Abstract
Molluscs have long been regarded as promising bioindicator and biomonitoring subjects for heavy metals as molluscs are highly tolerant to heavy metals and exhibit high accumulation in their body. In spite of several previous studies about the impact of cadmium on molluscs, little information exists in literatures concerning the toxic effects of cadmium on Lymnaea acuminata, especially pertaining to behavioral and hematological changes as these are considered effective bioindicators and biomonitoring variables for detecting heavy metals in polluted water bodies. In the present study, the median lethal concentrations of cadmium chloride to snail, Lymnaea acuminata, were estimated to be 9.66, 7.69, 6.26, and 5.54 mg/L at 24, 48, 72, and 96 h, respectively. For behavioral studies, variable test concentrations of cadmium from 0.00 to 10 mg/L were used. The clumping tendency, crawling activity, and touch reflex in the exposed snails were gradually decreased with higher concentrations at 72 and 96 h. For measuring the hemocyte numbers in the circulating hemolymph of snail during chronic cadmium exposure, two sublethal doses of cadmium (10 and 20% 96-h LC50—0.55 and 1.11 mg/L, respectively) were used. A significant variation (p < 0.05) from the control at all exposure times (7, 14, 21, and 28 days) was recorded at 1.11 mg/L concentration. The total count of circulating hemocytes was significantly reduced (p < 0.05) compared to the controls at both concentrations of cadmium exposure at all time periods except 14 and 21 days exposure at 0.55 mg/L where values were non-significantly increased. In comparison between two sublethal doses, blood cells were significantly (p < 0.05) lowered at 1.11 mg/L cadmium treatment. Considering the behavioral and hematological data, it seems possible to forecast the physiological state of snails in cadmium-contaminated water bodies and these findings can be used in determining the safe disposal level of cadmium in aquatic ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid industrialization is one of the major causes of heavy metal pollution in aquatic ecosystem. Among heavy metals, cadmium possesses a significant ecological problem for its ability to be accumulated in living organisms in a cumulative manner (Jensen and Bro-Rasiriussen 1992; Alazemi et al. 1996). Cadmium can enter the environment from various anthropogenic sources, such as by-products of zinc, lead, and copper mining, and smelting, coal combustion in the thermal power plants, iron and steel production, pigments, fertilizers, and pesticides (Hodgson 2010; Gad 2005). Though cadmium occurs naturally in the environment in insignificant amounts, its release in the environment is steadily increasing due to increase in anthropogenic activities causing severe pollution of soil and aquatic ecosystems. Aquatic organisms mainly absorb cadmium from polluted water through respiratory and digestive systems and body surface without significant excretion and are prone to be affected with this heavy metal (Rainbow and White 1989; van Hatton et al. 1989).
Aquatic organisms are very much susceptible to any change or stress in their environment as they are in direct contact with water (Al-Attar 2005). In general, molluscs play an important role in the balance of nature, and also act as good bioindicator, in determining the degree of pollution of water among the aquatic organisms especially to monitor heavy metal toxicity (Nurnberg 1984; Kambale and Potdar 2010). Among the freshwater molluscs, Lymnaea sp. has been used as a suitable test organism in ecotoxicological studies (Russo and Lagadic 2004; Das and Khangarot 2010) and acts as a promising heavy metal toxicity bioindicator in pollution research.
In aquatic organisms like molluscs, the blood remains in the direct contact with their surroundings as molluscs have open circulatory system. Thus, blood physiology of aquatic organisms may be considered as a vital index to monitor health status of the organisms as well as an indicator of water pollution (Kori-Siakpere et al. 2006). Toxic metal ions are known to cause deleterious impact on the various blood parameters in the aquatic organisms including molluscs as they form complexes with the structural proteins, enzymes, and nucleic acids and interrupt their normal physiological functions (Chakraborty et al. 2008; Vinodhini and Narayanan 2008; Chakraborty and Ray 2009). Gastropods possess a well-developed innate defense system where hemocytes play an important role. Its role in the metal transportation and metabolism in molluscs is also well established (Suresh and Mohandas 1989; Suresh 1990). Since gastropods have an open circulatory system, any change in relation to stress immediately gets reflected in the blood. Variations in the hematocyte number present in the hemolymph in gastropods due to the changing external environment thus act as a sensitive indicator to monitor stress and also the physiological state of the organism (Mohandas et al. 1989; Guria et al. 2003; Ray et al. 2013).
In the present study, chronic toxicity of cadmium to mature freshwater snail, Lymnaea acuminata (class: Gastropoda; family: Lymnaeidae), was performed to determine the alteration in circulating hemocytes in the hemolymph. Behavioral changes were also assessed in this snail as an acute effect of the same toxicant.
Materials and methods
For bioassays, healthy, active, and mature freshwater snails, Lymnaea acuminata with mean shell height 1.98 ± 0.12 cm and mean weight 0.89 ± 0.36 g, were used as test organisms. The snails were collected from the unpolluted local ponds, washed up with tap water, and acclimatized in the laboratory condition for 72 h. For acclimatization, they were kept in well-aerated aquarium filled with unchlorinated tap water at room temperature (27 ± 0.45 °C) and provided with some aquatic macro-vegetation (Hydrilla sp. and Pistia sp.) as their natural food and resting place. The water was changed at every 24 h to avoid detritus load.
Analytical grade of cadmium chloride, CdCl2·H2O (purity 98%, molecular weight 201.32 g/mol; E. Merck, Germany), was used as the test chemical. Water chemical analysis and the bioassays were done following the standard methods outlined in the American Public Health Association (APHA 2012). Static replacement bioassays were used for the 96-h tests (acute toxicity tests and behavioral study) in the laboratory. The underground water stored in the overhead tank was used as a diluent medium. The test medium was replaced every 24 h by freshly prepared test solution to avoid the interference of different abiotic factors with the animals’ performance. The cadmium content of water was analyzed on a regular basis by flame atomic absorption spectrophotometry (Varian model spectrAA–10, AAS) according to the modified method of Brewer et al. (1969) as described in FAO (1975). The cadmium content of water was detected very near to the expected values.
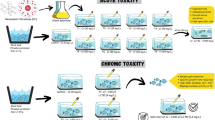
During acute (96 h) bioassay, tests with snails were conducted in 15-L glass aquaria each holding 10 L of water for the determination of acute toxicity. Tap water stored in the glass aquaria (temperature 27 ± 0.45 °C, pH 7.4 ± 0.21, free CO2 8.0 ± 0.21 mg/L, DO 5.54 ± 0.42 mg/L, alkalinity 176 ± 7.01 mg/L as CaCO3, hardness 120 ± 7.0 mg/L as CaCO3) was used as a diluent medium. A set of four aquaria was exposed to one single concentration of cadmium chloride to make four replicates per concentration while determining acute toxicity of the pollutant. Each set of tests was accompanied by four replicates of control. Ten test organisms were used in each replicate. While conducting acute toxicity test, different concentrations of the toxicant were prepared by weighing the required quantity of the chemical compound which was then added directly to the test medium. The test medium was then stirred with a magnetic stirrer for uniform mixing of the test chemical compound. Initially, rough range finding tests were conducted for the test organisms to determine the dose range at which mortality occurs. The selected test concentrations of cadmium chloride finally used for the determination of median lethal concentrations (LC50) to the snails were 0, 1.5, 3.0, 4.0, 5.5, 6.0, 6.5, 7.0, 8.5, 9.0, 9.5, and 10.0 mg/L. Test organisms were not fed 24 h before and during the bioassays. The vitality of the snails was checked frequently (four times/day) using soft tweezers, and they were considered dead when there was no response to physical stimulation. The number of dead organisms was counted every 24 h and removed immediately from the test medium to avoid any organic decomposition and oxygen depletion.
Mortality rate at different concentrations and at different time of exposure (24, 48, 72, 96 h) was analyzed using the computer software R version 2.14.0 (US EPA 1999) and probit analysis by Finney (1971) for determining median lethal concentrations (LC50) with 95% confidence limits of cadmium to the test organisms at different time of exposure. Correlation analysis of mortality rate with different concentrations and different time of exposure (24, 48, 72, and 96 h) was analyzed following the methods described by R Development Core Team (2011) and Gomez and Gomez (1984). The behavioral changes of the test organisms in respect of their clumping tendency (lower snail strongly attached to the substratum whereas the top snail bound to the shell surface of the lower one), crawling activity (extremely slow-paced movement either on the surface or on the wall of the aquarium), and touch reflex (a slight stimulus eliciting a local contraction of the musculature under the shell) exposed to the toxicant during acute toxicity were recorded systematically by naked-eye observation following the method of Rand (1985). Scoring of behavioral changes was performed independently by two observers and the scores were collated and average was calculated and quantified in terms of −/+/++/+++ (− = none; + = mild; ++ = moderate; +++ = strong).
Chronic toxicity tests were also conducted in 15-L glass aquaria having similar water qualities as used in acute (96 h) studies, each holding 10 L of water in the laboratory condition for 28 days using two different sublethal concentrations (10 and 20% of the 96-h LC50 value) of cadmium and a control. The aquaria were arranged in three blocks each with three aquaria as per randomized block design (Gomez and Gomez 1984), thereby giving three replicates for each of the two sublethal concentrations and control. Each aquarium was stocked with ten healthy, mature, active, and properly acclimatized snails for hematological study under sublethal exposure. In aquaria, they were provided with their natural food like Hydrilla sp. and Pistia sp. The blood samples of the treated and non-treated L. acuminata were analyzed for hemocyte count. The hemolymph samples of the snails were analyzed at every 7 days interval with a total exposure period of 28 days following the method described by Suresh (1990). For this study, the snails were taken out from the aquaria and were washed with cold water in order to remove the feces and excess mucus. Before collection of hemolymph, the water adhering to the snail was soaked and the foot cleaned with tissue paper. The hemolymph was collected from the sinus using a needle fitted with heparinized capillary tube. Hemolymph was also collected by touching the foot with the tip of a micropipette. As a result, the snail was forced to retract deeply into its shell and extruded hemolymph. In this way, about 0.2 mL of oozing hemolymph was collected aseptically from each snail and was immediately stored at 4 °C to avoid hemocyte clumping. An aliquot of fresh extracted hemolymph of each specimen of L. acuminata was placed on Neubauer hemocytometer for determination of total hemocyte count per milliliter of hemolymph (Caprette 2007). Post-collection, the first two drops of hemolymph were expelled while the succeeding drops were discharged in one of the V-shaped wells of hemocytometer with a capillary tube. The cells were allowed to settle for 1 to 2 min and then determination of cell numbers was carried out after 2 min.
The mean values of hematological parameter for the control and experimental snails were subjected to one-way analysis of variance (ANOVA) at 0.01 and 0.05 levels using the computer software R version 2.14.0 followed by Duncan’s Multiple Range Test (DMRT) for determining significant differences among the means (Gomez and Gomez 1984).
Results
The median lethal concentrations (LC50) at different time of exposure (24, 48, 72, and 96 h) with 95% confidence limits of cadmium to Lymnaea acuminata are given in Table 1. No mortality was observed in the control group during the experiment. Significant co-relationship (p < 0.01) was observed between mortality rate of the snails and exposure concentrations at all the exposure times (24, 48, 72, 96 h) as tabulated in Table 2. On the other hand, the co-relationship between mortality rate and exposure times was significant (p < 0.01) at all concentrations except 3.0 and 5.5 mg/L (Table 3).
The behavioral responses observed in the test organisms exposed to cadmium chloride are summarized in Table 4. The clumping tendency and crawling activity of the snail were low to moderate at control and lower doses of cadmium but were increased with the increasing concentrations of the toxicant at 24 h exposure. With the passage of time, the clumping tendency and the crawling activity gradually decreased with higher concentrations and increased at lower concentrations at 72 and 96 h exposure. No such clumping and crawling effects were recorded at 8.5–9.5 mg/L of 96 h exposure. Touch reflex of the snail was observed at all the concentrations of 24 and 48 h exposures but it was found to decrease at the higher concentrations with the progress of time. Touch reflex was completely absent at 9.0 and 9.5 mg/L of 72 h and at 8.5–9.5 mg/L of 96-h treatments (Table 4).
Single-factor ANOVA was carried out with mean values of hemocyte numbers in the circulating hemolymph of L. acuminata exposed to two sublethal doses (10 and 20% 96-h LC50 value) of cadmium (0.55 and 1.11 mg/L). The results showed a significant variation of hemocyte numbers (p < 0.05) at all time period (7, 14, 21, and 28 days) only at 1.11 mg/L cadmium treatment (Table 5). In this experimental setup, the number of hemocytes in control snails (0.00 mg/L) at 7,14, 21, and 28 days remained constant which lowered significantly (p < 0.05) at 1.11 mg/L cadmium treatment as the cadmium exposure period increased from 7 to 28 days. However, at 0.55 mg/L cadmium treatment, no lowering of hematocyte numbers in comparison to control was observed at 14 and 21 days. Thus, significant variation (p < 0.05) was only observed during the experiment period at higher dose of treatment. During the study, no mortality of the snails in the control was observed and the mortality rate of the exposed snails was also insignificant in respect of control group.
Discussion
Pollution of the aquatic ecosystem due to cadmium has been a serious concern for quite some time (Gad 2005). In aquatic ecosystem, cadmium is readily absorbed by the organisms directly from the water in its free ionic form (AMAP 1998). In this connection, snails are the most susceptible aquatic group to cadmium toxicity (Kaviraj and Das 1990). This may be due to direct contact of their vital organs to the toxicant or to the absence of operculum in cases of Lymnaeidae which is in agreement with the result of the present investigation. The 96-h median lethal concentration of cadmium to Lymnaea acuminata was found to be 5.54 mg/L in the present study which nearly corresponds with the 48-h LC50 value of cadmium to the snail, Filopaludina martensi (5.01 mg/L) (Piyatiratitivorakul and Boonchamoi 2008), and 72-h LC50 value to Melanoides tuberculata (5.24 mg/L) (Shuhaimi-Othman et al. 2012). Similar findings were also recorded to the mud crab, Eurypanopeus depressus (72-h LC50 4.9 mg/L) (Collier et al. 1973), tropical grass shrimp, Palaemon northropi (96-h LC50 6 mg/L) (Chung 1980), and Atlantic oyster drill, Urosalpinx cinerea (96-h LC50 6.6 mg/L) (WHO 1992). This near correspondence in median lethal concentration values between Lymnaea acuminata and already mentioned invertebrates (crabs, shrimps, and oysters) may be attributed to the fact that all these organisms have open circulatory systems; open circulatory systems evolved primarily in phylum molluscs and arthropods, thus similar impact due to cadmium exposure may be expected in such animals. However, it was observed in our experimental setup the fresh water snails were insensitive to cadmium in acute exposure, with a very high LC50 value of 5.54 in 120 mg/L hardness water which is several times greater than other sensitive freshwater organisms in 100 mg/L hardness water (US EPA 2016). This high LC50 value can be attributed to the increased hardness of water and the difference in hardness of water can influence the median lethal concentration as evidenced by Datta et al. (2003). In addition to the physicochemical properties of water, another contributing factor—the age of organisms (Weir and Walter 1976; Wang et al. 2010)—can also cause variation in LC50 values among species (Kaviraj and Das 1990) or even between closely related species (Okocha and Adedeji 2011). Susceptibility of L. acuminata to the lethal effect of cadmium was duration and concentration dependent as recorded in the present study. This result corresponds with the findings of Das and Khangarot (2010) on L. luteola exposed to cadmium. Many workers have also reported similar findings in other aquatic organisms exposed to cadmium (Nelson et al. 1976; Singh et al. 2010; Tiwari et al. 2011; Dhara et al. 2014).
The alteration in the behavioral pattern is considered the most sensitive indicator of potential toxic effects of cadmium in aquatic organisms (Tiwari et al. 2011). In our present study, Lymnaea acuminata showed dose- and duration-dependent behavioral changes highlighting their avoidance reaction to the cadmium toxicity. This observation was in accordance to earlier studies showing similar behavioral activities in the water snail, Physa integra and Taphius glabratus with their inability to crawl when exposed to such polluted water (WHO 1992; Tantulvesn and Pornprapa 1995). These avoidance reactions may be contributed to the narcotic effects or to the change in sensitivity of chemo-receptors in the exposed organisms (Suterlin 1974). Enzymatic as well as ionic disturbances in the blood or tissue may also be associated with such alteration in animal behavior (Larsson et al. 1981).
Changes in hematological values of aquatic organisms are known to occur in relation to physiological stress, disease, and toxic environmental conditions (Kori-Siakpere et al. 2006). One of the known manifestations of stress in molluscs is the significant fluctuation in the total hemocyte count (Sminia 1981). The number of hemocytes is a factor directly linked with innate defense system of the gastropods which alters defense mechanism, mainly at the cellular level (Suresh and Mohandas 1989). Thus, the alteration of total hemocyte count in gastropods can be considered as one of the most reliable indicators to monitor stress factor and also to assess their physiological state (Mohandas et al. 1989; Auffreta et al. 2006). In this context, mercury is known to cause significant hemocyte mortality in Pacific oyster, Crassostrea gigas (Gagnaire et al. 2004). Reduced hemopoiesis was observed in copper-stressed oysters C. virginica molluscs (Cheng 1988). Bindya Bhargavan (2008) found time-dependent decrease of hemocyte count in green mussel, Perna viridis exposed to copper and mercury. In a separate study by Suresh (1990), the hemocyte count also decreased significantly in Indoplanorbis exustus and Lymnaea acuminata exposed to copper. In accordance to these previous studies, our present study on cadmium-treated L. acuminata showed a decrease in hemocyte count (Table 5) confirming disturbance to the organisms. This particular observation is also in agreement with a previous experimental study by George et al. (1983) on oyster, Ostrea edulis, exposed to cadmium. Significant difference in total hemocyte counts as observed in our L. acuminata exposed to different doses of cadmium acts as a good indicator of ecological difference. The decrease in hemocyte count in our experimental snails exposed to cadmium may have resulted from hemodilution as a result of mechanism that reduces the concentration of the toxicant/pollutant in the circulatory system (Kori-Siakpere et al. 2006). Bindya Bhargavan (2008) have opined that heavy metals usually get accumulated in the granular hemocytes and overloading results in the decrease in cell viability or cell death in molluscs due to degenerated enzymes and reactive oxygen species. Decrease in total hemocyte count can also be a result of reduced proliferation of hemocytes or movement of cells from circulation into damaged tissues (Pipe and Coles 1995). Besides blood cell death, low inflow of hemocytes from other sites into the hemolymph could also reduce the total hemocyte count (Bindya Bhargavan 2008). Besides, Mohandas et al. (1989) has suggested that hemocytes under stress may also migrate from the circulatory system to the gonads to phagocytose, resulting in lower count of hemocytes in the hemolymph. In contrary to this, total hemocyte count in molluscs has also been reported to increase by heavy metal pollution in some earlier studies (Pickwell and Steinert 1984; Pipe et al. 1995, 1999; Fisher et al. 2000). Pipe and Coles (1995) recorded significantly higher number of circulating hemocytes in mussels under chronic exposure of cadmium. Interestingly, similar trends were also obtained in our current study in the treated snails at lower dose of cadmium for certain period of exposure (Table 5). Such increase in the number of circulating hemocytes may be due to migration of cells from the reservoir compartment to the hemolymph as a result of stress elicited by cadmium ions (Pipe et al. 1999). It may also be due to mitosis of leukocytes or to the continuous hemopoiesis from the amoebocyte-producing organ as reported in Biomphalaria glabrata (Jeong et al. 1983). Similarly, Cheng (1988) reported that cadmium stimulated the process of hemopoiesis in molluscs resulting in the elevation of hemocyte level. In freshwater gastropods, the majority of hemocytes are amoebocytes with granules in the cytoplasm (Sminia 1972, 1981; Sminia et al. 1983; Ottaviani 1983; Dikkeboom et al. 1985). It was reported that although metal stress causes reduction in hemocyte counts, mature granulocytes are seldom affected. Molluscs exposed to toxicant would need increased number of granulocytes to remove the overload of toxicants as such granulocytes play important roles in internal defense (Suresh 1990). This is reflected in the increase in the number of hemocytes.
Conclusion
The present findings, thus, highlight the toxicity of cadmium to Lymnaea acuminata during their acute and chronic exposure. Acute toxicity studies are among the first steps in determining the water quality required for the sustenance of snails. These studies reveal the toxicant concentrations (viz. LC50) that cause snail mortality even at short-term exposure. The observation on the behavioral responses of the snail in the present study may be an indicative parameter for assessing the toxicity of cadmium in the ecosystem as suggested by Doving (1992). The hematological study clearly shows that heavy metals like cadmium pose a serious threat to the biological functions of snails during their chronic exposure. On the basis of hematological changes, it might be possible to forecast the physiological state of snails in natural water bodies as well as developing new vistas pertaining to the mechanism of action of a toxicant in a molluscan body.
References
Al-Attar AM (2005) Changes in haematological parameters of the fish, Oreochromis niloticus treated with sublethal concentration of cadmium. Pak J Biol Sci 8(3):421–424
Alazemi BM, Lewis JW, Andrews EB (1996) Gill damage in the freshwater fish Gnathonemus petersii (family: Mormyridae) exposed to selected pollutants: an ultrastructural study. Environ Technol 17:225–238
AMAP (1998) Assessment report: arctic pollution issues. Arctic Monitoring and Assessment Programme, Oslo
American Public Health Association (APHA) (2012) Rice EW, Baird RB, Eaton AD, Clesceri LS (eds) Standard methods for the examination of water and wastewater, 22nd edn, American Public Health Association, American Water Works Association, Water Environment Federation: Washington DC
Auffreta M, Rousseaua S, Bouteta I, Tanguya A, Baronb J, Moragaa D, Duchemina M (2006) A multiparametric approach for monitoring immunotoxic responses in mussels from contaminated sites in Western Mediterranean. Ecotoxicol Environ Saf 63:393–405
Bindya Bhargavan PV (2008) Haematological responses of green mussel, Perna viridis (Linnaeus) to heavy metals copper and mercury. Ph.D Thesis Cochin University of Science and Technology, Kochi-682016, India
Brewer PG, Spencer DW, Smith CL (1969) Determination of trace metals in seawater by atomic absorption spectrophotometry. Atomic Absorption Spectroscopy, ASTM STP 443, American Society for Testing and Materials pp 70–77.
Caprette DR (2007) Experimental bioscience; laboratory methods: using a Counting Chamber http://www.ruf.rice.edu/~biolabs/methods/microscopy/cellcountin html
Chakraborty S, Ray S (2009) Nuclear morphology and lysosomal stability of molluscan hemocytes as possible biomarkers of arsenic toxicity. Clean 37(10):769–775
Chakraborty S, Ray M, Ray S (2008) Sodium arsenite induced alteration of hemocyte density of Lamellidens marginalis—an edible mollusc from India. Clean 36(2):195–200
Cheng TC (1988) In vitro effects of heavy metals on cellular defense mechanisms of C. virginica: phagocytic and endocytic indices. J Invert Pathol 51:215–220
Chung KS (1980) Effects of selected heavy metals on the survival of tropical grass shrimp (Palaemon northropi). Biol Inst Oceanogr Univ Oriente, Cumana 19:53–58
Collier RS, Miller JE, Dawson MA, Thurberg EP (1973) Physiological response of the mud crab, Eurypanopeus depressus to cadmium. Bull Environ Contam Toxicol 10:378–382
Das S, Khangarot BS (2010) Bioaccumulation and toxic effects of cadmium on feeding and growth of an Indian pond snail Lymnaea luteola L. under laboratory conditions. J Hazard Mater 182(1-3):763–770
Datta S, Masala SH, Das RC (2003) Influence of some abiotic factors on the acute toxicity of cadmium to Cyprinus carpio. J Indian Fish Assoc 30:41–52
Dhara K, Mukherjee D, Saha NC (2014) Acute and chronic toxicity of cadmium to male Clarias batrachus Linn. with special reference to their haematological changes. Int J Sci Res 3(12):28–30
Dikkeboom R, Van der Knaap WPW, Meuleman EA, Sminia T (1985) A comparative study on the internal defence system of juvenile and adult Lymnaea stagnalis. Immunology 55:547–553
Doving KB (1992) Assessment of animal behaviour as method to indicate environmental toxicity. Comp Biochem Physiol 100:247–252
FAO (1975) Manual of methods in aquatic environment research: part 1 Methods for detection, measurement and monitoring of water pollution. FAO Fisheries Technical paper no. 137. FIRI/T137 pp 211–218
Finney DJ (1971) Probit analysis. Cambridge University Press, London
Fisher WS, Oliver LM, Winstead JT, Long ER (2000) A survey of oysters Crassostrea virginica from Tampa Bay, Florida: associations of internal defense measurements with contaminant burdens. Aquat Toxicol 51:115–138
Gad SC (2005) Cadmium. In: Wexler P (ed) Encyclopedia of Toxicology, vol 1-4. Academic Press, Elsevier, Cambridge, p 376
Gagnaire B, Thomas-Guyonb H, Renaulta T (2004) In vitro effects of cadmium and mercury on Pacific oyster, Crassostrea gigas (Thunberg), haemocytes. Fish Shellfish Immunol 16:501–512
George SG, Pirie BJS, Frazier JM (1983) Effects of cadmium exposure on metal containing amoebocytes of the oyster, Ostrea edulis. Mar Biol 76:63–66
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. John Wiley and Sons, New York
Guria K, Ray S, Chattopadhyay S (2003) Methylparathion and fenvalerate induced alteration of hemocyte function of Bellamya bengalensis: a threat to indigenous human diet. Sci Cult 69(9–10):357–358
Hodgson E (2010) A textbook of modern toxicology, 4th edn. John Wiley & Sons., Inc., Hoboken http://www.wiley.com/go/permission
Jensen A, Bro-Rasiriussen F (1992) Environmental cadmium in Europe. Rev Environ Contam Toxicol 125:101–181
Jeong KH, Lie KJ, Heyneman D (1983) The ultrastructure of the amoebocyte-producing organ in Biomphalaria glabrata. Dev Comp Immunol 7:217–228
Kambale NA, Potdar VV (2010) Hematological analysis of Molluscan species Bellamya bengalensis and Lamiellidens marginalis. Biol Forum- An Int J 2(1):70–72
Kaviraj A, Das BK (1990) Bioaccumulation and toxicity of cadmium to aquatic organisms—a review. Growth, Dev Nat Res Conserv 3:177–186
Kori-Siakpere O, Ake JEG, Avworo UM (2006) Sub lethal effects of cadmium on some selected haematological parameters of Heteroclarias (a hybrid of Heterobranchus bidorsalis and Clarias gariepinus). Int J Zool Res 2(1):77–83
Larsson A, Bengtsson BE, Haux C (1981) Disturbed ion balance in flounder, Platichthys flesus L. exposed to sublethal levels of cadmium. Aquat Toxicol 1:19–35
Mohandas A, Suresh K, Ramanathan K (1989) Total haemocyte counts in two size groups of Lamallidens marginalis. In: Banerjee et al.,(ed) Proceedings of National Symposium on Emerging trends in Animal Haematology, Department of Zoology, Patna University, 53–56
Nelson DA, Calabrese A, Nelson BA, Maclnnes JR, Wenzloff DR (1976) Biological effects of heavy metals on juvenile Bay Scallops, Argopecten irradians in short term exposures. Bull Environ Contam Toxicol 16:275–282
Nurnberg HW (1984) Bio-accumulation of heavy metals by bivalves from Lim Fjord (North Adriatic Sea). J Mar Biol Sci 81(2):177–188
Okocha RC, Adedeji OB (2011) Overview of cadmium toxicity in fish. J Appl Sci Res 7(7):1195–1207
Ottaviani E (1983) The blood cells of the freshwater snail Planorbis corneus (Gastropoda, Pulmonata). Dev Comp Immunol 7(2):209–216
Pickwell GV, Steinert SA (1984) Serum biochemical and cellular responses to experimental cupric ion challenge in mussels. Mar Environ Res 14:245–265
Pipe RK, Coles JA (1995) Environmental contaminants influencing immune function in marine bivalve molluscs. Fish Shellfish Immunol 5:581–595
Pipe RK, Coles JA, Thomas ME, Fossato VU, Pulsford AL (1995) Evidence for environmentally derived immunomodulation in mussels from the Venice lagoon. Aquat Toxicol 32:59–73
Pipe RK, Coles JA, Carissan FMM, Ramanathan K (1999) Copper induced immunomodulation in the marine mussel Mytilus edulis. Aquat Toxicol 46:43–54
Piyatiratitivorakul P, Boonchamoi P (2008) Comparative toxicity of mercury and cadmium to the juvenile freshwater snail, Filopaludina martensi martensi. Sci Asia 34:367–370
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria ISBN 3-900051-07-0, URL http://www.Rproject.org/.Accessed 8 Oct 2012
Rainbow PS, White SL (1989) Comparative strategies of heavy metal accumulation by crustaceans: zinc, copper and cadmium in a decapod, an amphipod and a barnacle. Hydrobiologia 174(3):245–262
Rand GM (1985) Behavior. In: Rand GM, Petrocelli SR (eds) Fundamentals of aquatic toxicology methods and applications. Hemisphere Publishing Corporation, Washington, p 221
Ray M, Bhunia AS, Bhunia NS, Ray S (2013) Density shift, morphological damage, lysosomal fragility and apoptosis of hemocytes of Indian molluscs exposed to pyrethroid pesticides. Fish Shellfish Immunol 35:499–512
Russo J, Lagadic I (2004) Effects of environmental concentrations of atrazine on hemocyte density and phagocytic activity in the pond snail Lymnaea stagnalis (Gastropoda, Pulmonata). Environ Pollut 127(2):303–311
Shuhaimi-Othman M, Nur-Amalina R, Nadzifah Y (2012, [125785]) Toxicity of metals to a freshwater snail, melanoides tuberculata. Sci World J 2012. https://doi.org/10.1100/2012/125785
Singh A, Jain DK, Kumar P (2010) Determination of LC50 of cadmium chloride in Heteropneustes fossilis. GERF Bull Biosci 1(1):21–24
Sminia T (1972) Structure and function of blood and connective tissue cells of the freshwater pulmonate Lymnaea stagnalis studies by electron microscopy and enzyme histochemistry. Z Zellforsch Mikrosk Anat 130:497–526
Sminia T (1981) Gastropods. In: Ratcliffe NA, Rowley AF (eds) Invertebrate blood cells, vol l. Academic Press, New York, pp 191–232
Sminia T, Van der Knaap WPW, Van Assett LA (1983) Blood cell types and blood cell formation in gastropod molluscs. Dev Comp Immunol 7:665–668
Suresh PG (1990) Studies on haemolymph constituents of Indoplanorbis exustus (Deshayes) and Lymnaea acuminata (Lamarck) f. rufescens (gray) and the effects of copper on the activity pattern of selected transaminases and phosphatases. Ph.D. Thesis submitted to the Cochin University of Science and Technology, Cochin-682016
Suresh P, Mohandas A (1989) Effect of pH on the number of circulating haemocytes in the freshwater gastropod, Indoplanorbis exustus (Deshayes). In: Banerjee et al., (ed) Proceedings of National Symposium on Emerging trends in Animal Haematology, Department of Zoology, Patna University, 63–66
Suterlin AM (1974) Pollutants and chemicals of aquatic animals prospective. Chem Senses Flavor 1:167–178
Tantulvesn M, Pornprapa P (1995) Water management and waste water treatment in fish pond no.1. Water quality management, 2nd edn. Chulalongkorn Press, Bangkok
Tiwari M, Napure NS, Saksena DN, Kumar R, Singh SP, Kushwaha B, Lakra WS (2011) Evaluation of acute toxicity levels and ethological responses under heavy metal cadmium exposure in freshwater teleost, Channa punctata (Bloch). Int J Aquat Sci 2(1):36–47
US EPA (1999) Probit Program Version 1.5. Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory, U. S. Environmental Protection Agency, Cincinnati, Ohio 45268. http://www.epa.gov/nerleerd/stat2.htm
US EPA (2016) Aquatic life—ambient water quality criteria cadmium 2016. U S Environmental protection Agency, Office of Water, Office of Science and Technology, Health and Ecological Criteria Division, Washington D C. https://www.epa.gov/sites/production/files/2016-03/documents/cadmium-final-report-2016.pdf
van Hatton B, De Voogt P, Van Den Bosch L, Van Straalen NM, Joosse EN, Govers H (1989) Bioaccumulation of cadmium by the freshwater isopod Asellus aquaticus (L.) from aqueous and dietary sources. Environ Poll 62(2–3):129–151
Vinodhini R, Narayanan M (2008) Effect of heavy metals induced toxicity on metabolic biomarkers in common carp (Cyprinus Carpio L.) Mj Int J of Sci Tech 2(01):192–200
Wang N, Ingersoll CG, Ivey CD, Hardesty DK, May TW, Augspurger T, Roberts AD, van Genderen E, Barnhart MC (2010) Sensitivity of early life stages of freshwater mussels (Unionidae) to acute and chronic toxicity of lead, cadmium, and zinc in water. Environ Toxicol Chem 29(9):2053–2063
Weir CF, Walter WM (1976) Toxicity of cadmium in the freshwater snail Physa gyrina. J Environ Qual 5:359–362
WHO (1992) Cadmium. Environmental Health Criteria. World Health Organisation, International Programme on Chemical Safety (IPCS), Geneva, p 134
Acknowledgements
The authors are thankful to the Principal and Head, P.G. Department of Zoology, Jhargram Raj College, Paschim Medinipur, West Bengal, for providing infrastructural facilities to complete the work. Authors are also thankful to the Department of Fisheries, Govt. of West Bengal, India, for providing the necessary permission to carry out the research work.
Funding
The authors thank the Principal, Jhargram Raj College, Paschim Medinipur, West Bengal, for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Dhara, K., Saha, N.C. & Maiti, A.K. Studies on acute and chronic toxicity of cadmium to freshwater snail Lymnaea acuminata (Lamarck) with special reference to behavioral and hematological changes. Environ Sci Pollut Res 24, 27326–27333 (2017). https://doi.org/10.1007/s11356-017-0349-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0349-8