Abstract
The removal of hexavalent and trivalent chromium from hydroponic solution by plants to changes in temperature was investigated. Pre-rooted hybrid willows (Salix matsudana Koidz × alba L.) were exposed to a nutrient solution spiked with potassium chromate (K2CrO4) or chromium chloride (CrCl3) for 4 days. Ten different temperatures were tested ranging from 11 to 32°C. Total Cr in solutions and in plant materials were all analyzed quantitatively. The results revealed that large amounts of the applied Cr were removed from the hydroponic solution in the presence of the plants. Significantly faster removal of Cr(III) than Cr(VI) was achieved by hybrid willows from the hydroponic solutions at all temperatures (P < 0.01). The removal rates of both chemical forms of Cr by plants increased linearly with the increase of temperatures. The highest removal rate of Cr(VI) was found at 32°C with a value of 1.99 μg Cr/g day, whereas the highest value of Cr(III) was 3.55 μg Cr/g day at the same temperature. Roots were the main sink for Cr accumulation in plants at all temperatures. Translocation of both chemical forms of Cr from roots to lower stems was only found at temperatures ≥24°C. The temperature coefficient values (Q 10) were 2.41 and 1.42 for Cr(VI) and Cr(III), respectively, indicating that the removal of Cr(VI) by hybrid willows was much more susceptible to changes in temperature than that of Cr(III). This information suggests that changes in temperature have a substantial influence on the uptake and accumulation of both chemical forms of Cr by plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) is considered a serious environmental pollutant. Despite this, it is a high volume production chemical with a wide variety of uses (Kimbrough et al. 1999; Dixit et al. 2002). Cumulative Cr production has been estimated to be 105.4 million tons globally in 2000 and has been significantly increased since the 1950s (Han et al. 2002). The major form of Cr in the environment due to anthropogenic inputs is hexavalent chromium [Cr(VI)], the most toxic species to animals and humans (Katz and Salem 1994). Another common and stable form is the trivalent species [Cr(III)], which is less mobile, less toxic and is mainly found in soil and aquatic environments adsorbed with organic matter (Becquer et al. 2003). The recommended guideline is 1 μg Cr(VI)/l and 8 μg Cr(III)/l for freshwater life, and 1 μg Cr(VI)/l and 50 μg Cr(III)/l for marine life (Zayed and Terry 2003). In the United States, 14.6 μg/l in ground water and 25.9 g/kg in soils have been found in the vicinity of chrome production sites (Shanker et al. 2005).
Cr compounds are highly toxic to plants and are detrimental to their growth and development (Shanker et al. 2005). Cr concentration of 100 μM/kg dry weight has been suggested as the threshold value to cause toxic symptoms to most plants (Davis et al. 2002). Reduction and inhibition of plant growth, chlorosis in young leaves, transpiration, antioxidant enzymes, nutrient balance, wilting of tops and root injury have been observed frequently as an end point of toxicity determination (Hunter and Vergnano 1953; Chatterjee and Chatterjee 2000; Dixit et al. 2002; Sharma et al. 2003; Scoccianti et al. 2006). Metabolic responses of Cr to plants were determined (Yu and Gu 2007; Yu et al. 2007a), in which soluble proteins and the activity of catalase (CAT) in leaves of hybrid willows were the most sensitive bioindicators to Cr(VI) and Cr(III) exposure, respectively. Additionally, the application of EDTA had a strong influence on the uptake and translocation of Cr by willows exposed to Cr(III) rather than Cr(VI) (Yu and Gu 2008).
Phytoextraction is the process of assimilating pollutants from the contaminated sites and subsequent translocation to harvestable plant parts (Kumar et al. 1995). Most studies with Cr accumulation have focused on extreme examples representing genotypes native to highly Cr-rich environments or screening hyperaccumulator species of plants. Plants selected belong to either grasses or cultivated species, which possess potential risk to high animals through the food chain (Yu et al. 2007a). It is evident that there are differences in tolerance, uptake and accumulation of Cr among plant species (Shahandeh and Hossner 2000; Yu et al. 2008) and poor translocation of Cr from roots to vegetative parts has been often reported (Pulford et al. 2001; Scoccianti et al. 2006). So far, most works on Cr in plants have been concerned with its effects on plant growth, uptake, toxicity, translocation, and/or soil-plant relationships (Zayed et al. 1998). There was very little attention paid to the effect of temperature on the phytoextraction of Cr. Plants are affected by numerous abiotic factors, and temperature is one of particular significance. Temperature affects transpiration, growth and metabolism of plants and therefore both uptake and elimination of pollutants (Yu et al. 2005).
Temperature may influence water chemistry, and the plant growth (Fritioff et al. 2005). Indeed, the fastest growth for most plants occurs between 15 and 30°C (Larcher 1995). In field application higher temperatures can also minimize the time period required to reach clean-up goals. Baghour et al. (2001) found that Potato plants (Solanum tuberosum L. var. Spunta) showed higher uptakes of Cr at high temperature than plants grown at low temperature. Additionally, higher temperatures may increase accumulation of toxicants, followed eventually by phytotoxic effects or an increased risk of contamination for consumers of plant materials (Yu et al. 2007b). In this study, uptake and bioaccumulation of Cr(VI) and Cr(III) in hybrid willows in the temperature range of 11–32°C was investigated to provide quantitative information necessary for the implementation of phytoremediation in field and for ecological risk assessment.
Materials and methods
Trees specimens and exposure regimes
Hybrid willows (Salix matsudana Koidz × alba L.) were taken from those grown naturally on the Dongting Lake, Hunan, P. R. China. Tree cuttings (40 cm in length) were removed from a mature tree and all cutting used in this study were obtained from a single tree. The cuttings were placed in buckets of tap water maintained at room temperature of 15–18°C under natural sunlight until new roots and leaves appeared. After a 2-month period of growth, each young rooted cutting was transferred into a 250 ml Erlenmeyer flask filled with approximately 200 ml of aerated aqueous Cr solution (deionized oxygen-saturated water). Modified ISO 8692 nutrient solution was used to support tree growth as described in the study by Yu et al. (2008). The flasks were all sealed with cork stoppers and silicon sealant (Dow Chemical Co, Midland, Michigan) to prevent escape of water, and wrapped with aluminum foil to inhibit potential growth of algae in the flask. The two chemical forms of Cr, potassium chromate (K2CrO4) and chromium chloride (CrCl3), were of analytical grade with ≥95% purity. The initial concentrations of spiked solutions were 1.51 (±0.01) and 1.53 (±0.01) mg Cr/l for the treatment with Cr(VI) and Cr(III), respectively. For each chemical form, ten different temperatures (11, 14, 17, 20, 22, 24, 26, 28, 30 and 32°C) were tested. The flasks were put in a climate control chamber with a constant temperature and a relative humidity of 60 ± 2% under continuous artificial light (a rack of 24 fluorescent tubes 60 W with 5 cm distance between each). For each temperature, the treatment containing willows and aqueous Cr were conducted in five replicates. Controls (five replicates) consisted of cutting in nutrient solution without Cr, to compare transpiration with the treated trees, and to determine the Cr concentrations in the non-treated plants. The concentration of total Cr in the aqueous solution was measured before the tree cuttings were transferred. Exposure periods were 4 days for all treatments. At the end of the exposure, the remaining solution, roots, lower and higher stem, and leaves were analyzed for total Cr. It should be noted that the lower part of the cutting with 12 cm length refers to the lower stem and the upper part is the higher stem.
Chemical analysis
The concentration of total Cr in the aqueous solution was analyzed quantitatively by flame atomic absorption spectrophotometry. Preparation and extraction of root, stem and leaf samples for total Cr determination were conducted according to the method described by Banks et al. (2006). Plant materials from the treated plants were harvested after exposure. The plants were washed with tap and distilled water followed by thorough rinsing, and then oven dried at 90°C for 48 h. Dried plant samples were ground in an electrical blender, except for the roots due to the small quantity of the total harvested materials. The biomass was sieved to pass 2 mm before use. Then, the ground tissue were placed in clean glass bottles and dried for an additional 24 h at 65°C to remove any moisture absorbed during the processing steps. The bottles were sealed and placed in a desiccator. Root, stem and leaf samples were extracted for total Cr using a nitric/perchloric acid digestion method. Exactly 0.25 g of oven dried and ground plant materials were placed in 50 ml digestion tubes, mixed with 10 ml of 1:1 HNO3/HClO4, and allowed to stand overnight. The samples were then placed in a digestion block and heated for 2 h at 200°C until the digested liquid was clear. The digested liquid was diluted to 25 ml with deionized water and filtered (Whatman #1 filter paper, Fisher Scientific, Pittsburgh, Pennsylvania) into 100 ml Erlenmeyer flasks. The filtrates were analyzed using flame atomic absorption spectrophotometry. The detection limits, determined as mean blank plus three times the standard deviation of ten blanks, was 0.001 mg Cr/l for water samples and 0.005 mg Cr/kg DW for plant materials, respectively. The sample preparation methods used were also checked against samples spiked with certified solution standards; mean recovery was 96.49%. The precision of Cr determination, based on variations of replicate analyses (n = 2) for the same sample, was <15%.
Determination of the removal rate
In the absence of volatilization and negligible background Cr in the controls with plants, all loss from the hydroponic solution can be contributed to removal by plants. The removal velocity v p (μg Cr/g day) was calculated from final and initial Cr mass using the formula
where m (0) is the total mass (μg) of Cr in the solution at the beginning, and m (t) is the total mass (μg) of Cr in solution at time t, Δt is 4 days, and M is the biomass of the plant (g).
Determination of the transpiration rate
The toxic effect of Cr solutions was evaluated by measuring transpiration rate by trees. The transpiration of plants is coupled to photosynthesis, and an inhibition of transpiration is a reliable and rapid hint of toxic effects (Trapp et al. 2000). The transpiration rate of plants T (g water/g plant day) was determined from the weight loss of the system (flask with solution and plant) during the exposure period using the formula
where M (0) is the total weight (g) of the system at the beginning, and M (t) is the total weight (g) of the system at time t, Δt is 4 days, and M is the biomass of the plant (g).
Determination of the temperature coefficient Q 10
The influence of temperature on the removal rate was quantified by calculating the temperature coefficient Q 10, which is defined as the ratio of removal rates at a 10 degree difference in temperature. The temperature coefficient was derived using the equation of Atkin et al. (2002)
where ΔT is 10°C, and slope is the slope of the linear fit curve of log v P versus temperature T.
Statistical analysis
The students t-test (two-tailed) and the Pearson’s product–moment correlation and regression were done in excel. The significance of the correlations was judged using tabled values for critical r (degree of freedom is n−2, significance level was 0.01 or 0.05) from Sachs (1992). The partial correlation was used to determine whether a correlation between two variables was due to a common correlation to a third variable as described by Yu et al. (2007b), with the equation
where r xy·z is the partial correlation coefficient between variables x and y under the assumption of a constant variable z, and r xy is the bivariate Pearson correlation coefficient between variables x and y etc.
Results
Removal of Cr from hydroponic solution by willows
Figure 1 shows the measured total Cr concentrations in the nutrient solution with hybrid willows exposed to Cr(VI) or Cr(III) at different treatment temperatures after a 4-days period of exposure. Between 12.54 and 61.30% of the applied Cr(VI) and 63.56–97.17% of the initial Cr(III) were removed from the hydroponic solution by plants for the treatments with Cr(VI) and Cr(III), respectively, within 4 days of exposure. The total Cr in hydroponic solution increased from 1.51 to 1.57 mg Cr/l (±0.17 mg Cr/l) in the presence of hybrid willows exposed to Cr(VI) at 11°C after the 4-days of incubation, while the Cr concentration declined form 1.52 to 0.71 mg Cr/l (±0.05 mg Cr/l) in the hydroponic solution spiked with Cr(III). With the increase of temperature, a faster decrease of total Cr concentration in hydroponic solution was observed for the trees exposed to both chemical forms of Cr. At the highest test temperature of 32°C, the Cr(VI) and Cr(III) concentration in the plant growth media decreased from 1.51 to 0.76 mg Cr/l (±0.10 mg Cr/l) and from 1.54 to 0.08 mg Cr/l (±0.03 mg Cr/l) within a 4-days period, respectively, judged by the total Cr analyzed.
Uptake and translocation of Cr into plant materials
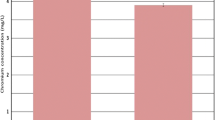
Figure 2 shows the concentrations of total Cr in the roots of willows exposed to Cr(VI) or Cr(III) after different treatment temperatures. The background Cr in non-treated control trees was 0.05 mg Cr/kg DW for roots (n = 2) and no Cr concentration above the detection limit was detected in other parts of the plants. Compared to the controls, only Cr concentrations in roots of exposed trees were significantly elevated under all treatment temperatures, while an increase of Cr concentration in lower stems were only observed at higher treatment temperatures. For the treatments ≤22°C, roots were the only sink for Cr accumulation in plants exposed to both chemical forms of Cr. Small amounts of Cr were translocated to the lower stems of plants at temperature ≥24°C. The concentration ratio of root to the initial solution (root concentration factor RCF) of both chemical forms of Cr generally increased with temperature from 11 to 32°C. The linear trends were significant (Figure not shown, r 2 values of 0.95 and 0.98 for the treatments with Cr(VI) and Cr(III), respectively) at α = 0.01. Considering the final concentrations in solution, the RCF increased with temperature and the trends were also significant (Figure not shown, r 2 values of 0.97 and 0.59 for the treatments with Cr(VI) and Cr(III), respectively) at α = 0.01. Although translocation of Cr from roots to lower stems was possible at higher treatment temperatures, there was no significant trend for the lower stems (stem concentration factor SCF, data not shown).
Measured total Cr concentrations (mg Cr/kg DW) in roots of hybrid willows (Salix matsudana Koidz × alba L.) exposed to Cr(VI) or Cr(III) at different treatment temperatures. The exposure period was 4 days. The values are the mean of five replicates. Error bars represent standard deviation; DW dry weight
Mass balance of Cr
The mass balance of total Cr in hybrid willows exposed to Cr(VI) is presented in Table 1. At the low temperature between 11 and 22°C, 12.53–38.97% of the applied Cr(VI) were removed from the hydroponic solution by plants and Cr above detection limit was only detected in roots of willows over the 4-days of test period judged by the total Cr analyzed. At the high temperature of 24–32°C, 44.82–61.30% of the applied mass was removed by plants. The majority of the Cr was accumulated in the roots and small amounts were detected in the lower stems after a 4-days of exposure. The Cr recovered from the plant biomass accounted for 87.56–116.96% of the amounts lost from the plant growth media. The calculated Cr(VI) removal rates are given in Table 1, showing a significant increase with temperatures (Figure not shown, r 2 = 0.99, significant at α = 0.01). The highest Cr(VI) removal rate was 1.99 μg Cr/g day at 32°C, which was more than 6-fold higher than that at 11°C.
The mass balance of Cr(III) was made from the total Cr in plant materials and that remaining in the solution (Table 2). The distribution of Cr(III) in plant materials followed a similar pattern as that of Cr(VI) judged by total Cr analyzed. At the low temperature of 11–22°C, 60.74–89.25% of the applied Cr(III) was removed from the growth media by plants over a 4-days test period. The applied Cr(III) analyzed in the form of total Cr was only associated with roots. The Cr recovered from roots accounted for 75.73–81.64% of the applied Cr(III) lost from the hydroponic solution by hybrid willows. At the temperature of 24–32°C, a large fraction of Cr was accumulated in roots and less than 30% of the Cr lost from growth media was recovered in the lower stems after the 4-days exposure. The calculated Cr(III) removal rates (Table 2) increased linearly with temperature (r 2 = 0.99, significant at α = 0.01). The highest Cr(III) removal rate was found at 32°C with a value of 3.55 μg Cr/g day, which was more than twofold higher than at 11°C.
Response of plant transpiration to Cr exposure at different temperatures
Figure 3 shows the transpiration of control trees (without Cr exposure) compared to exposed trees under the different temperature range from 11 to 32°C. Significant difference in the transpiration rate between treated plants and non-treated control trees was found for those exposed to Cr(VI) or Cr(III) (P < 0.05), respectively. Significant decrease of the transpiration was observed in Cr(VI) trees compared with the Cr(III) exposed trees (P < 0.05), implying that hybrid willows exposed to Cr(VI) are more sensitive to changes in temperature than that to Cr(III). The results also indicate that with the increase of temperature, the transpiration rates of treated and non-treated plants increased linearly as expected, with r 2 values of 0.79, 0.87 and 0.80 for control trees, Cr(VI) exposed trees and Cr(III) exposed trees (P < 0.01), respectively. Although a remarkable decrease of the plant transpiration rate was found for the plants exposed to both chemical forms of Cr, symptoms of chlorosis of leaves were not observed in any plant for during the entire period of exposure.
Temperature coefficient Q 10
Figure 4 shows the linear lines from which the temperature coefficient Q 10 was calculated. With a slope of 0.0382 for hybrid willows exposed to Cr(VI), a Q 10 of 2.41 results. The slope for trees exposed to Cr(III) is 0.0151 and a Q 10 of 1.42 results. This indicates that the uptake of Cr(VI) by hybrid willows is more susceptible to the change of temperature than that of Cr(III).
Discussion
Phytoextraction of both chemical forms of Cr by various plants is well documented. One conclusive point is that Cr(VI) is much more mobile and soluble than Cr(III) and the internal concentrations of Cr in plants were many fold higher than those of plants exposed to Cr(III), thus at elevated levels of Cr(VI) (Mei et al. 2002; Shahandeh and Hossner 2000). However, in our current study, significantly higher removal rates of Cr(III) than Cr(VI) were observed from the hydroponic solution by hybrid willows at all treatment temperatures (P < 0.01). Similar conclusions were also reached by Ramachandran et al. (1980), McGrath (1982) and Mei et al. (2002) previously. For example, soybean (Glycine max L., var. Mitchell-450) tended to absorb less Cr(VI) than Cr(III) (Mei et al. 2002). This is probably due to the internal mechanisms for Cr tolerance. The oxidant, Cr(VI), may damage biological membranes, whereas Cr(III), a reductant, causes little membrane damage and is more easily absorbed into the plant and tolerated (Mei et al. 2002). Poor translocation of Cr to the shoot is also expected, as plants are exposed with either form of Cr and the tendency to accumulate Cr in the roots seems to be a common phenomenon in most plant species. Skeffington et al. (1976) illustrated that Cr(III) and Cr(VI) enter the vascular tissue with difficulty; however, once in the xylem, Cr moves more readily. In out study, due to the remarkable difference in the uptake capacity between the two chemical species of Cr by plants, the reduction of Cr(VI) to Cr(III) in the nutrient solution before uptake by plant roots was unlikely to take place. Our results are in agreement with the earlier report by McGrath (1982). Additionally, the same patterns of Cr accumulation and translocation in willow trees were observed in both chemical forms of Cr at all treatment temperatures in this study, implying that the reduction of Cr(VI) to Cr(III) might take place in roots, but translocation of Cr from root to shoot was extremely limited because of the propensity of Cr(III) to bind to cell walls.
Differences in Q 10 values occur in plants (Fitter et al. 1998). These values are in the range of previously reported Q 10-values of 1.1–2.9 (Azcón-Bieto 1992; Atkin et al. 2000). A Q 10-value of 2.7 for the respiration of Populus tremuloides was found by Tjoelker et al. (1999). Yu et al. (2005) used detached leaves of plants exposed to free cyanide to determine Q 10 values in a temperature range from 11 to 32°C in which Q 10 values of 1.84 and 2.09 were found for Chinese elder (Sambucus chinensis L.) and weeping willow (Salix babylonica L.), respectively. The influence of temperature on the removal of cyanide by weeping willows was also studied by Yu et al. (2007b) and the Q 10 value was determined for intact trees to be 1.46. The Q 10 values obtained in this study fall into this range, even though the category of chemical is very different.
The movement of metals from external solution into root cells is either due to diffusion of metal iron along the concentration gradient formed or due to mass flow driven by transpiration stream (Greger 1999). The linear correlation between removal rates and temperature is highly significant for the treatment with Cr(VI) (r 2 = 0.99, P < 0.01). The correlation between temperature and transpiration rate is also highly significant (r 2 = 0.87, P < 0.01). Since humidity and light were kept constant during the experiments, an improved correlation might be expected, but the size of the individual trees varied and had some influence on the correlation results between temperature and transpiration. The correlation between transpiration and Cr(VI) loss from the system is also highly significant (r 2 = 0.86, P < 0.01). However, a partial correlation between temperature, removal rates of Cr(VI) and transpiration unveils that the correlation between temperature and removal rate, assuming transpiration rates a constant, is significant (r 2 = 0.91, P < 0.01). On the other hand, the partial correlation between removal rates and transpiration (assuming temperature a constant) is insignificant (r 2 = 0.001, P > 0.01), suggesting that Cr(VI) uptake into roots was mainly dependent on diffusion rather than transpirations. A similar data analysis was conducted for the treatments supplied with Cr(III), in which transpiration rates of plants showed a stronger influence on the uptake of Cr(III) by plants. These results are constant with our previous studies (Yu and Gu 2007; Yu et al. 2007a).
Conclusion
Results from this study demonstrated that the removal of Cr(III) from hydroponic solution by hybrid willows was faster than Cr(VI) at all treatment temperatures judged by the total Cr analyzed. Root was the exclusive sink for Cr accumulation for both chemical forms of Cr from 11 to 22°C, while small amounts of Cr were translocated to the lower stems with increasing temperature ≥24°C. Results from the calculation of the temperature coefficient revealed that the uptake of Cr(VI) by plants is more sensitive to changes in temperature than the uptake of Cr(III), and metal accumulation increases with temperature for both chemical forms of Cr.
References
Atkin OK, Evans J, Ball M, Lambers H, Pons T (2000) Leaf respiration of snow gum in the light and dark: interactions between temperature and irradiance. Plant Physiol 122:915–923
Atkin OK, Zhang Q, Wiskich J (2002) Effect of temperature on rates of alternative and cytochrome pathway respiration and their relationship with the redox poise of the Quinone pool. Plant Physiol 128:212–222
Azcón-Bieto J (1992) Relationships between photosynthesis and respiration in the dark in plants. In: Barber J, Guerrero MG, Medrano H (eds) Trends in photosynthesis research. Intercept Ltd, Andover, pp 241–253
Baghour M, Moreno DA, Herna′ndez J, Castilla N, Romero L (2001) Influence of root temperature on phytoaccumulation of As, Ag, Cr and Sb in potato plants (Solanum tuberosum L. var. spunta). J Environ Sci Health A 36:1389–1401
Banks MK, Schwab AP, Henderson C (2006) Leaching and reduction of chromium in soil as affected by soil organic content and plants. Chemosphere 62:255–264
Becquer T, Quantin C, Sicot M, Boudot JP (2003) Chromium availability in ultramafic soils from New Caledonia. Sci Total Environ 301:251–261
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74
Davis FT, Puryear JD, Newton RJ, Egilla JN, Grossi JAS (2002) Mycorrhizal fungi increase chromium uptake by sunflower plants: influence on tissue mineral concentration, growth, and gas exchange. J Plant Nutr 25:2389–2407
Dixit V, Pandey V, Shyam R (2002) Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L.cv. Azad) root mitochondria. Plant Cell Environ 25:687–693
Fitter AH, Graves JD, Self GK, Brown TK, Bogie DS, Taylor K (1998) Root production, turnover and respiration under two grassland types along with an altitudinal gradient-influence of temperature and solar radiation. Oecologia 114:20–30
Fritioff A, Kautsky L, Greger M (2005) Influence of temperature and salinity on heavy metal uptake by submersed plants. Environ Pollut 133:265–274
Greger M (1999) Metal availability, uptake, transport and accumulation in plants. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants: from molecules to ecosystems. Springer-Verlag, Heidelberg, pp 51–57
Han FX, Banin A, Su Y, Monts DL, Plodinec MJ, Kingery WL, Triplett GB (2002) Industrial age anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften 89:497–504
Hunter JG, Vergnano O (1953) Trace element toxicities in oat plants. Ann Appl Biol 4:761–777
Katz SA, Salem H (1994) The biological and environmental chemistry of chromium. VCH Publishers, New York
Kimbrough DE, Cohen Y, Winer AM, Creelam L, Mabuni C (1999) A critical assessment of chromium in the environment. Crit Rev Environ Sci Technol 29:1–46
Kumar PBAN, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Larcher W (1995) Physiological plant ecology, 3rd edn. Springer, Berlin
McGrath SP (1982) The uptake and translocation of tri- and hexavalent chromium and effects on the growth of oat in flowing nutrient solution and in soil. New Phytol 92:381–390
Mei BJ, Puryear JD, Newton RJ (2002) Assessment of Cr tolerance and accumulation in selected plant species. Plant Soil 247:223–231
Pulford ID, Watson C, McGregor SD (2001) Uptake of chromium by trees: prospects for phytoremediation. Environ Geochem Health 23:307–311
Ramachandran V, D’Souza TJ, Mistry KB (1980) Uptake and transport of chromium in plants. J Nucl Agric Biol 9:126–129
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Scoccianti V, Crinelli R, Tirillini B, Mancinelli V, Speranza A (2006) Uptake and toxicity of Cr (Cr3+) in celery seedlings. Chemosphere 64:1695–1703
Shahandeh H, Hossner LR (2000) Plant screening for chromium phytoremediation. Int J Phytorem 2:31–51
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Sharma DC, Sharma CP, Tripathi RD (2003) Phytotoxic lesions of chromium in maize. Chemosphere 51:63–68
Skeffington RA, Shewry PR, Peterson PJ (1976) Chromium uptake and transport in barley seedlings (Hordeum vulgare L.). Planta 132:209–214
Tjoelker MG, Oleksyn J, Reich PB (1999) Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob Chang Biol 5:679–691
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Environ Contam Toxicol 39:154–160
Yu XZ, Gu JD (2007) Accumulation and distribution of trivalent chromium and effects on hybrid willow (Salix matsudana Koidz × alba L.) metabolism. Arch Environ Contam Toxicol 52:503–511
Yu XZ, Gu JD (2008) The role of EDTA in phytoextraction of hexavalent and trivalent chromium by two willows tress. Ecotoxicol 17:143–152
Yu XZ, Trapp S, Zhou PH, Hu H (2005) The effect of temperature on the rates of cyanide metabolism of two woody plants. Chemosphere 59:1099–1104
Yu XZ, Gu JD, Huang SZ (2007a) Hexavalent chromium induced stress and metabolic responses of in hybrid willows. Ecotoxicol 16:299–309
Yu XZ, Trapp S, Zhou PH, Chen L (2007b) Effect of temperature on the uptake and metabolism of cyanide by weeping willows. Int J Phytorem 9:243–255
Yu XZ, Gu JD, Xin LQ (2008) Differences in uptake and translocation of hexavalent and trivalent chromium by two species of willows. Ecotoxicol 17:747–755
Zayed AM, Terry N (2003) Chromium in environment: factors affecting biological remediation. Plant Soil 249:135–156
Zayed A, Lytle CM, Terry N, Qian JH (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Acknowledgments
This work was financially supported by The National Science Foundation of China (NSFC: 30770389).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Peng, XY. & Xing, LQ. Effect of temperature on phytoextraction of hexavalent and trivalent chromium by hybrid willows. Ecotoxicology 19, 61–68 (2010). https://doi.org/10.1007/s10646-009-0386-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0386-2