysis to detect and localize major elements including F and S in tissues. Lastly, levels of leaf phytometabolites, i.e. chlorophylls, anthocyanins and epidermal flavonols, were estimated using non-destructive field equipment for fluorescence measurement with the aim of selecting indicators of the physiological status of this plant species when exposed to fluoride pollution.
Materials and methods
The study area
The study area is located along the east coast of Tunisia near the superphosphate factories of Gabes and Skhira for the contaminated sites and at Smara for the reference site, as described in Boukhris et al. (2015). In more details, located on the coast and near the industrial phosphate factories of Tunisia for the first two, the site areas of Gabes, Skhira and Smara belong to the arid bioclimate of Tunisia with only 167 mm of rain per year (Zahran 2010). The average annual temperature from 1997 to 2011 was 21 °C, ranging from 13 °C in January to 28 °C in August as revealed by the climagram (Fig. 1). The sites are almost entirely covered with chamaephytic vegetation. The soils are calcic-magnesic and calcareous containing gypsum and limestone.
Element (fluoride and metals) contamination of the soils was previously analysed in March 2011 (Boukhris et al. 2015) and the analyses showed a gradient of fluoride contamination in the following decreasing concentration order: Gabes, Skhira and Smara. Vegetation cover and communities correspond to typical arid rangelands, as previously described (Boukhris et al. 2015). A. serratuloides Sieber ex Cass. (Asteraceae) is a typical plant species of gypsum crust from southern Tunisia (Pouget 1968) and is an abundant chamaephyte at the three sites.
Soil and plant collection
In June 2011, three samples of 500 mg of topsoil (0–20 cm depth) around the roots of A. serratuloides individuals per site were collected and analysed separately to assess fluoride content, granulometry, texture composition, cation exchange capacity and major nutrient content. Soil samples were stored in plastic bags until the return to the laboratory, where they were dried at room temperature until analysis.
A. serratuloides samples were collected at the same locations as soil sampling for each site. Aboveground parts of plants were collected for fluoride analyses and physiological biomarkers measurements. Root samples were randomly taken on each individual for assessment of AMF and DSE colonizations. Additional samples of leaf, stem and root were stored at 5 °C and then prepared for SEM analysis on fresh organs.
Fluoride analysis
Both soil and plant samples were analysed in triplicate. Soil samples were sieved through a 2-mm mesh. The extraction was performed using 5 g of soil sample mixed with HCl (1/2) during 90 min. Then, the HCl extract was mixed with total ionic strength adjustment buffer (TISAB) and analysed using a fluoride-specific ion electrode (inlab/Model WTW) coupled to a pH meter (pH ION R503). Dried plant samples were ground (<1.0 mm) in a stainless steel mill. For each species, 0.5 g of powdered sample was heated in an electric oven with a mixture of carbonate potassium and carbonate sodium at 700 °C. Then samples were mineralized by hydrochloric acid wet process. After filtration and adjustment with distilled water, F concentrations were determined by potentiometry, as described by Mezghani et al. (2005) on other Tunisian soils with high levels of F, Al and Fe. Standards solutions of NaF were used for calibration. Enrichment factor for fluoride was determined according to Branquinho et al. (2007), i.e. the ratio of total F concentration in shoot tissue on total F concentration in soil.
Granulometry, texture composition, cation exchange capacity and major nutrient content
All parameters of soil fertility were performed at the INRA Laboratoire d’Analyses des Sols (LAS, Arras, France) using standard methods (LAS INRA 2007) except for total organic carbon and total Kjeldahl nitrogen, analysed at the laboratory of Environmental Chemistry from Aix Marseille University. The main physico-chemical properties of the soil samples (<2 mm fraction) were characterized as follows: exchangeable phosphorus (NF X 31-161), cation exchange capacity (CEC) and the exchangeable bases were determined by the co-standardized baltihexamine saturation method (NF X 31-130 and NF X 31-108). Particle-size distribution was determined analytically using wet sieving and the pipette method (NF X 31-107). Total organic carbon (TOC) quantifications were carried out with a Jena Analytic TOC-N/C2100S and total Kjeldahl nitrogen (TKN) following ISO 11261 norm (1995).
Scanning electron microscopy and elemental analysis
Leaf, stem and root samples were prepared as previously described by Rabier et al. (2008). Transversal sections 30 mm thick were cut at −25 °C using a cryomicrotome (Cryo-cut II microtome Reichert-Jung), then immediately placed on SEM specimen holders and carbon metallized (10–15 nm) for observation under an ESEM Philips XL 30 microscope with detector EDAX sdd apollo 10. X-ray mapping was performed for 20 min to give the elemental distribution for each selected element (F, Ca, K, Mg, Cl, Mn, S, P, Ni, Al, Mg, Fe and Si). In all cases, the voltage was 20 kV. SEM images were obtained with back-scattered electron (BSE) or secondary electron (SE) imaging. BSE imaging was used to study the ultrastructure and micromorphology of the plant tissues. To help in the identification of the different tissues of the analysed organs, transversal histological sections were stained with carmino-green staining and observed with an optical microscope.
Root symbiosis observations
Root samples of three individuals per site were taken. The roots were rinsed under the tap and then with desionized water and stored in alcohol (60 %, v/v) at room temperature until proceeding. The percentage of symbiotic colonization was estimated by visual observation of fungal colonization after clearing the washed roots in 10 % KOH and staining with lactophenol blue solution according to Phillips and Hayman’s method (1970). A minimum of 50 root segments (1-cm long) per plant were counted. AM and DSE frequencies were recorded and expressed as a percentage of colonization length per root sample.
Stress biomarkers
Plant physiological indices were optically estimated using non-destructive measurements of constitutive and induced epidermal phenols, flavonols, anthocyanins, chlorophylls and the chlorophyll-to-flavonoids ratio named nitrogen balance index (NBI) of in situ plants, using a Multiplex® 3 equipment (FORCE-A, Orsay, France). This portable fluorimetric device uses fluorescence technology with multiple excitations. Different combinations of the blue-green, red and far-red fluorescence signals at the various excitation bands could be used as indices of various compounds (Cerovic et al. 2008; Agati et al. 2011). For practical reasons, the measurements were done on excised leaf microwave dried samples using the same procedure for each sample. Moisture loss was recorded by removing the shoot sample and their filter paper support from the microwave and weighing it on a digital balance. The microwave power was applied until the weight of the sample was reduced to a level corresponding to a moisture content of about 0.10 dry basis (Soysal 2004; Özbek and Dadali 2007). Fifteen different sample surfaces were measured for each site.
Statistical analysis
Statistical analyses were performed for all data using JMP 9 statistical software (SAS Institute, Cary, NC, USA). Differences between element concentrations in plant parts and soils, plant health biomarkers and fungal colonization percentages in the three populations of A. serratuloides were compared using the parametric Tukey test when data distribution was normal. The non-parametric Dunn All Pairs for Joint Ranks was used for non-normal distributions of data.
Results
Soil characterization
Soil analyses confirmed the calci-magnesic composition of soils (Table 1). In our field study, a widespread gypsum-calcareous crust was observed at the three selected sites. Soils were for the most part sandy with a major percentage of fine sand (50–200 μm). Gabes had a higher level of fine sand than Smara and the lowest percentage of medium sand. For silt content, Smara had higher percentage of fine (2–20 μm) and medium (20–50 μm) silts than Gabes. No significant difference was observed for clay content.
Available phosphate concentrations were high but significantly higher in Gabes than in Skhira and Smara. CEC was very low (ca 3–5 cmol+/kg) but higher in Smara than in Gabes, notably with more K+ and Mg2+ available in Smara than in Gabes soils. Total organic carbon contents varied from 0.13 to 0.65 % and total Kjeldahl nitrogen, from 0.31 to 0.58 mg/g. Fluoride concentrations showed a low contamination in Smara with only 33 mg/kg of F, Skhira was more contaminated and, Gabes, the most contaminated with 1519 mg/kg of F (Table 2).
Tissue element localization
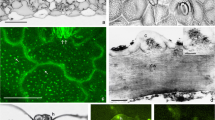
EDXS cartography was performed for all the detected peaks of the X-ray spectra on leaf, stem and root samples of A. serratuloides to determine the precise element localization in plant tissues and punctually, point analyses on specific tissues were done.
Amongst the elements detected by EDAX point analyses, fluorine represented 4.8 % for leaf and only an average of 1.5 % in root and stem from Gabes, although elements such as Ca were concentrated in some tissues at more than 39 % of the detected elements. However, even with high total F content in the organs, F concentrations were below the threshold of detection at the level of a 30-μm thick transversal section mapping (Figs. 2, 3 and 4).
We focused on Ca, S, Mg and Si in leaf, stem and root sections from Gabes, the most polluted site, and from Smara, the reference site for which a differential balance was revealed in the different organs between Gabes and Smara. The maps of distribution of Cu, Cl, Fe, K, Na and P are not shown here since these elements were equally distributed between polluted and reference samples.
As shown in Figs. 2, 3 and 4, the general structure of the fluoride-polluted plant organs (leaf, stem and root) did not differ from those of the control. Therefore, no structural alterations were observed due to fluoride exposure.
Leaf lamina was generally narrow and spiny with a thick-walled epidermis (Fig. 2). The epidermis was composed of two layers of epidermal cells. The mesophyll is differentiated into palisade and spongy parenchyma. Surrounding the midvein is a thick layer of sclerenchyma. No cell collapse was observed. However, Ca and S were more strongly accumulated in the mesophyll around the midvein of the Gabes leaf section than in that from Smara, and Mg was more accumulated all over the mesophyll of the Gabes leaf section, leading to a modified element balance in leaves from the polluted site. Bright crystals in the mesophyll are clearly visible in BSE observations. Calcium oxalates were detected in the peripheral leaf tissues of Gabes samples. Otherwise, a Si-deposition was mainly observed in leaf thorns (Fig. 2e) as needle-like bodies. Point analyses were done in the mesophyll of leaf margins from Gabes and Smara. The results showed a significantly higher contents in F, Na, Mg, Al, Si and P and lower content in S in Gabes samples compared to Smara ones (Table 3). However, no significant difference in Ca content was detected.
Stems of A. serratuloides were characterized by the occurrence of bundles of schlerenchymatous cells in the cortical parenchyma and fibrous pith (Fig. 3). Ca was preferentially stored in the fibrous pith and the cork in Gabes stem sections. S seemed more intensively located in the cortical parenchyma of Smara stem sections and diffuse in those from Gabes, with a high background effect. In contrast, a major deposition of Mg was observed in all tissues except the pith in stem sections from Gabes and not observed in the samples from Smara. Thus, a disruption of element distribution in stems between both sites was observed.
In root sections, an imbalance of S between polluted and reference sites was also encountered with a diffuse but intensive S deposition in Smara root sections and a not easily detectable occurrence of S in those from Gabes (Fig. 4). Moreover, a diffuse distribution of Mg was also observed and this element was more intensively present in Smara root sections and a greater deposition of Si was located in the peripheral tissues of Smara root sections.
Calcium oxalate crystals were found in all plant organs from Gabes samples.
Stress biomarkers of fluoride exposure
No variation of chlorophyll and leaf epidermal phenol content was detected between the different sites (Table 2). However, flavonol and anthocyan indices were significantly lower in leaves from Gabes than in leaves from the two other sites. In contrast, the nitrogen balance index was higher at Gabes than at the other sites.
Root symbioses
Root symbioses were observed in all root systems of A. serratuloides (Table 2), even in Gabes, the most polluted site of our study. To the best of our knowledge, this is the first report dealing with root symbioses in A. serratuloides. Two main types of root symbioses were encountered, i.e. arbuscular endomycorrhizae (AM) and interactions with dark septate endophytes (DSE). However, the percentage of AM and DSE colonizations were low with no more than 23 and 2 % of root length colonized, respectively. No DSE were observed in the root fragments at Skhira. No significant differences of AM colonization were observed between all the sites.
Discussion
Fluoride contamination of soils and plants
Soils may be characterized as gypsum-calcareous soils according to Mtimet (2001). The granulometry differed between the sites particularly for sand and silt contents with a higher percentage of fine sand percentage in Gabes. This may be related to the fact that the surrounding soils are affected by a long-term exposure to fluoride atmospheric deposits in Gabes due to uncovered phosphogypsum deposits (Boukhris et al. 2015). Fluoride concentrations of the soils at the vicinity of A. serratuloides individuals at the three sites confirmed the gradient of pollution already reported in the three sites (Boukhris et al. 2015). The average F concentrations in the soils around the plant species for the three sites were similar to the average F concentrations found in pooled samples previously analysed, with Gabes as the most contaminated site (ca. 1519 compared to 1431 mg/kg for the pooled soil samples in March 2011), followed by the site of Skhira with ca. 150 mg/kg (instead of 144 mg/kg) and the site of Smara considered as reference as the least polluted with ca. 33 mg/kg (instead of 35 mg/kg). These results are in agreement with long-term contamination of the topsoil with a fairly homogeneous level of contamination. This is very different from many soils contaminated by heavy metals due to metallurgic industrial activities in which element contamination is highly heterogeneous (Affholder et al. 2013; Testiati et al. 2013).
As previously reported, fluorine accumulated in aboveground parts of A. serratuloides. With an increased accumulation of fluoride in aboveground parts from 37 mg/kg at the Smara site and ca 89 mg/kg at Skhira to almost three-fold more concentrated at Gabes, with 252 mg/kg, A. serratuloides appears to be tolerant of a wide range of fluoride concentrations in soils. However, since enrichment factors were <1 in Gabes and Skhira and equal to 1 in Smara (Table 2), A. serratuloides may be considered as an excluder in the F-polluted sites and indifferent in Smara according to Baker (1981).
Fluoride tolerance mechanisms in A. serratuloides
Probable role of calcium crystals
At tissue level, a lack of alteration of leaf as previously observed in Hypericum perforatum (Fornasiero 2001) was observed even in the most F-contaminated site of Gabes. However, the distribution of Ca, S and Mg varied differently between Gabes and Smara depending on plant organs and may thus be considered as elements acting in the physiological responses of A. serratuloides to fluoride pollution. According to Tayibi et al. (2009), the main component of phosphogypsum is calcium oxide (CaO), reaching ca 31 %, and sulphates expressed as SO3 with ca 46 %, which can increase the available S and Ca that plants may absorb. S and Ca were majorly deposited around the midvein of leaf lamina and in the pith and peripheral tissues in the stems from Gabes and no preferential Ca deposits were detected in root sections. One hypothesis is that leaf surfaces are more exposed to wind-borne polluted deposition than through absorption and translocation mechanisms. In root and stems parts from Gabes, an exclusion mechanism restricting S absorption may also occur. Many calcium oxalates were detected in tissues of Gabes samples. This crystal formation has been well documented by White and Broadley (2003) when excessive Ca is present in rhizosphere solution and by Nakata and McConn (2000), confirming the role of calcium oxalate in ion balance, plant defence and detoxification. Furthermore, in polluted environments, available calcium may play a role in lowering fluorine toxic effects by trapping it in the sequesterable form of CaF2, preventing fluorine impact on plant metabolism (Weinstein and Davison 2004). This result is in agreement with the results previously reported by Ben Abdallah et al. (2006) and Álvarez-Ayuso et al. (2011), confirming the important role of Ca in limiting the translocation of F to the aboveground parts. In the leaf sections from Gabes, Ca and S distributions in cartographies were mainly superimposable in mesophyll and detected as components of the druse crystals aggregates inside cells (Fig. 2g, k) that may be interpreted as calcium sulphate inclusions.
Responses to reactive oxygen species
One of the suggested roles of flavonoids in plants is the scavenging of reactive oxygen in stressed environments (Türkan and Demiral 2009). Previous results on the protective mechanism of the antioxidant activities on leaf senescence suggest that the presence of flavonoids and particularly anthocyanins in Mediterranean plant species protects them against a variety of reactive oxygen species (ROS)-producing stresses. Because of their induction by osmotic stress, drought-tolerant plants may also contain more anthocyanins in their tissues (Chalker-Scott 1999). The toxicity of fluoride is associated with ROS induction by the generation of NO and the reduction of cellular antioxidant defences against oxidative damage (Barbier et al. 2010; Fornasiero 2001; Li et al. 2011). Thus, it would have been congruent to detect higher levels of anthocyanins in Gabes and Skhira leaves. However, in Gabes, a reduced level of anthocyans had been measured. Since it has been previously reported that HF may destroy anthocyanins in Coleus (Lamprecht and Powell 1977), the observed reduction of anthocyanins may be linked to fluoride toxicity. Thus, we may suppose that the main mechanism of fluoride tolerance is not linked to a nonenzymatic antioxidative defence system against ROS which includes phenols and flavonoids as reported by Sytar et al. (2013).
In our study, S had been identified as one of the elements involved in A. serratuloides response to fluoride-polluted environments. The role of sulphur in the metabolism is also important in redox control mechanisms and especially as a source of glutathione, a significant compound involved in first step-responses to ROS (Leusteck and Saito 1999). Moreover, C/N balance may be modified in response to fluoride exposure, as described in wheat by Asthir et al. (1998), in which the disruption in carbon metabolism was compensated by the nitrogen metabolism. Our results showed a significantly higher nitrogen balance index in A. serratuloides leaves at Gabes than at the two other sites, although the soils from these sites are nitrogen-deficient. We may hypothesize that the fluoride tolerance mechanism involved is linked to redox active enzyme activities such as glutathione metabolism.
A. serratuloides potential for fluoride-polluted soil stabilization
In soils like those of our study sites, in which aluminium is one of the major elements (Boukhris et al. 2015), fluoride complexes tightly with Al and may affect the phosphate absorption by plants (Façanha and Okorokova-Façanha 2002). However, no decrease of chlorophyll contents, highly dependant of phosphate resource, was detected between the three sites, and this suggests a high tolerance of A. serratuloides to the particular conditions of those fluoride-polluted soils under arid climate. Moreover, according to Delgado-Baquerizo et al. (2013), aridity has a direct negative effect on the organic matter component and phosphatase activity, but a positive effect on total P in drylands. These authors thus suggest that an increase in aridity in drylands, as forecast with climate change, is expected to lead to severe nutrient depletion in these environments and reduction of plant cover. Therefore, empowering populations of fluoride-tolerant native plant species such as A. serratuloides in the field may help to offset this process.
Moreover, soil quality is not only linked to its structure and chemical composition but also to its microbial diversity. It is interesting to notice the occurrence of root symbiotic fungal strains in such drastic soil conditions. Furthermore, the low-observed colonization by AM fungi and DSE in roots of A. serratuloides may probably be due to the calcareous soil properties (Table 1) and a negative effect of CaCO3 on symbioses which reduces and drastically disturbs the main life cycle of AM fungi, according to Labidi et al. (2011). Moreover, high available phosphorus concentrations are present in soils especially at Gabes due to dust and waste by-product from the superphosphate factory. One of the most important roles of AM symbiosis, i.e. improving phosphorus nutrition, may be thus reduced (Renker et al. 2005) and AM occurrence may be affected in the particular conditions of the gypsum-calcareous soils. Furthermore, it has been demonstrated that nitrogen/phosphorus balance in soils may affect AM colonization. However, Blanke et al. (2005) reported an enhanced AM colonization of Artemisia vulgaris roots in nitrogen-deficient but phosphorus-rich soils. Under low resource availability, it appears that root physiological adaptations occur in order to maximize resource acquisition capacity, amongst these, endomycorhizal symbioses (Cruz et al. 2008). However, a recent work by Palacio et al. (2012) on other gypsophytes reported low percentage of colonization due to other adaptations to cope with the restrictive conditions of gypsum in soil. Moreover, following Trotta et al. (2006), whenever colonization occurs, even to a small extent, it induces beneficial effects in the host plants. Nevertheless, little is known about the potential of AM fungi and DSE to enhance plant tolerance under fluoride pollution, and there is a need for ex situ experiments to better understand their possible roles.
Conclusion
This study is a first insight into the physiological mechanisms of A. serratuloides involved in its fluoride tolerance. Its tolerance to fluoride seems to use the protective effect of calcium due to a Ca-F interaction on and within plant tissues, increasing tissue tolerance to fluoride and suggesting a potential role of enzyme-dependant reactions reducing oxidant stresses. The results demonstrated the contribution of element balance with a calcium accumulation in leaf and stem parts at the most polluted site in Gabes accompanied with more magnesium in stems and leaves and of phytometabolite balance with less anthocyanins and flavonols in leaf parts from Gabes in the in situ responses of A. serratuloides to fluoride. This is also the first report on root symbioses of A. serratuloides that should be taken into account in an eventual future use of this species for fluoride-polluted soil stabilization. This first contribution suggests a great potential for application in southern Mediterranean countries concerned by superphosphate exploitations.
References
Affholder MC, Prudent P, Masotti V, Coulomb B, Rabier J, Nguyen-The B, Laffont-Schwob I (2013) Transfer of metals and metalloids from soil to shoots in wild rosemary (Rosmarinus officinalis L.) growing on a former lead smelter site: human exposition risk. Sci Total Environ 454–455:219–229
Agati G, Cerovic ZG, Pinelli P, Tattini M (2011) Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ Exp Bot 73:3–9
Álvarez-Ayuso E, Giménez A, Ballesteros JC (2011) Fluoride accumulation by plants grown in acid soils amended with flue gas desulphurisation gypsum. J Hazard Mater 192:1659–1666
Asthir B, Basra AS, Batta SK (1998) Fluoride-induced alteration of carbon and nitrogen metabolism in developing wheat grains. Biol Plant 41:287–292
Baker AJM (1981) Accumulators and excluders: strategies in the response of plants to trace metals. J Plant Nutr 3:643–654
Barbier O, Arreola-Mendoza L, Del Razo LM (2010) Molecular mechanisms of fluoride toxicity. Chem Biol Interact 188:319–333
Ben Abdallah F, Elloumi N, Mezghani I, Boukhris M, Garrec J-P (2006) Survival strategies of pomegranate and almond trees in a fluoride polluted area. C R Biologies 329:200–207
Benaradj A, Boucherit H, Hasnaoui O, Mederbal K, Sehli A (2013) Rehabilitation of the steppe Lygeum spartum in the region of Naama (western Algeria). Energy Procedia 36:349–357
Blanke V, Renker C, Wagner M, Füllner K, Held M, Kuhn AJ, Buscot F (2005) Nitrogen supply affects arbuscular mycorrhizal colonization of Artemisia vulgaris in a phosphate-polluted field site. New Phytol 166:981–992
Boukhris A, Laffont-Schwob I, Mezghani I, Kadri E, Lefi P, Pricop A, Tatoni T, Chaieb M (2015) Screening biological traits and fluoride contents of native vegetations in arid environments to select efficiently fluoride-tolerant native plant species for in-situ phytoremediation. Chemosphere 119:217–223
Braen SN, Weinstein LH (1985) Uptake of fluoride and aluminium by plants grown in contaminated soils. Water Air Soil Pollut 24:215–223
Branquinho C, Serrano HC, Pinto MJ, Martins-Loução MA (2007) Revisiting the plant hyperaccumulation criteria to rare plants and earth abundant elements. Environ Pollut 146:437–443
Brougham KM, Roberts SR, Davison AW, Port GR (2013) The impact of aluminium smelter shut-down on the concentration of fluoride in vegetation and soils. Environ Pollut 178:89–96
Cerovic ZG, Moise N, Agati G, Latouche G, Ben Ghozlen N, Meyer S (2008) New portable optical sensors for the assessment of winegrape phenolic maturity based on berry fluorescence. J Food Compos Anal 21:650–654
Chaieb M, Floret C, Le Floc’h E, Pontanier R (1992) Life history strategies and water resource allocation in five pasture species of the Tunisian arid zone. Arid Land Res Manag 6(1):1–10
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Choi DS, Kayama M, Jin HO, Lee CH, Izuta T, Koike T (2006) Growth and photosynthetic responses of two pine species (Pinus koraiensis and Pinus rigida) in a polluted industrial region in Korea. Environ Pollut 139:421–432
Cruz C, Correia P, Ramos A, Carvalho L, Bago A, Martins Loução MA (2008) Arbuscular mycorrhiza in physiological and morphological adaptations of Mediterranean plants, In: Varma A, editor. Mycorrhiza—State of the Art, Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics. Springer, 733–752. DOI:10.1007/978-3-540-78826-3-34
Davison AW, Weinstein LH (2006) Some problems relating to fluorides in the environment: effects on plants and animals, in fluorine and the environment, atmospheric chemistry, emissions and lithosphere, Elsevier, Tressaud ed. Vol 1, p251-298
Delgado-Baquerizo M, Maestre FT, Gallardo A, Bowker MA, Wallenstein MD, Quero JL, Ochoa V, Gozalo B, García-Gómez M, Soliveres S, García-Palacios P, Berdugo M, Valencia E, Escolar C, Arredondo T, Barraza-Zepeda C, Bran D, Carreira JA, Chaieb M, Conceição AA, Derak M, Eldridge DJ, Escudero A, Espinosa CI, Gaitán J, Gatica MG, Gómez-González S, Guzman E, Gutiérrez J,R, Florentino A, Hepper E, Hernández RM, Huber-Sannwald E, MJankju M, Liu J, Mau RL, Miriti M, Monerris J, Naseri K, Noumi Z, Polo V, Prina A, Pucheta E, Ramírez E, Ramírez-Collantes DA, Romão R, Tighe M, Torres D, Torres-Díaz C, Ungar ED, Val J, Wamiti W, Wang D, Zaady E (2013) Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 502:672–676
Façanha AR, Okorokova-Façanha AL (2002) Inhibition of phosphate uptake in corn roots by aluminum-fluoride complexes. Plant Physiol 129:1763–1772
Fornasiero RB (2001) Phytotoxic effects of fluorides. Plant Sci 161:979–985
Guerrero-Campo J, Alberto F, Hodgson J, García-Ruiz JM, Montserrat-Martí G (1999) Plant community patterns in a gypsum area of NE Spain. I. Interactions with topographic factors and soil erosion. J Arid Environ 41:401–410
Jauffret S, Visser M (2003) Assigning life-history traits to plant species to better qualify arid land degradation in Presaharian Tunisia. J Arid Environ 5:1–28
Koblar A, Tavčar G, Ponikvar-Svet M (2011) Effects of airborne fluoride on soil and vegetation. J Fluor Chem 132:755–759
Labidi S, Calonne M, Ben Jeddi F, Debiane D, Rezgui S, Laruelle F, Tisserant B, Grandmougin-Ferjani A, Sahraoui AL (2011) Calcareous impact on arbuscular mycorrhizal fungus development and on lipid peroxidation in monoxenic roots. Phytochemistry 72:2335–2341
Lamprecht WO, Powell RD (1977) The effect of hydrogen fluoride on two pigments in coleus. Econ Bot 31:148–152
LAS INRA (2014) Laboratoire d’analyses des sols d’Arras. Méthodes applicables aux sols. http://www6.lille.inra.fr/las/Methodes-d-analyse/Sols
Le Houérou H-N (1995) Bioclimatologie et biogéographie des steppes arides du Nord de l'Afrique : diversité biologique, développement durable et désertisation. In : Le Houérou H.-N. (ed.). Bioclimatologie et biogéographie des steppes arides du Nord de l’Afrique : diversité biologique, développement durable et désertisation. Montpellier : CIHEAM, p. 1–39 6
Leung HM, Ye ZH, Wong MH (2007) Survival strategies of plants associated with arbuscular mycorrhizal fungi on toxic mine tailings. Chemosphere 66:905–915
Leung HM, Wu FY, Cheung KC, Ye ZH, Wong MH (2010) Synergistic effects of arbuscular mycorrhizal fungi and phosphate rock on heavy metal uptake and accumulation by an arsenic hyperaccumulator. J Hazard Mater 181:497–507
Leusteck T, Saito K (1999) Sulfate transport and assimilation in plants. Plant Physiol 120:637–643
Li C, Zheng Y, Zhou J, Xu J, Ni D (2011) Changes of leaf antioxidant system, photosynthesis and ultrastructure in tea plant under the stress of fluorine. Biol Plant 55:563–566
Loganathan P, Hedley MJ, Wallace GC, Roberts AHC (2001) Fluoride accumulation in pasture forages and soils following long-term applications of phosphorus fertilisers. Environ Pollut 115:275–282
Mezghani I, Elloumi N, Ben Abdallah F, Chaieb M, Boukhris M (2005) Fluoride accumulation by vegetation in the vicinity of a phosphate fertiliser plant. Fluoride 38:69–75
Mtimet A (2001). Soils of Tunisia, In: P. Zdruli, P. Steduto, C. Lacirignola, L. Montanarella (Eds.), Soil resources of southern and eastern Mediterranean countries. CIHEAM, Bari, pp. 243–262
Nakata PA, McConn MM (2000) Isolation of Medicago trunculata mutants defective in calcium oxalate formation. Plant Physiol 124:1097–1104
Navarro T, El Oualidi J, Taleb MS, Pascual V, Cabezudo B (2009) Dispersal traits and dispersal patterns in an oro-Mediterranean thorn cushion plant formation of the eastern High Atlas, Morocco. Flora 204(9):658–672
Özbek B, Dadali G (2007) Thin-layer drying characteristics and modelling of mint leaves undergoing microwave treatment. J Food Eng 83:541–549
Palacio S, Johnson D, Escudero A, Montserrat-Martí G (2012) Root colonisation by AM fungi differs between gypsum specialist and non-specialist plants: links to the gypsophile behaviour. J Arid Environ 76:128–132
Phillips JM, Hayman DS (1970) Improved procedures for clearing and staining parasitic and vesicular arbuscular fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Pouget M (1968) Contribution à l’étude des croûtes et encroûtements gypseux de nappe dans le sud tunisien. Cah ORSTOM, Sér Pédologie 6:309–365
Rabier J, Laffont-Schwob I, Notonier R, Fogliani B, Bouraïma-Madjebi S (2008) Anatomical element localization by EDXS in Grevillea exul var. exul under nickel stress. Environ Pollut 156:1156–1163
Rabier J, Laffont-Schwob I, Pricop A, Ellili A, D’enjoy-Weinkammerer G, Salducci MD, Prudent P, Lotmani B, Tonetto A, Masotti V (2014) Heavy metal and arsenic resistance of the halophyte Atriplex halimus L. along a gradient of contamination in a French Mediterranean spray-zone. Water Air Soil Pollut. doi:10.1007/s11270-014-1993-y
Renker C, Blanke V, Buscot F (2005) Diversity of arbuscular mycorrhizal fungi in grassland spontaneously developed on area polluted by a fertilizer plant. Environ Pollut 135(2):255–66
Rodríguez AR, Mora JL, Arbelo C, Bordon J (2005) Plant succession and soil degradation in desertified areas (Fuerteventura, Canary Islands, Spain). Catena 59:117–131
Ronel M, Néeman G, Lev-Yadun S (2010) Spiny east Mediterranean plant species flower later and in a drier season than non-spiny species. Flora 205:276–281
Rouis MJ, Bensalah A (1990) Phosphogypsum management in Tunisia: environmental problems and required solutions. In: Proceedings of the Third International Symposium on Phosphogypsum, Orlando, FL, FIPR Pub. No. 01-060-083; 1, 87–105
Rutherford PM, Dudas MJ, Samek RA (1994) Environmental impacts of phosphogysum. Sci Total Environ 149:1–38
Schechter SP, Bruns TD (2008) Serpentine and non-serpentine ecotypes of Collinsia sparsiflora associate with distinct arbuscular mycorrhizal fungal assemblages. Mol Ecol 17:3198–3210
Singh J, Singh P, Singh A (2014) Fluoride ions vs removal. A Study, Arabian Journal of Chemistry, Technologies. doi:10.1016/j.arabjc.2014.06.005
Slimani H, Aidoud A, Rozé F (2010) 30 years of protection and monitoring of a steppic rangeland undergoing desertification. J Arid Environ 74:685–691
Soysal Y (2004) Microwave drying characteristics of parsley. Biosyst Eng 89(2):167–173
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999
Tayibi H, Choura M, López FA, Alguacil FJ, López-Delgado A (2009) Environmental impact and management of phosphogypsum. J Environ Manag 90:2377–2386
Testiati E, Parinet J, Massiani C, Laffont-Schwob I, Rabier J, Pfeifer H-R, Lenoble V, Masotti V, Prudent P (2013) Trace metal and metalloid contamination levels in soils and in two native plant species of a former industrial site: evaluation of the phytostabilization potential. J Hazard Mater 248–249:131–141
Treshow M, Anderson FK (1989) Plant stress from air pollution. Wiley, Chichester, p 283
Trotta A, Falaschi P, Cornara L, Minganti V, Fusconi A, Drava G, Berta G (2006) Arbuscular mycorrhizae increase the arsenic translocation factor in the as hyperaccumulating fern Pteris vittata L. Chemosphere 65:74–81
Türkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Valdez-Jiménez L, Soria Fregozo C, Miranda Beltrán ML, Gutiérrez Coronado O, Pérez Vega MI (2011) Effects of the fluoride on the central nervous system. Neurologia 26(5):297–300
Wainwright M, Supharungsun S (1984) Release by fungi of F− from insoluble fluorides. Trans Br Mycol Soc 82(2):289–292
Weinstein LH, Davison AW (2004) Fluorides in the environment. CABI Publishing, Cambridge
White PJ, Broadley RM (2003) Calcium in plants. Rev Ann Bot 92:487–511
Wu FY, Bi YL, Leung HM, Ye ZH, Lin XG, Wong MH (2010) Accumulation of As, Pb, Zn, Cd and Cu and arbuscular mycorrhizal status in populations of Cynodon dactylon grown on metal-contaminated soils. Appl Soil Ecol 213–218
Zahran MA (2010) Climate vegetation. Afro-Asian Mediterranean and Red Sea coastal lands. Plant and Vegetation. London New York: Springer Dordrecht Heidelberg, 344
Acknowledgments
This study was partly funded by the Action Intégrée Franco-Tunisienne of the French Ministère des Affaires Etrangères et Européennes and the Tunisian Ministère de l’Enseignement Supérieur, de la Recherche Scientifique. The authors thank Imed Mezghani for his help in harvesting and sampling in the field, and thanks are also due to Oceane Beaufort for helping with root staining in the laboratory. The authors are grateful to Laurent Vassalo for his efficient help in TOC and TKN analyses in a high-performance time. Also, the authors thank the Groupe Chimique Tunisien, in particular the Direction Centrale de la Recherche, for its assistance and logistical support in carrying out this study. Lastly, the authors would like to thank Michael Paul for revising the English of this text.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Boukhris, A., Laffont-Schwob, I., Rabier, J. et al. Changes in mesophyll element distribution and phytometabolite contents involved in fluoride tolerance of the arid gypsum-tolerant plant species Atractylis serratuloides Sieber ex Cass. (Asteraceae). Environ Sci Pollut Res 22, 7918–7929 (2015). https://doi.org/10.1007/s11356-014-3957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-3957-6