Abstract
Because exposure to toxicants not only results in mortality but also in multiple sublethal effects, the use of life-table data appears particularly suitable to assess global effects on exposed populations. The present study uses a life table response approach to assess population-level effects of two insecticides used against mosquito larvae, spinosad (8 μg/l) and Bacillus thuringiensis var. israelensis (Bti, 0.5 μl/l), on two non target species, Daphnia pulex and Daphnia magna (Crustacea: Cladocera), under laboratory versus field microcosms conditions. Population growth rates were inferred from life table data and Leslie matrices under a model with resource limitation (ceiling). These were further used to estimate population risks of extinction under each tested condition, using stochastic simulations. In laboratory conditions, analyses performed for each species confirmed the significant negative effect of spinosad on survival, mean time at death, and fecundity as compared to controls and Bti-treated groups; for both species, population growth rate λ was lower under exposure to spinosad. In field microcosms, 2 days after larvicide application, differences in population growth rates were observed between spinosad exposure conditions, and control and Bti exposure conditions. Simulations performed on spinosad-exposed organisms led to population extinction (minimum abundance = 0, extinction risk = 1), and this was extremely rapid (time to quasi-extinction = 4.1 one-week long steps, i.e. one month). Finally, D. magna was shown to be more sensitive than D. pulex to spinosad in the laboratory, and the effects were also detectable through field population demographic simulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Population-level ecotoxicology has gained much interest during the past decade and its relevance for ecological risk assessment is now well recognized and documented (e.g., Forbes 1999; Forbes et al. 2001; Kammenga and Laskowski 2000; Akçakaya et al. 2008). Assessment of toxicant impact on populations is essential for the understanding of ecological and evolutionary processes in ecosystems. Applying the theories and methods of population ecology to questions related to the viability of species in ecosystems exposed to xenobiotics has proven to be relevant in an ecotoxicological context, as reflected for example by the Life Table Response Experiment approach (Caswell 2001; Mauri et al. 2003; Bøhn et al. 2010).
The effects of pesticides and other toxicants on organisms are usually estimated using simplistic estimates such as LD50 or LC50 (lethal dose or concentration for 50% of an exposed group of individuals), based upon survivorship to a range of concentrations over short periods of time (acute toxicity), and survival, growth rate, and individual reproductive performance under chronic exposures to sublethal concentrations (Forbes and Calow 1999). Acute toxicity tests represent a straightforward approach to assess and compare the toxicity of various compounds with regard to their effect at the organismic level, in a given species, as well as an affordable screening of several target and non-target species for their sensitivity to a particular compound. However, this approach has no concern on the outcome of the individuals that survive exposure (Stark et al. 2007). Chronic exposures can sometimes result in much higher mortality levels in populations than predicted by acute, short-term exposures, because lethal effects can be delayed and because sublethal effects can occur that may affect several population traits, most notably by decreasing fecundity (Forbes and Calow 1999; Stark and Banks 2003). On the other hand, inferences on population-level effects based upon extrapolations from individual-level endpoints may also be overprotective, for example when a strongly impacted trait has little influence on population growth rate (Forbes et al. 2001).
In the case of mosquito larvae control, insecticide dosing is chosen in order to minimize impacts on non-target organisms. However, aquatic invertebrates may be submitted to repeated exposures because of successive treatments. Life-table data therefore appear particularly suited to estimate sublethal effects on non-target organisms chronically exposed to such compounds. Cladocerans and other zooplankton groups are water column-dwelling organisms that share the habitat and, at least in part, food resources of mosquito larvae (Blaustein and Chase 2007). They may thus be exposed to larvicides in treated areas, and there is a need for methods that can be used for the monitoring of impacts of mosquito control programmes on these non-target organisms. Moreover, cladocerans such as Daphnia species are important components of aquatic foodwebs because they are primary consumers feeding on algae and bacteria, and serve as food resource for other aquatic organisms including fish and invertebrates. Although Daphnia species are not threatened or endangered, they are often used as indicator species of aquatic ecosystem pollution. Furthermore, due to their extremely high reproductive rates and short generation times, and to the fact that they are easily reared in laboratory conditions, waterfleas are commonly used as test models for ecotoxicity testing.
The present study was undertaken to assess population-level effects of two larvicides used for mosquito control, spinosad and Bti (Bacillus thuringiensis var. israelensis), on two non target species, Daphnia pulex and Daphnia magna (Crustacea: Cladocera) under laboratory versus field conditions. Comparison of these two situations aimed at providing empirical arguments on the relevance of extrapolations from individual endpoints measured in laboratory conditions to field-relevant population-level effects in ecological risk assessment (Forbes et al. 2001). In order to reach more generic conclusions (or to limit idiosyncratic effects), two species were used, D. pulex and D. magna, which were also chosen because they both naturally occur in biotopes where mosquito larvae develop and where larvicides are applied. Bacillus thuringiensis (Bt) is a rod-shaped, positive Gram, endospore-forming aerobic bacterium. Its insecticidal activity is due to crystal (Cry) proteins associated with sporulation. The crystal proteins need the alkaline pH of insect midgut to be activated into toxins that bind to specific receptors of the epithelial cell wall, causing membrane perforations of the gut, leaking of gut internal fluids, and eventually death (Whalon and Wingerd 2003). The serovar Bacillus thuringiensis israelensis (Bti) is well-known for its selectivity to Nematocera dipterans, and it is widely used for mosquito control all over the world (Boisvert and Lacoursière 2004). Laboratory tests and field studies have shown that Bti may be considered as safe to the environment due to its selectivity (Mulla et al. 1982; Barnes and Chapman 1998; Boisvert and Lacoursière 2004). However, larvae of some species of non-target Nematocera (Chironomidae) have been shown to be susceptible to Bti (Kondo et al. 1992; Rey et al. 1998), and results of in situ studies on the impact of Bti-containing larvicides on non-target organisms remain controversial (Hershey et al. 1995, 1998; Liber et al. 1998; Niemi et al. 1999; Vinnersten et al. 2009). Spinosad is a new biological insecticide that is currently evaluated as a candidate larvicide for mosquito control. It is a mixture of spinosyns A and D known as fermentation products of a soil bacterium (Saccharopolyspora spinosa, Actinomycetes; Crouse et al. 2001). Spinosad acts as a contact and stomach poison (Salgado 1998). It persistently stimulates the insect central nervous system by interacting with nicotinic acetylcholine receptors through a mechanism distinct from those of other nicotinic agonists (Watson 2001). Spinosad is considered as a selective insecticide for insect pest species (Miles and Dutton 2000), but it may be toxic to non-target species (Nasreen et al. 2000; Tillman and Mulrooney 2000; Consoli et al. 2001), especially the zooplanktonic crustaceans D. pulex (Stark and Vargas 2003; Duchet et al. 2008) and D. magna (Duchet et al. 2010).
Demographic parameters were estimated in D. pulex and D. magna exposed to spinosad and Bti, in laboratory and field microcosms. Population growth rates were inferred from life table data and Leslie matrices under a model with resource limitation (ceiling). These were further used to estimate population risks of extinction under each tested condition, using stochastic simulations (RAMAS, Akçakaya 2005). Outcomes of the demographic analysis are discussed in terms of relevance of extrapoling complex field population effects from laboratory-assessed endpoints for two larvicides which bear different modes of action.
Materials and methods
Larvicides
Bacillus thuringiensis var. israelensis (Bti) was applied as the flowable formulation VectoBac® 12AS (1.2% AI, i.e. 1200 ITU/mg; CAS #68038-71-1) produced by Valent Biosciences (Libertyville, IL, USA). Spinosad was applied as Conserve® 120SC (11.6% AI, factor A CAS #131929-60-7 and factor D CAS #131929-63-0; DowAgroSciences LLC, Indianapolis, IN, USA). Nominal larvicide concentrations were prepared from serial dilutions in water of freshly prepared stock solutions (800 μg/l for spinosad; 50 μl/l for Bti).
Laboratory tests
Laboratory experiments were carried out using the 4th to 6th brood offspring of D. pulex and D. magna collected in the field and maintained under laboratory conditions for one year prior to testing. Each species was reared in 20 l glass aquaria filled with dechlorinated, charcoal-filtered tap water at 20 ± 1°C in a light:dark regimen of 16:8, with light intensity of ~15 μE m−2 s−1 (Organisation for Economic Cooperation and Development 1998). They were fed three times a week with a suspension (equivalent to ~0.1 mg carbon. Daphnia −1 day−1; Organisation for Economic Cooperation and Development 1998) of green microalgae (Desmodesmus subspicatus for D. pulex and Chlorella vulgaris for D. magna) batch-cultured according to AFNOR T90-304 (AFNOR 1980).
Tests were performed in 125 ml polystyrene beakers containing 100 ml of exposure medium (1 ml of stock solution was added to 99 ml of demineralised water containing green microalgae suspension). One nominal concentration was tested for each compound: 0.50 μl/l Bti (maximum rate registered for aerial treatments; ACTA 2009) and 8 μg/l spinosad, which corresponds to the lowest concentration allowing D. pulex population recovery after the first week of exposure under field microcosm conditions (Duchet et al. 2008). Pulse exposure to each compound was performed in 5 replicates, and 5 beakers remained as untreated controls. Neonates (<24 h old) of D. pulex (20 per beaker) or D. magna (15 per beaker) were introduced into each beaker at the beginning of the test (Sanchez et al. 2000). The duration of the test was 14 days for both species (time necessary to observe at least 3 broods in our systems). Every 2 days, immobile adults and newborns were counted and removed to measure survival and reproduction. For adult body length measurements, surviving individuals were collected separately with a pipette and transferred to a polystyrene cup where they were briefly deposited into a drop of the exposure medium and photographed using a binocular dissecting microscope fitted with a digital camera (S40 PowerShot, Canon Inc., Tokyo, Japan). Body length, from the eye to base of the tail spine (Boronat and Miracle 1997), was measured on the pictures using an image analysis software (Ellix® software, Microvision Instruments, Evry, France). Test organisms were then transferred to newly-made medium every other day, using stock solutions of the toxicants prepared at the beginning of the experiment, and fed with green microalgae.
Field studies
The field studies were carried out in 2005 and 2006, using enclosure-type microcosms, as described in Duchet et al. (2008, 2010). Cube-shaped plexiglas enclosures (50 × 50 × 50 cm) were placed in shallow temporary oligohaline marshes in Camargue (Bouches-du-Rhône, France) and in Morbihan (Brittany, France), in order to isolate fractions of natural populations of D. magna and D. pulex, respectively. The treatments were performed on August 10, 2005 in Camargue and on May 30, 2006 in Morbihan. Each larvicide was diluted in tap water before spraying at the water surface using a portable spraying apparatus, as previously described (Duchet et al. 2008, 2010). In order to carry out the experimentation in a realistic way, Bti was applied at 0.16 and 0.50 μl/l, and spinosad was applied at 8, 17 and 33 μg/l. These concentrations encompassed to the recommended rates for field applications (ACTA 2009). Only the results obtained for the concentrations of 0.50 μl/l Bti and 8 μg/l spinosad were used in the present study (same concentrations as those used in laboratory experiments). Each treatment was applied to 5 microcosms (replicates), and 5 microcosms remained as untreated controls.
Daphnids were sampled using home-made PVC tube samplers (70 cm length, 6 cm inner diameter) equipped with a 2 × 4 mm mesh screen-covered one-way valve at the bottom (Roucaute and Quemeneur 2007). Water column samples were collected from twenty regularly spaced locations within each enclosure. The resulting composite sample (mean volume = 88.68 ± 2.23 ml, n = 360, depending on the water level in the microcosm) was filtered through 30-μm mesh nylon net. The retained daphnids were transferred to a 500 ml plastic vial and preserved using neutral aqueous formaldehyde/sucrose (4%, v/v; 40 g/l) containing 250 μg/l Bengal pink dye. All sampled daphnids were identified using a taxonomic key (Amoros 1984). They were counted using a stereomicroscope (Stemi SV 6, Zeiss, Thornwood, NY, USA), and their body length was measured from the eye to base of the tail spine (Boronat and Miracle 1997). At each sampling date, water quality parameters (temperature, dissolved oxygen, salinity, pH, water level, suspended matter (SM) and chlorophyll a) were measured as previously described (Duchet et al. 2008, 2010).
Population model
Estimated demographic parameters were l x , the proportion of females surviving at age x (the start of the age interval [x; x + 1]) and m x , the average number of female offspring produced per female by age x (Caswell 2001). Vital rates l x and m x were used to build a Leslie matrix under each treatment (Bti, spinosad, control), with seven age-classes (2-day length each; Table 1) using PopTools (Hood 2006). For each treatment, fecundity of age-class i (F i ) was calculated under the hypothesis of post-breeding census (Caswell 2001) as P i × m i (with P i = (l i+1)/l i). Leslie matrices were used to calculate the population intrinsic growth rate λ and stable age structure. Under laboratory conditions, l x and m x were calculated from a horizontal life table, i.e. a single cohort was followed through the time span of interest. The net reproductive rate (R 0) was also calculated, as Σ l x m x . In field microcosms, as several cohorts were sampled on each sampling date, a time-specific life-table, also called vertical or current, was used. It is based on the fate of a virtual cohort found by determining the age structure, at one instant in time, of a sample of individuals from a population. This population is assumed to be stationary with considerable overlapping of generations, i.e. a multi-stage population (Southwood and Henderson 2000). Thus, the method must be used on population samples of large size, under the assumption that sampling is random across age-classes. As age determination is a prerequisite for time-specific life-tables, an age-body length relationship, as estimated under laboratory conditions (Table 1), was used. Field population matrices were based on age-specific survival (P i) as estimated from these vertical life-tables. Since fecundity data could not be obtained in the field, age-class fecundity of the Leslie matrices was estimated from the formula P i × m i, using laboratory m i values. Vertical life-tables were elaborated separately for two sampling dates, before (d0) and 2 days after treatment (d2).
Population models were developed using RAMAS-GIS (Akçakaya 2005). Density-dependence was accounted for by using a ceiling model (resource limitation), setting the carrying capacity to K = 500,000 individuals. Population growth was projected starting with 700 water fleas distributed at equilibrium (stable age vector). Following Stark (2008), population recovery was also determined, by comparing the time needed for Bti- or spinosad-exposed populations to reach K, relative to the control population.
Estimates of the extinction risks and associated parameters were based on simulations started with 700 initial fleas distributed at equilibrium. The method is based on stochastically simulated population growth, through the sampling of P i and F i-values within a normal distribution, of which parameters are mean and SD calculated among replicates of a given treatment. Terminal extinction risks were estimated as the probability that at least one population crashes by the end of the simulation duration. Expected minimum abundance, the smallest population size attained during the run, and time to quasi-extinction, the number of steps needed to reach extinction in 50% of the simulated replicates were also provided. Ten thousand populations were simulated using the same parameter distribution (mean and SD), and their trajectories were followed for 45–52 steps (weeks) depending on the population.
Statistical analysis
Normality of data was tested using the Shapiro-Wilks test. Whenever possible, linear models and ANOVA (ANalysis Of VAriance) were applied, and when significant, followed by a post hoc test (Tukey, α = 0.05). Variance homogeneity among groups was checked using Bartlett’s test. When data transformation failed, the influence of larvicide treatment on demographic parameters was tested using a Kruskal–Wallis (KW) test for each date, followed by the appropriate post hoc test: Least Significant Difference (LSD) test (Sprent et al. 1992) or R commands nparcomp and pgirmess. For analyses performed date by date, a Bonferroni correction was applied. All tests were performed using R for Windows Version 2.9.0 (R Development Core Team 2009). Significance was accepted at α = 0.05 for all tests.
Survival data under laboratory conditions were analysed using a survival model with censoring (all individuals were not followed until death) using a Weibull distribution for the error term, which allows non-constant hazard (function survfit in R). This analysis allowed inferring the age at death, even though all individuals were not surveyed until death (lab experiments lasted 14 days). The effect of treatment on survival functions and mean age at death was tested using survreg procedure in R, and a post hoc multiple comparison test (Tukey, α = 0.05). Model testing was performed using a deviance analysis (likelihood ratio test and χ2 distribution). Replicates were nested within treatments. Details on the method are given in Crawley (2007).
Results
Laboratory tests
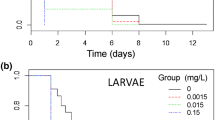
From the 6th post-contamination day to the end of the experiment, D. pulex exposed to spinosad survived significantly less than those exposed to Bti and to control conditions (KW test followed with post hoc LSD: p < 0.01, Fig. 1a). Survival of spinosad-exposed D. pulex dramatically decreased during the first week and was as low as 60% at the end of experiment, whereas mortality in the Bti treatment stayed close to that of controls and was only 20% at the end of the experiment (Fig. 1a). In D. magna, spinosad also caused significantly higher mortality than Bti or control conditions, after 6 days of exposure (KW and post hoc LSD: p < 0.01, Fig. 1b). However, survival curves differed between the two species, with a sharper decline within the first week than during the next one in D. pulex. The opposite was observed in D. magna, which also showed lower survival at the end of experiment (40%, Fig. 1b). For each species, survival analyses confirmed the significant negative effect of spinosad on survival and mean time at death, as compared to controls and Bti-treated groups (deviance analysis and Tukey’s post hoc test on survreg analysis, p < 0.001 in both species). Replicates did not differ significantly within treatments (p = 0.999 in D. pulex and p > 0.999 in D. magna). When analysed together, species did not differ significantly in their response to the treatments (species effect: p = 0.472, species by treatment interaction: p = 0.152). Estimated mean age at death was strongly reduced by spinosad exposure in both species (Fig. 1).
Female survival (L x ) as a function of age-class (x) of D. pulex (a) and D. magna (b), under laboratory exposure to Bti at 0.5 μl/l, spinosad at 8 μg/l, and control conditions. Significant difference from the control (KW test followed by LSD test): * p < 0.05; ** p < 0.01. Numbers at the right of the curves indicate mean age at death, as estimated from survival analysis (see text)
In both species, fecundity was also reduced in individuals exposed to spinosad, as compared to the other conditions. However, this was significant only on day 8 in D. pulex, and from day 8 to day 10 in D. magna (Fig. 2). Moreover, in D. pulex, the offspring of spinosad-exposed individuals was produced with a slight delay, which was significantly overcompensated after 10 days of exposure (see maxima on fecundity curves, Fig. 2a). Comparatively, D. magna exposed to spinosad showed a much more delayed fecundity, which started to be expressed after 8 days only and never reached the maximum values observed under Bti and control conditions during the course of the experiment. Finally, it is also noteworthy that Bti-exposed daphnids exhibited slightly higher fecundity than control individuals in both species, although this was significant only in one case (D. magna, day 6, Fig. 2b). The net reproductive rate R 0 was affected by the treatment (ANOVA on Boxcox transformed data, p < 0.001), with significantly lower values in spinosad-treated individuals (Tukey’s post hoc comparison), whatever the species. Species also significantly differed in their reproductive rate (p = 0.001), but this difference was mainly expressed under spinosad exposure, where D. magna prove to be significantly more affected than D. pulex (treatment by species interaction, p < 0.001 and Tukey’s post hoc comparison).
Number of offspring per surviving female (M x ) as a function of age-class (x) of D. pulex (a) and D. magna (b), under laboratory exposure to Bti at 0.5 μl/l, spinosad at 8 μg/l, and control conditions. Significant difference from control (KW test followed by LSD test): * p < 0.05; ** p < 0.01; *** p < 0.001. Mean values and standard-deviation of R 0, the net reproductive rate (ΣL x M x ), are presented in boxes
In both species, population growth rate λ was significantly lower under exposure to spinosad as compared to other conditions (KW and LSD post hoc test: p = 0.009 and p = 0.002 for D. pulex and D. magna, respectively; Fig. 3). However in D. pulex, λ-values were greater than 1, whatever the treatment, which means positive population growth, whereas in D. magna, exposure to spinosad was responsible for an increased variance among replicates and a mean switch from positive growth (Bti and control conditions) to population decline (λ < 1). Furthermore, in this species, Bti-exposed individuals led to a higher population growth rate than did their control counterparts, although this difference was not significant.
Mean population intrinsic growth rate (λ) of D. pulex (a) and D. magna (b) exposed to larvicides (Bti 0.5 μg/l, hatched bars, and spinosad 8 μg/l, grey bars), as compared to untreated controls (white bars), under laboratory and field conditions. Error bars indicate standard errors to the mean (n = 5). Statistically significant difference from the control: nonparametric multiple comparison test following KW test. * 0.05 > p > 0.01; ** 0.01 > p > 0.001
Extinction rates were not calculated under laboratory conditions, since no extinction occurred during the course of simulations (52 time steps, 10,000 replications). Using demographic stochasticity, the expected minimum abundances under control, Bti and spinosad treatment conditions, were 185.8, 186.8, and 164.9 individuals, respectively in D. pulex and 171.9, 194.2 and 118.4 individuals respectively in D. magna. Given that simulations were based upon the use of among-replicate variance in age-class survival, no variance could be obtained within treatments for these expected minimum numbers, thus precluding any statistical comparison.
Comparing the time needed to reach the carrying capacity (ceiling value, K = 500,000 individuals) among treatments showed that exposure to spinosad led to a delay of 2 weeks in D. pulex (13 versus 11 weeks under Bti and control treatments). In D. magna, this delay was much more important, with 38 weeks versus 12 and 10 weeks under Bti and control treatments, respectively (Fig. 4).
Field studies
Daphnia pulex––Before treatment (d0), no significant difference was observed in survival (l x ) among pre-assigned treatments (KW, p > 0.05). Two days after treatment (d2), the only significant difference was observed between l4–6 (p = 0.021) in spinosad and control enclosures but this difference was no more significant after Bonferroni correction (Fig. 5a, b).
Female survival (L x ) over age-class (x) under control conditions and after exposure of D. pulex and D. magna to Bti at 0.5 μl/l and spinosad at 8 μg/l in field microcosms, before treatment (d0) and 2 days after treatment (d2). Significant difference from control (KW test followed by LSD test): * p < 0.05; ** p < 0.01
Globally, population growth rates λ at d0 and d2 were similar (no significant difference), and ≤1 for the three treatments (control, Bti, spinosad; Fig. 3a) indicating that the populations were decreasing or remained stable. Expected minimum abundances were 93.5, 4.1 and 526.3 individuals, for the control, Bti and spinosad treatments, respectively, at d0, and 3.7, 673.4 and 15.8, respectively, at d2 (Table 2). Extinction risks increased from d0 to d2 under control and spinosad exposure conditions (13.6–51.3% and 0–64%, respectively), whereas Bti-exposed populations showed an opposite pattern (67.5–0%). When calculable and reached, time to quasi-extinction was similar among populations, irrespective of time or treatment (44–52 weeks).
Daphnia magna––before treatment (d0), l x curves differed between replicates assigned to Bti and spinosad treatments on one side, and those alloted to controls on the other side, for the three oldest age-classes (Fig. 5c). However, KW p-values were marginal (0.038, 0.045 and 0.045, respectively) and the post hoc test could not locate the difference in two of the tests. After Bonferroni correction, these differences were no more significant. On the contrary, 2 days after treatment (d2), survival curves differed significantly among microcosms from l6–8 to the final age-class, even after Bonferroni corrections (Fig. 5d). Post hoc multiple comparisons following KW tests, showed that survival to age-class 6–8 days was lower under spinosad than under Bti exposure (p = 0.007) and that all subsequent l x -values were significantly lower under spinosad than under Bti and control conditions, which on their side were similar (l8–10: p = 0.008, l10–12: p = 0.009, l12+: p = 0.008).
Before treatment, no significant difference in population growth rate was observed between treatments (KW, p = 0.068) and λ-values were all above 1 (Fig. 3b). At d2, significant differences were observed between population growth rates, and spinosad-exposed populations were significantly affected relatively to the controls, which exhibited growth rates similar to those observed under Bti-exposure (KW test, p = 0.003, post hoc comparison). Expected minimum abundances were similar, and ranged from 800 to 1,000 individuals. After treatment, Bti-exposed replicates remained stable (minimum abundance = 811.5). Population dynamics parameters of the controls led to a minimum abundance which was halved as compared to pre-treatment conditions, and some extinction rate could be calculated (0.007), although its confidence interval showed that it did not differ from zero (Table 2). In contrast, simulations performed on spinosad-exposed replicates led to population extinction (minimum abundance = 0, extinction risk = 1), and this was extremely rapid (time to quasi-extinction = 4.1 one-week long steps, i.e. one month).
Discussion
The present study confirmed the negative impact of spinosad on daphnid populations whereas Bti had little or no effect on demographic toxicological endpoints. However, effects were differentially expressed according to the species and environmental conditions. Bti and spinosad are known to have limited persistence in water, both compound undergoing photolysis and adsorption to particulate material or sediment within a few days following application (Yousten et al. 1992; Cleveland et al. 2002; Hajaij et al. 2005; Ping et al. 2005; Duchet et al. 2010). Species sensitivity and exposure scenario (laboratory versus field conditions) therefore appear as the main factors that determine population responsiveness.
Differences in species sensitivity to spinosad and Bti in laboratory conditions
Daphnid populations exposed to 8 μg/l spinosad were significantly impaired in many respects, although this concentration corresponds to D. magna chronic NOEC (WHO 2007) and to an acute LC11 in D. pulex (Stark 2008). Survival was unequivocally decreased in both species under laboratory conditions, and was as low as 50% on average after 2 weeks of exposure (60% and 40% in D. pulex and D. magna, respectively). Survival analysis showed that the effects of species and species by treatment interaction were not significant, meaning that D. pulex and D. magna responded very similarly in terms of survival, under laboratory standard conditions. Although individuals were not monitored until death, the use of a survival model with censoring allowed estimation of the mean age at death under the different treatments. As compared to control conditions, the estimated mean age at death was more strongly reduced by spinosad in D. magna (from 40 to 14 days) than in D. pulex (from 41 to 19 days), although this interspecific difference was not significant.
In D. pulex, l x under spinosad exposure was higher than previously observed (0.40 for the age-range 14 days; Stark 2008), and this probably results from experimental differences. First, exposure was repeated every other day with freshly made solutions in Stark’s study, whereas in the present one, the medium renewal was based on a stock solution prepared only once, i.e., at the beginning of the experiment. In addition, different commercial preparations, that may contain different types and amounts of impurities, were used (Success® in Stark’s study; Conserve® in the present investigation). Results of both experiments are also expected to differ depending on spinosad solubility and persistence in water. Indeed, half-life for the sum of spinosyns A and D is estimated at 1–2 days (Saunders and Bret 1997; Cleveland et al. 2002) in water. Second, individuals were kept isolated in Stark’s study, while grouped by 20 in the present one. Finally, there may also be some fitness differences among the strains used in the two studies. The dataset analysed by Stark (2008) stemmed from a previous study (Walthall and Stark 1999) that used test organisms from a laboratory culture, whereas our dataset was obtained with test organisms collected in the field and maintained in laboratory conditions (Duchet et al. 2008, 2010). Barata et al. (2000) showed that tolerance of field populations was strongly influenced by genetic factors and could modify the responses to toxicants in comparison with laboratory strains.
Fecundity was also affected by the treatments, both in terms of delay and total offspring, on the timescale of the laboratory experiment. For this trait, D. magna was found to be much more sensitive to spinosad than D. pulex (Fig. 2), in which fecundity was delayed for 2 days, and followed with a compensatory effect after 10 days. This particular sublethal effect observed in D. pulex adults may indirectly result from negative density-dependence, which strength might have been reduced, due to mortality, and triggering a higher reproductive investment by surviving individuals. In addition, a hormetic effect (higher reproductive investment) cannot be ruled out as it has been documented widely in pharmacological and toxicological studies (Stebbing 1982). For example, higher numbers of nauplii per copepod female at low lindane concentrations as compared to controls, were observed by Brown et al. (2003).
In both species, the net reproductive rate R 0 (number of offspring by which a newborn individual will be replaced by the end of its life; Caswell 2001) was significantly reduced under spinosad exposure, and the effect was significantly stronger in D. magna than in D. pulex. Compared to the study of Stark and Vargas (2003), R 0 values estimated in D. pulex were generally lower, but they were found to be less reduced between control conditions and exposure to 8 μg/l spinosad (13–9 offspring in the present study, against 260–50, as estimated from Fig. 1 in Stark and Vargas’work). Once again, such discrepancies are probably related to differences in experimental conditions. In particular, individual density may have played a role, if the expression of fecundity is density-dependent. The amount of food provided also differed between the two studies. Alternatively, some fitness differences or contrasted levels of genetic variability among the strains used in the two studies may also affect R 0. From a population dynamics point of view, comparing the two studies on D. pulex suggests that highly growing populations are more severely impacted by spinosad, at the tested concentration of 8 μg/l. Such an observation points to the need for experiments designed to include interactions between toxicants and other ecologically and demographically relevant factors, such as density, amount of resources, competition, predation, etc. (Liess 2002; Rohr et al. 2004; Bøhn et al. 2010). Population structure and density at the time of toxicant exposure may also affect the outcome, as shown in D. pulex (Pieters and Liess 2006; Stark and Banken 1999; Hanazato and Hirokawa 2004) and other organisms (Forbes et al. 2003; Kramarz et al. 2005).
It is also to be noted that, as compared to control conditions, R 0 increased under Bti exposure in both species, although the difference was significant in D. magna only (Fig. 2). A similar effect on R 0 was previously observed in D. pulex exposed to low concentrations of the synthetic insecticide diazinon, which was however much more toxic at higher concentrations (Stark and Vargas 2003). The possibility that Bti has a hormetic effect should be investigated more thoroughly before concluding on the effect of this product in daphnids. According to previous studies, Bti is highly selective for Nematocera (Diptera) like Culicidae, Simuliidae and Chironomidae (Boisvert and Boisvert 2000), whereas other aquatic organisms such as molluscs, crustaceans, other insects, fish and amphibians are not sensitive to this insecticide. Ali (1981) and Miura et al. (1981) showed that Ephemeroptera, Amphipoda, Cladocera and Copepoda were not affected by Bti. Therefore, it was not surprising to find no negative impact of this larvicide on both daphnid species, as shown by the population trajectories estimated under laboratory conditions (Fig. 4). In contrast, vital rates were reduced in D. magna fed with Bt-transgenic maize (expressing Cry1Ab Bt-toxin; Bøhn et al. 2008, 2010). However, toxins from Bt-transgenic maize are different from Bti toxins since they are produced by Bacillus thuringiensis kurstaki (Btk) (Gill et al. 1992). Although Bti had a positive effect on fecundity in D. magna, this did not lead to significantly higher population growth rate, as compared to the control. Such a result was consistent with elasticity analyses of the Leslie matrices (data not shown), which show that the main impact on λ resulted from changes in the two-first age-classes survival, and that changes in F i had much less effect, regardless of the treatment. This is consistent with previous results on short-lived, high-fecundity invertebrates (Forbes et al. 2001), such as springtails (Widarto et al. 2007) or freshwater snails (Jensen et al. 2001; Coutellec et al. 2008).
Projection matrices revealed a more negative impact of spinosad on D. magna (λ = 0.9) than on D. pulex (λ = 1.64), relatively to their respective controls (1.736 and 1.837). However, in both cases, λ values were significantly smaller than under control conditions. Similarly, time to recovery was also differentially increased among species, with a much longer time in D. magna (28 weeks) than in D. pulex (2 weeks). Compared to Stark’s study (2008), the present results reveal better recovery performances in D. pulex (2 weeks against 11 weeks in the former), but this comparison is probably hazardous and useless, because of strong differences in life tables (age-class lengths), carrying capacity and population dynamics, in addition to differential exposure conditions. Nevertheless, from our study, the index of recovery (Stark 2008) appears as highly relevant in summarizing demographic effects on positively growing populations, such as those usually studied under laboratory conditions.
As a general feature, ignoring the sign of the effect, sensitivity to the two studied larvicides appeared higher in D. magna than in D. pulex. This result does not confirm the observed similar sensitivity of both species to many synthetic chemicals (immobilization test, 30 reference chemicals, Canton and Adema 1978; LC50 and reproduction test, 15 compounds, Lilius et al. 1995). The present observation may be used as an argument in favour of toxicity tests performed on several species, if these are taken as representatives of a whole taxonomic group.
Laboratory versus field conditions
Some discrepancies in the effects of the two larvicides were detected between laboratory and field microcosms. This result was not surprising, since population dynamics modelling was based on life history traits, which expression is known to be highly environment-dependent in many organisms (see Stearns 1992; Begon et al. 2006).
Laboratory conditions could be considered as very favourable to daphnid population growth, at least on a short time-scale, because all λ values were well above 1, except in the case of D. magna exposed to spinosad. Even in this situation where λ mean value was 0.9 (which means population decline), some replicates performed better, as reflected by the very high variance observed among them (SD = 0.58) for this parameter. Consistently, the Leslie matrix based on mean l x and m x values had a λ-value of 1.19, and thus expressed positive growth (Fig. 3b, spinosad trajectory). It may therefore be suggested that laboratory conditions allowed the estimation of spinosad and Bti effects in the absence of other environmental stressors acting in the short term, with the exception of density effects, such as those discussed above (although other uncontrolled factors may still have been effective but undetected on the traits studied).
On the contrary, field conditions include many uncontrolled interactions between the tested larvicides and environmental parameters. Although this specificity entails difficulties to interpret observations, field studies are the conditions allowing ecologically realistic effects to be estimated. Therefore, such studies are highly desired, while laboratory studies should be considered as complementary, and used to help explain the patterns observed in natura. In this study, one might have expected stronger effects to be emphasized in the field, due to the potentially large amount of interacting factors (e.g., Duchet et al. 2010; Kim et al. 2010).
Field survival curves were estimated from an age-size relationship established under laboratory conditions. As a consequence, age-class survival may have been either overestimated (if field growth is slower, leading to individuals artificially staying too long in a given class) or underestimated (if field growth is faster, leading to individuals artificially leaving an age-class too rapidly). Estimation of age-class fecundity under field conditions is very difficult and prone to error, due to micro-environmental variations and caging potential effects. We avoided such a drawback by using estimates based on homogeneous conditions. As a consequence, the present results on field populations have to be interpreted as reflecting an intermediate condition between laboratory beakers and true natural populations: laboratory-based relationships between some demographic key-parameters, and temporal sampling of field individuals. As a general rule, survival curves substantially differed between laboratory and field, and this was revealed through a higher mortality in juvenile and pre-adult age-classes (compare curves on Figs. 1, 5). It might be suggested that laboratory conditions favour juvenile survival beyond natural limits. This was also observed in other invertebrates (e.g., Coutellec et al. 2008). Nevertheless, laboratory and field results were consistent, in terms of effects of spinosad on estimated population fate. The decrease in D. pulex population growth observed in the laboratory was not significant in the field, as a result of increased variance in field replicates exposed to spinosad. It might then be advocated to increase the number of replicates from lab to field. It may also result partly from the fact that D. pulex was found less sensitive than its counterpart to spinosad. It might then be suggested that recovery to spinosad exposure is more likely in D. pulex than in D. magna, relying on a fecundity rebound in survivors, which can act very quickly.
Daphnid survival was shown to be lower in the field than in laboratory conditions. This could be easily explained by differences in experimental conditions. Indeed, laboratory experiments are under controlled conditions, chosen to be close to optimal preferences of the organisms (optimal temperature and light, non restricted food conditions, no competition or predation) whereas the experiments in field microcosms took place in shallow temporary oligohaline marshes, i.e., a rather unfavourable environment for daphnid populations, with lower concentrations of dissolved oxygen (1.87 ± 0.08 mg/l in Morbihan and 5.5 ± 0.2 mg/l in Camargue at d0 both, versus 8 mg/l in laboratory conditions), higher salinity (0.4 ± 0.00 g/l in Morbihan and 3.2 ± 0.01 g/l in Camargue at d0 both, versus 0 g/l in laboratory conditions), etc. For example, the peak of salinity (>4 g/l) observed during the 21-day observation period in our field microcosm study in Camargue (Duchet et al. 2010), may have been partly responsible for the decrease of D. magna population density observed in all the enclosures (Fig. 6b). It was also probably responsible for the absence of recovery in the enclosures treated with spinosad, which caused a sharp decrease of D. magna abundance within the first 2 days following treatment (Fig. 6b), suggesting that it may be difficult for a field population of daphnids to cope simultaneously with natural (water salinity and temperature) and anthropogenic (larvicides) stressors.
Change in mean values (+SE; n = 5) of D. pulex (a) and D. magna (b) abundance (expressed as the number of individuals per litre) in control microcosms, in microcosms treated with Bti at 0.5 μl/l, and microcosms treated with spinosad at 8 μg/l. Significant difference from control (Duncan’s post hoc test): * p < 0.05; ** p < 0.01; *** p < 0.001
Finally, predation and competition are not taken into account in single species test in laboratory conditions, unlike with field microcosms, and therefore, several indirect effects may not be detectable under laboratory conditions (Beketov and Liess 2005; Beketov and Liess 2006; Coutellec et al. 2008). In our study in field microcosms in Morbihan (Duchet et al. 2008), population of Chaoborus sp. was larger in microcosms treated with Bti than in spinosad-treated microcosms and controls. These Diptera larvae are huge zooplankton consumers (Lüning-Krizan 1997). In addition, more Culex sp. larvae were observed in microcosms treated with Bti at 0.50 μl/l than in the controls. Mosquito larvae are filter feeders and thus can compete with D. pulex for food, leading to decrease of the daphnid population, sensible to food privation (Fig. 6a). On the contrary, the lack of predation and competition in spinosad-treated microcosms allowed the D. pulex population to recover at the concentration of 8 μg/l (Fig. 6a; Duchet et al. 2008). This points to the interest of ecological relevance of outdoor meso/microcosm or field studies for ecotoxicity testing.
Population extinction risks
Daphnia magna and D. pulex population growth under laboratory conditions were exponential, which is clearly unrealistic for natural populations. Nevertheless, applying a carrying capacity to this model allowed estimating the recovery time due to treatments (Stark 2008). This clearly showed that spinosad increased the time needed to reach K, especially in D. magna. Thus, elementary “population” dynamics under laboratory conditions can be very informative, if restricted to a comparative interpretation between treatments. As expected with such dynamics, no extinction risk could be calculated using the time span of simulations (around one year, beyond which other seasonal processes are likely to be more effective on the population dynamics).
Under field conditions, population growth rates were estimated using vertical life-tables. l x values were averaged over replicates, and m x values were taken from the laboratory experiment. We hypothesize that, due to benign laboratory conditions, the main consequence would be an underestimation of population extinction risks. In both species, minimum abundances expected after 52 steps decreased from d0 to d2, under control and spinosad conditions. Simulations from Bti exposed replicates led to a notable population rebound after 52 weeks in D. pulex, or no change in D. magna. Compared to the change of replicate size during the 3 weeks following treatment (Fig. 6), although not in contradiction and owing to the time difference, this result appears rather unrealistic, and may be (as already said) a consequence of high fecundities estimated in laboratory conditions.
In D. magna exposed to spinosad in the laboratory, in accordance with the shift between positive growth and population decline, estimated field population dynamics showed that, under the hypothesis that conditions characteristic of d2 are maintained, populations would go to extinction. Extinction was estimated to occur rapidly in the field, i.e. within 4 weeks (under “d2” conditions), which, this time, is consistent with observations from d0 to d21 (Fig. 6).
Conclusion
The present study allowed assessing population-level effects of two larvicides used for mosquito control, spinosad (Conserve® 120SC) and Bti (Vectobac® 12AS), in two non target species, D. pulex and D. magna (Crustacea: Cladocera) under two contrasted situations, laboratory versus field microcosms.
From the present results, it is concluded that population-level inferences based on laboratory tests are protective, in the sense that they allow effects to be detected, whereas field population estimates have higher variability and require more replicates to increase statistical power. The comparison of D. pulex and D. magna populations illustrated well this pattern: D. magna was shown to be more sensitive than D. pulex to spinosad in the laboratory, and the effect was also detectable through field population demographic simulations. Therefore, studies performed in the field, although ecologically more relevant, are more prone to experimental error, and likely to lead to a lack of statistical significance simply as a result of the design, instead of innocuousness of the tested chemical. It might thus be recommended to combine laboratory and field conditions in order to come closer to real effects.
References
ACTA (2009) Index phytosanitaire. Association de Coordination Technique Agricole, Paris
AFNOR (1980) Détermination de l’inhibition de croissance de Scenedesmus subspicatus par une substance. Norme expérimentale T 90–304
Akçakaya HR (2005) RAMAS metapop: viability analysis for stage-structured metapopulations (version 5.0). Applied biomathematics, Setauket, New-York
Akçakaya HR, Stark JD, Bridges TS (2008) Demographic toxicity––methods in ecological risk assessment. Oxford University Press, New York
Ali A (1981) Bacillus thuringiensis serovar israelensis (ABG-6108) against chironomids and some nontarget aquatic invertebrates. J Invert Pathol 38:264–272
Amoros C (1984) Crustacés Cladocères. Bull Mens Soc Linn Lyon 53:72–145
Barata C, Baird DJ, Amat F, Soares AMVM (2000) Comparing population response to contaminants between laboratory and field: an approach using Daphnia magna ephippial egg banks. Funct Ecol 14:513–523
Barnes PB, Chapman MG (1998) Effects of the larvicide (Vectobac) on assemblages of benthic invertebrates in Bicentennial Park. Centre for Research on Ecological Impacts of Coastal Cities, Sydney
Begon M, Townsend CR, Harper JL (2006) Ecology from individuals to ecosystems, 4th edn. Blackwell Publishing Ltd
Beketov MA, Liess M (2005) Acute contamination with esfenvalerate and food limitation: chronic effects on the mayfly, Cloeon dipterum. Environ Toxicol Chem 24:1281–1286
Beketov MA, Liess M (2006) The influence of predation on the chronic response of Artemia sp. populations to a toxicant. J Appl Ecol 43:1069–1074
Blaustein L, Chase JM (2007) Interactions between mosquito larvae and species that share the same trophic level. Annu Rev Entomol 52:489–507
Bøhn T, Primiciero R, Henssen DO, Traavik T (2008) Reduced fitness of Daphnia magna fed a Bt-transgenic maize variety. Arch Environ Contam Toxicol 55:584–592
Bøhn T, Traavik T, Primiciero R (2010) Demographic responses in Daphnia magna fed transgenic Bt-maize. Ecotoxicology 19:419–430
Boisvert M, Boisvert J (2000) Effects of Bacillus thuringiensis var. israelensis on target and non target organisms: a review of laboratory and field experiments. Biocont Sci Tech 10:517–561
Boisvert J, Lacoursière JO (2004) Le Bacillus thuringiensis et le contrôle des insectes piqueurs au Québec. Ministère de l’Environnement Québécois, Québec
Boronat MD, Miracle MR (1997) Size distribution of Daphnia longispina in the vertical profile. Hydrobiologia 360:187–196
Brown RJ, Rundle SD, Hutchinson TH, Williams TD, Jones MB (2003) A copepod life-cycle test and growth model for interpreting the effects of lindane. Aquat Toxicol 63:1–11
Canton JH, Adema DMM (1978) Reproducibility of short-term and reproduction toxicity experiments with Daphnia magna and comparison of the sensitivity of Daphnia magna with Daphnia pulex and Daphnia cucullata in short-term experiments. Hydrobiologia 59:135–140
Caswell H (2001) Matrix population models. Sinauer, Sunderland
Cleveland CB, Bormett GA, Saunders DG, Powers FL, McGibbon AS, Reeves GL, Rutherford L, Balcer JL (2002) Environmental fate of spinosad. 1. Dissipation and degradation in aqueous systems. J Agric Food Chem 50:3244–3256
Consoli FL, Botelho PSM, Parra JRP (2001) Selectivity of insecticides to the egg parasitoid Trichogramma galloi Zucchi, 1988 (Hym. Trichogrammatidae). J Appl Entomol 125:37–43
Coutellec MA, Delous G, Cravedi JP, Lagadic L (2008) Effects of the mixture of diquat and a nonylphenol polyethoxylate adjuvant on fecundity and progeny early performances of the pond snail Lymnaea stagnalis in laboratory bioassays and microcosms. Chemosphere 73:326–336
Crawley MJ (2007) The R book. John Wiley and Sons Ltd
Crouse GD, Sparks TC, Schoonover J, Gifford J, Dripps J, Bruce T, Larson L, Garlich J, Hatton C, Hill RL, Worden TV, Martynow JG (2001) Recent advances in the chemistry of spinosyns. Pest Manag Sci 57:177–185
Duchet C, Larroque M, Caquet Th, Franquet E, Lagneau C, Lagadic L (2008) Effects of spinosad and Bacillus thuringiensis israelensis on a natural population of Daphnia pulex in field microcosms. Chemosphere 74:70–77
Duchet C, Caquet Th, Franquet E, Lagneau C, Lagadic L (2010) Influence of environmental factors on the response of a natural population of Daphnia magna (Crustacea: Cladocera) to spinosad and Bacillus thuringiensis israelensis in Mediterranean coastal wetlands. Environ Pollut 158:1825–1833
Forbes VE (1999) Genetics and ecotoxicology. Taylor and Francis, Philadelphia
Forbes VE, Calow P (1999) Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environ Toxicol Chem 18:1544–1556
Forbes VE, Calow P, Sibly RM (2001) Are current species extrapolation models a good basis for ecological risk assessment? Environ Toxicol Chem 20:442–447
Forbes VE, Sibly RM, Linke-Gamenick I (2003) Joint effects of population density and toxicant exposure on population dynamics of Capitella sp. I. Ecol Appl 13:1094–1103
Gill SS, Cowles EA, Pietrantonio PV (1992) The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol 37:615–636
Hajaij M, Carron A, Deleuze J, Gaven B, Setier-Rio M-L, Vigo G, Thiéry I, Nielsen-LeRoux C, Lagneau C (2005) Low persistence of Bacillus thuringiensis serovar israelensis spores in four mosquito biotopes of a salt marsh in southern France. Microb Ecol 50:475–487
Hanazato T, Hirokawa H (2004) Changes in vulnerability of Daphnia to an insecticide application depending on the population phase. Freshwater Biol 49:402–409
Hershey AE, Shannon L, Axler R, Ernst C, Mickelson P (1995) Effects of methoprene and Bti (Bacillus thuringiensis var. israelensis) on non-target insects. Hydrobiologia 308:219–227
Hershey AE, Lima AR, Niemi GJ, Regal RR (1998) Effects of Bacillus thuringiensis israelensis (Bti) and methoprene on non-target macroinvertebrates in Minnesota wetlands. Ecol Appl 8:41–60
Hood GM (2006) PopTools version 2.7.5. http://cse.csiro.au/poptools
Jensen A, Forbes V, Parker ED Jr (2001) Variation in cadmium uptake, feeding rate, and life histories effects in the gastropod Potamopyrgus antipodarum: linking toxicant effects on individuals to the population level. Environ Toxicol Chem 20:2503–2513
Kammenga J, Laskowski R (2000) Demography in ecotoxicology. John Wiley and Sons, Chichester, UK
Kim J, Park J, Kim PG, Lee C, Choi K, Choi K (2010) Implication of global environmental changes on chemical toxicity-effect of water temperature, pH, and ultraviolet B irradiation on acute toxicity of several pharmaceuticals in Daphnia magna. Ecotoxicology 19:662–669
Kondo S, Ohba M, Ishii T (1992) Larvicidal activity of Bacillus thuringiensis serovar israelensis against nuisance chironomid midges (Diptera: Chironomidae) of Japan. Lett Appl Microbiol 15:207–209
Kramarz P, Zwolak M, Laskowski R (2005) Effect of interaction between density dependence and toxicant exposure on population growth rate of the potworm Enchytraeus doerjesi. Environ Toxicol Chem 24:537–540
Liber K, Schmude KL, Rau DM (1998) Toxicity of Bacillus thuringiensis var. israelensis to chironomids in pond mesocosms. Ecotoxicology 7:343–354
Liess M (2002) Population response to toxicants is altered by intraspecific interaction. Environ Toxicol Chem 21:138–142
Lilius H, Hästbacka T, Isomaa B (1995) A comparison of the toxicity of 30 reference chemicals to Daphnia magna and Daphnia pulex. Environ Toxicol Chem 14:2085–2088
Lüning-Krizan J (1997) Selective feeding of third- and fourth-instar larvae of Chaoborus flavicans in the field. Arch Hydrobiol 140:347–365
Mauri M, Barladi E, Simonini R (2003) Effects of zinc exposure on the polychaete Dinophilus gyrociliatus: a life-table response experiment. Aquat Toxicol 65:63–100
Miles M, Dutton R (2000) Spinosad: a naturally derived insect control agent with potential use in glasshouse integrated pest management systems. Meded Fac Landbouwkundige Toegepaste Biol Wet Univ Gent 65:393–400
Miura T, Takahashi RM, Mulligan FS III (1981) Impact of the use of candidate bacterial mosquito larvicides on some selected aquatic organisms. In: CMC Association (ed) Proceeding annual conference of the californian mosquito control association, pp 45–48
Mulla MS, Federici BA, Darwazeh HA (1982) Larvicidal efficacy of Bacillus thuringiensis ser. H-14 against stagnant-water mosquitoes and its effects on nontarget organisms. Environ Entomol 11:788–795
Nasreen A, Ashfaq M, Mustafa G (2000) Intrinsic toxicity of some insecticides to egg parasitoid Trichogramma chilonis (Hym. Trichogrammatidae). Bull Inst Trop Agr Kyushu Univ 23:41–44
Niemi GJ, Hershey AE, Shannon L, Hanowski JM, Lima A, Axler RP, Regal RR (1999) Ecological effects of mosquito control on zooplankton, insects, and birds. Environ Toxicol Chem 18:549–559
Organisation for Economic Cooperation and Development (1998) Daphnia magna reproduction test. OECD guidelines for testing of chemicals
Pieters BJ, Liess M (2006) Population developmental stage determines the recovery potential of Daphnia magna populations after fenvalerate application. Environ Sci Technol 40:6157–6162
Ping L, Wen-Ming Z, Shui-Yun Y, Jin-Song Z, Li-Jun L (2005) Impact of environmental factors on the toxicity of Bacillus thuringiensis var. israelensis IPS82 to Chironomus kiiensis. J Am Mosq Control Assoc 21:59–63
R Development Core Team (2009). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Rey D, Long A, Pautou MP, Meyran JC (1998) Comparative histopathology of some Diptera and Crustacea of aquatic alpine ecosystems, after treatment with Bacillus thuringiensis var. israelensis. Entomol Exp Appl 88:255–263
Rohr JR, Elskus AA, Shepherd BS, Crowley PH, McCarthy TM, Niedzwiecki JH (2004) Multiple stressors and salamanders: effects of an herbicide, food limitation, and hydroperiod. Ecol Appl 14:1028–1040
Roucaute M, Quemeneur A (2007) Echantillonnage de la colonne d’eau dans les écosystèmes aquatiques peu profonds. Les Cahiers Tech de l’INRA 60:5–10
Salgado VL (1998) Studies on the mode of action of spinosad: insect symptoms and physiological correlates. Pestic Biochem Physiol 60:91–102
Sanchez M, Ferrando MD, Sancho E, Andreu E (2000) Physiological perturbations in several generations of Daphnia magna Straus exposed to diazinon. Ecotoxicol Environ Safe 46:87–94
Saunders DG, Bret BL (1997) Fate of spinosad in the environment. Down Earth 52:14–20
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell Science, Oxford, UK
Sprent P, Ley J trad (1992) Pratique des statistiques non paramétriques. INRA Éditions
Stark JD (2008) Water flea Daphnia pulex: population recovery after pesticide exposure. In: Akçakaya HR, Stark JD, Bridges TS (eds) Demographic toxicity––methods in ecological risk assessment. Oxford University Press, New-York, pp 143–151
Stark JD, Banken JAO (1999) Importance of population structure at the time of toxicant exposure. Ecotoxicol Environ Safe 42:282–287
Stark JD, Banks JE (2003) Population-level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol 48:505–519
Stark JD, Vargas RI (2003) Demographic changes in Daphnia pulex (Leydig) after exposure to the insecticides spinosad and diazinon. Ecotoxicol Environ Safe 56:334–338
Stark JD, Sugayama RL, Kovalesky A (2007) Why demographic and modelling approaches should be adopted for estimating the effects of pesticides on biocontrol agents. Biocontrol 52:365–374
Stearns SC (1992) The evolution of life histories. Oxford University Press, New-York
Stebbing ARD (1982) Hormesis––the stimulation of growth by low-levels of inhibitors. Sci Total Environ 22:213–234
Tillman PG, Mulrooney JE (2000) Effects of selected insecticides on the natural enemies Coleomegilla maculata and Hippodamia convergens (Coleoptera: Coccinellidae), Geocoris punctipes (Hemiptera: Lygaeidae), and Bracon mellitor, Cardiochiles nigriceps, and Cotesia marginiventris (Hymenoptera: Braconidae) in cotton. J Econ Entomol 93:1638–1643
Vinnersten TZP, Lundström JO, Petersson E, Landin J (2009) Diving beetles assemblages of flooded wetlands in relation to time, wetland type and Bti-based mosquito control. Hydrobiologia 635:189–203
Walthall WK, Stark JD (1999) The acute and chronic toxicity of two xanthene dyes, fluorescein sodium salt and phloxine B, to Daphnia pulex. Environ Pollut 104:207–215
Watson GB (2001) Actions of insecticidal spinosyns on gamma-aminobutyric acid responses from small-diameters cockroach neurons. Pestic Biochem Physiol 71:20–28
Whalon ME, Wingerd BA (2003) Bt: mode of action and use. Arch Insect Biochem Physiol 54:200–211
WHO (2007) Spinosad. World Health Organization, Geneva, Switzerland
Widarto TH, Krogh PH, Forbes VE (2007) Nonylphenol stimulates fecundity but not population growth rate(λ) in Folmosia candida. Ecotoxicol Environ Safe 67:369–377
Yousten A, Genthner F, Benfield E (1992) Fate of Bacillus sphaericus and Bacillus thuringiensis serovar israelensis in the aquatic environment. J Am Mosq Control Assoc 8:143–148
Acknowledgements
Financial support for this work was provided by the French Ministry for Ecology, Sustainable Development and Spatial Planning through the National Programme for Ecotoxicology (PNETOX). The authors wish to thank Dow AgroSciences for the generous gift of Conserve® 120SC, Mr. Girand and Mr. Defois for giving access to the study sites, and Thierry Caquet for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duchet, C., Coutellec, MA., Franquet, E. et al. Population-level effects of spinosad and Bacillus thuringiensis israelensis in Daphnia pulex and Daphnia magna: comparison of laboratory and field microcosm exposure conditions. Ecotoxicology 19, 1224–1237 (2010). https://doi.org/10.1007/s10646-010-0507-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0507-y