Abstract
The insecticidal properties of delta-endotoxins from Bacillus thuringiensis (Bt) serotypes kurstaki and israelensis and crystal proteins of Bacillus sphaericus (Bs) serotype H5 have been used in insect control for decades. The availability of microbial toxins in biopesticides as well as in plants with incorporated protection has been increasing the concerns about biosafety. Acute toxicity to Danio rerio and cytotoxicity on mouse bone marrow cells and peripheral erythrocytes of Oreochromis niloticus were tested with Bt israelensis, Bt kurstaki and Bs H5 strains. The concentration and dose tested were 106 and 108 spores/ml, respectively. Neither lethality nor effects on mouse bone marrow were promoted by any strain. In necrosis–apoptosis study on peripheral erythrocytes of O. niloticus an increased frequency of necrotic cells caused by exposure to strains of B. thuringiensis was found. Exposure to B. sphaericus did not show cytotoxic effects in either tested system. None of the strains studied induced apoptosis in contrast with the chemical controls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The insecticidal properties of toxins from Bacillus have been used in insect control for a long time. The first commercial Bacillus thuringiensis product was produced in France in 1938 (Kumar et al. 1996). Advances in genetic engineering in recent years have led to the development of plants that present resistance to some insects through incorporation and expression of genes encoding δ-endotoxins from the bacterium B. thuringiensis. A number of plant species, particularly crops, such as cotton, corn, potatoes, tobacco, tomato, and sugarcane have been modified to produce δ-endotoxin proteins from B. thuringiensis (Prieto-Samsonov et al. 1997; Mendelsohn et al. 2003; Romeis et al. 2006; OECD 2007). Thus, the concern about exposure to microbial toxins from plants with incorporated protection as well as to biopesticides has been raised, due to potential adverse effects on non-target species in the environment, including aquatic ecosystems.
The toxicological database on B. thuringiensis shows no mammalian health effects attributable to δ-endotoxins (McClintock et al. 1995a, b). Short term feeding/toxicity studies on poultry, pigs, calves, and cattle also provided additional information on the behavior of Cry1Ab protein in the gastro intestinal tract. Cry1Ab was not completely degraded in the gastro-intestinal tract and fragments of the gene and/or immunoreactive protein fragments were still present in the intestinal content and in the feces, but no residual DNA/protein could be found in animal tissues nor in the peripheral blood, nor was any risk associated with these findings (Jennings et al. 2003; Chowdhury et al. 2003; Einspanier et al. 2004; Lutz et al. 2005; OECD 2007).
Bacillus sphaericus is a naturally occurring soil bacterium and that can effectively kill mosquito larvae. B. sphaericus spores that are eaten by mosquito larvae release toxins into the mosquito gut, causing the larvae to stop feeding and die. B. sphaericus is only effective against feeding larvae, and does not affect mosquito pupa or adults. No measurable health effects were seen in laboratory animals that were exposed to larvicidal concentrations of B. sphaericus by multiple routes of exposure. The larvicide property of B. sphaericus consists of two proteins of 51 and 42 kDa. Within the midgut the 51 and 42 kDa proteins are converted to 42 and 39 kDa proteins, respectively, resulting in a major toxicity (Baumann et al. 1991).
Oliveira-Filho (2007) presents an interesting review about the ecotoxicity of bioinsecticides indicating absence of adverse effects on aquatic and terrestrial species. But the release of microbial toxins in the environment constantly generates the need for more data on biosafety, and consequently new toxicological evaluations should be undertaken to support the risk assessment of each new strain and toxin discovered.
Bone marrow is a highly vascularized tissue, and it contains a population of rapidly cycling cells that can be readily isolated and processed. The kinetics of bone marrow cells’ progression can be measured through the ratio between polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE), which is about 1:1 in the young adult mouse. PCE is a transient cell precursor of NCE (mature erythrocyte). Declines in PCE frequency compared with NCE after 24 h of exposure to a toxic chemical compound means inhibition of the kinetics of cell progression, which can be recognized as cytotoxicity (Schmid 1975, Salamone and Heddle 1983; OECD 1997). The cell viability assay is recommended to study cell death induced by toxin exposure.
Cell viability assessment based on the double-stain with fluorescent DNA-binding acridine orange/ethidium bromide (AO/EtBr-1:1) allows viable cells to be distinguished from necrotic and apoptotic cells. Cell death caused by an exogenous damage will result in necrosis. Otherwise, cells with DNA severely damaged are arrested at the G1 phase. If the DNA repair process fails, the p53 gene is activated and initiates apoptosis (Cotran et al. 1999; Müllauer et al. 2001; Golstein and Kroemer 2007; Puttonen et al. 2008).
The aim of this study was to carry out an investigation on the acute toxicity and cytotoxicity of strains of B. thuringiensis serotypes kurstaki and israelensis and B. sphaericus serotype H5 on the survival of Danio rerio, and on cell viability of peripheral blood erythrocytes of Oreochromis niloticus and cell proliferation of mouse bone marrow.
Material and methods
Bacillus strains tested
Three Brazilian entomopathogenic strains were used in this study. Each one represented a different serotype: B. thuringiensis serotype kurstaki, encoding Cry1Aa, Cry1Ab, Cry1Ac, and Cry1B proteins, toxic to lepidopteran larvae (Monnerat et al. 2007), B. thuringiensis serotype israelensis, encoding Cry4A, Cry4B, Cry11, and cyt1 proteins, toxic to dipteran larvae (Monnerat et al. 2005) and B. sphaericus serotype H5 encoding 51 and 42 kDa proteins, toxic to dipteran larvae (Monnerat et al. 2004). These strains were isolated from Brazilian soils and are stored at a collection of entomopathogenic Bacillus spp. of Embrapa Genetic Resources and Biotechnology, Brazil.
The strains were tested in a maximum hazard concentration (106 spores/ml) as proposed by USEPA (1996) for fish acute toxicity and 108 spores/ml for cytotoxicity studies. Concentrations were determined by serial dilutions after counting the number of spores in a concentrate of around 108–1010 spores/ml in NYSM medium (Silva et al. 2002).
Acute toxicity assay in Danio rerio
Thirty day static-renewal acute assays with zebrafish (D. rerio) were conducted as reported in a standardized protocol (USEPA 1996). Zebrafish used in this study was a wild type purchased from a local commercial supplier in Brasília. After a week of acclimatization on laboratory conditions, tests were performed in 3,000 ml beakers containing synthetic softwater (pH 7.5 ± 0.1, water hardness 40–48 mg l−1 as CaCO3), maintained at 25 ± 1°C under a 16 h light/8 h dark cycle. Twenty fish were exposed in duplicate (10 per beaker) to each concentration (1 × 106 and 5 × 106 spores/ml−1) of the tested strains. Testing solutions were replaced once a week and fish mortality was recorded daily until the 30th day.
Necrosis/apoptosis assay in Oreochromis niloticus
Oreochromis niloticus used in this study were obtained from a fish farm of the local municipality, where breeding conditions were controlled and monitored constantly. The criterion for fish selection was body length of 7–10 cm. Fish were acclimatized in the Genetics Laboratory of the University of Brasilia for a week in tanks of 250 l volume, with filtered and dechlorinated tapwater continuously aerated. Fish were maintained at a constant temperature of 25 ± 2°C and fed with fish chow. The ammonium level in the water was constantly monitored and the water was periodically renewed. Fish were randomly placed in other aquaria in groups of 7 and treatments were carried out through intra-abdominal injection of 0.2 ml of the Bt israelensis, Bt kurstaki, and Bs H5, at a concentration of 1 × 108 spores/ml and observed for 72 h. For positive chemical control, fish were treated intra-abdominally with cyclophosphamide (CP) at 30 mg kg−1 body weight. CP (Genuxal–Asta Medica) is a well-known DNA alkylating agent commonly used as positive control in genotoxicity studies. For the apoptosis test, 0.1 ml of peripheral blood was obtained from cardiac puncture and diluted in 2.0 ml of fetal bovine serum at room temperature of 23°C. A smear of 15 μl of cell suspension was made immediately and 1 μl of AO/EtBr stain was added and the slides were covered with a coverslip. Slides were analyzed with a fluorescence Axioskop 2 Zeiss microscope with 1000× magnification using a wavelength of 510–560 nm. Around 500 peripheral erythrocytes were analyzed and classified as viable, necrotic or apoptotic.
Mouse bone marrow assay
Swiss mice from the Central Animal Facility of the University of Brasília were acclimatized to laboratory conditions for 1 week prior to the study. Males and females (10–12 weeks old), weighing 30 ± 2 g, were fed Purina mice chow and filtered water ad libitum. The negative control received filtered water (100 μl via gavage). They were housed at random, in groups of 6, dosed with test-solution of Bt israelensis, Bt kurstaki, and Bs H5 at a volume of 100 μl, via gavage for 24 h. Animals were sacrificed by cervical dislocation and the bone marrow preparations for polychromatic and normochromatic erythrocyte identification followed the protocol proposed by Schmid (1975). After smear, slides were fixed with methanol and stained with Giemsa. One thousand cells per animal were scored and classified as polychromatic (PCE) or normochromatic (NCE). The relationship between PCEs/NCEs was determined by the first 1,000 PCEs or NCEs counted.
Statistical analysis
Results of necrosis/apoptosis assay in O. niloticus were statistically analyzed by t-test for paired samples. Differences among treated and control groups of mice exposed to Bt and Bs-toxins were analyzed by t-test for paired samples. Both analysis were performed through computer software package SigmaStat 3.5.
Results
Neither mortality nor visible adverse effects were observed in D. rerio or O. niloticus exposed to tested concentrations of all bacterial strains. This observation supports that the lethal concentrations (LC50) of these strains to these fish species are higher than 5 × 106 spores/ml.
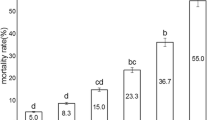
In the necrosis–apoptosis study on peripheral erythrocytes of O. niloticus, an increased frequency of necrotic cells caused by exposure to strains Bt israelensis and Bt kurstaki was found (Table 1, t-test P < 0.05). On the other hand, exposure to Bs H5 did not cause cytotoxic effect in either tested system, compared with the controls (t-test P > 0.05). Thus, none of studied strains induced apoptosis, which means no genotoxicity. In contrast, fish exposed to cyclophosphamide, a positive chemical control, presented a statistically significant increased number of both necrotic and apoptotic cells (Table 1, t-test P < 0.05).
The data on cell cycle kinetics in mouse bone marrow cells shown in Table 2 indicate no effect on cell proliferation by the three strains tested Bt israelensis, Bt kurstaki, and Bs H5 compared to control, which means absence of cytotoxicity. There was also no increased toxicity as a function of time, comparing results of 48 with 96 h of exposure. Our analysis was based in the higher variations of NCEs population in comparison with PCE population.
Discussion
The mode of action of Cry-toxins on susceptible insects is dependent on enzymatic activation after ingestion. The crystalline inclusions are dissolved and then converted to active toxins by insect proteases. The active Cry-toxins bind to specific receptor sites and produce pores in the insect gut which results in loss of homeostasis and septicemia, which are lethal to the insect (Broderick et al. 2006). There are no known equivalent receptor sites for binding of the δ-endotoxins in mammals (Noteborn et al. 1995; Gill and Ellar 2002; Broderick et al. 2006). The mode of action also appears to be insect specific due to the reliance of the lepidopteran midgut on unique ATPases for potassium influx regulation and the insect midgut’s unique susceptibility to ionic stress (Knowles and Ellar 1986), plus the observations that even when Cry toxin binding site proteins are expressed in mammalian cells, the mammalian cells are unable to express them in a form that allows the toxins to bind to them (Keeton and Bulla 1997; Gill and Ellar 2002; Broderick et al. 2006). On the other hand, Mizuki et al. (1999) and Kim et al. (2000), reported in vitro studies on cytotoxicity of Bt-protein on human cells. After inclusion into the cytoplasm, these Bt-proteins were activated through an alkali-proteolytic process becoming highly cytocidal. This study was carried out with leukaemic T cells, Hela cells, and normal T cells, and the major cytocidal activity was found only on leukemic T cells.
In the mouse bone marrow assay, evidence of toxicity is given by reduction of %PCE (Salamone and Hiddle 1983). Population of PCE was not affected because the relationship between PCEs/NCEs determined by the first 1,000 PCEs or NCEs counted always showed more PCEs than NCEs. Thus, no bone marrow suppression was noted.
Apoptosis is a distinct form of cell death that proceeds along a genetically determined execution program. It is a form of cell death designated to eliminate unwanted cells in a tissue through activation of a coordinated, internally programmed series of events genetically controlled (Cotran et al. 1999). Exposure of cells to genotoxic agents, such as radiation and chemotherapeutic compounds induces apoptosis by a mechanism that is initiated by DNA damage. Apoptosis–necrosis analysis to study cell viability and mode of cell death induced by toxins using fluorescent double stain is rapid, repeatable and easy to perform. It allows the determination of the viability/death ratio and also it is possible to distinguish apoptosis from necrosis. Acridine orange reaches the nucleus of intact cells and binds both RNA and DNA, whereas ethidium bromide is generally excluded from those cells with an intact plasma membrane. If the membrane is broken due to a necrotic effect, the dye readily reaches the nucleus to intercalate with DNA. Dead cells, necrotic and late apoptotic cells have disrupted cellular membranes, which allow EtBr reach the nucleic acid in the nucleus. The incoming DNA-dye complex intensifies the level of fluorescence over that of the dye alone. In the early apoptosis only AO reaches the nucleus showing the fragmentation of chromatin and the apoptotic bodies, through green fluorescence given exclusively by AO. This method provides more realistic information about the mode of action of cell death (Puttonen et al. 2008). Necrosis is a cell death due to progressive degradative action of enzymes, characterized by denaturation of cytoplasmic proteins caused by exogenous injury. Necrosis can be started by non-specific external stimuli, such as ischemia, trauma, infection, cellular membrane receptor blockade or surface receptor damage (Cotran et al. 1999).
Fish treated with CP at 30 mg kg−1 presented necrotic as well as apoptotic effects, probably due to the high dose administered. Lower doses probably could cause more apoptosis than necrosis, because CP works as a clastogenic agent causing chromatin fragmentation. Probably, 30 mg kg−1 is above the apoptotic threshold dose. Brockmann et al. (2006) showed that apoptosis initiates at much lower doses than cytotoxic doses when cells are exposed to alkylating chemicals. Positive control is used to demonstrate the sensitivity of the test-system. No induction of apoptosis could mean that toxins from B. thuringiensis and B. sphaericus to not reach the cell nucleus causing DNA or chromatin damage. It should be pointed that cell death by necrosis involved a large population of cells due to the presence of the Bt and Bs-toxins in high concentrations, contrasting to apoptosis, which is an individual event. Thus, necrosis was observed due to an extremely invasive route for fish exposure.
Conclusions
This study showed that acute toxicity of Bacillus strains against fish were not observed in the concentrations tested, suggesting that LC50 s of these strains are higher than 5 × 106 spores/ml. On the contrast, by non-conventional routes of exposure (e.g., injection) Bt israelensis and Bt kurstaki toxins showed toxicity causing peripheral erythrocyte cells death by necrosis. Thus, our results are coherent with the toxicological mechanism of action of such toxins on target cells, which means binding with the cell receptor causing cell membrane disruption. In fish exposed through whole body, no toxicity was found confirming literature data that Bt and Bs have low toxicity to non-target aquatic species.
Acknowledgments
Research supported by SEG/Embrapa. Madaí Cruz Lopes received a fellowship from PIBIC/CNPq (Brazilian Ministry of Science and Technology).
References
Baumann P, Clark MA, Baumann L, Broadwell AH (1991) Bacillus sphaericus as a mosquito pathogen: properties of the organism and its toxins. Microbiol Rev 55:425–436
Broderick N, Raffa FK, Handelsman J (2006) Midgut bacteria required for Bacillus thruingiensis insecticidal activity. Proc Natl Acad Sci USA 103:15196–15199. doi:10.1073/pnas.0604865103
Brockmann WG, Kostoryz EL, Eick JD (2006) Correlation of apoptotic potential of simple oxiranes with cytotoxicity. Toxicol In Vitro 20:729–735. doi:10.1016/j.tiv.2005.10.009
Chowdhury EH, Kuribara H, Hino A, Sultana P, Mikami O, Shimada N et al (2003) Detection of corn intrinsic and recombinant DNA fragments and Cry1AB protein in the gastrointestinal contents of pigs fed genetically modified corn Bt11. J Anim Sci 81:2546–2551
Cotran RS, Kumar V, Collins T (1999) Cellular pathology I: cell injury and cell death. In: Cotran RS, Kumar V, Collins T (eds) Robbins pathologic basis of disease. Saunders, Philadelphia, pp 1–28
Einspanier R, Lutz B, Rief S, Berezina O, Zverlov V, Schwarz W et al (2004) Tracing residual recombinant feed molecules during digestion and rumen bacterial diversity in cattle fed transgene maize. Eur Food Res Technol 218:269–273. doi:10.1007/s00217-003-0842-9
Gill M, Ellar D (2002) Transgenic Drosophila reveals a functional in vivo receptor for the Bacillus thuringiensis toxin Cry1Ac1. Insect Mol Biol 11:619–625. doi:10.1046/j.1365-2583.2002.00373.x
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32:37–43. doi:10.1016/j.tibs.2006.11.001
Jennings JC, Albee LD, Kolwyck DC, Surber JB, Taylor ML, Hartnell GF et al (2003) Attempts to detect transgenic and endogenous plant DNA and transgenic protein in muscle from broilers fed YieldGard Corn Borer corn. Poult Sci 82:371–380
Keeton TP, Bulla LA Jr (1997) Ligand specificity and affinity of BT-R1, the Bacillus thuringiensis Cry1A toxin receptor from Manduca sexta, expressed in mammalian and insect cell cultures. Appl Environ Microbiol 63:3419–3425
Kim HS, Yamashita S, Akao T, Saitoh H, Higuschi K, Park YS et al (2000) In vitro cytotoxicity on non-Cyt inclusion proteins of a Bacillus thringiensis isolate against human cells, including cancer cells. J Appl Microbiol 89:16–23. doi:10.1046/j.1365-2672.2000.01087.x
Knowles BH, Ellar DJ (1986) Characterization and partial purification of a plasma membrane receptor for Bacillus thuringiensis var kurstaki lepidopteran-specific delta-endotoxin. J Cell Sci 83:89–101
Kumar PA, Sharma PP, Malik VS (1996) The insecticidal proteins of Bacillus thuringiensis. Adv Appl Microbiol 42:1–43
Lutz B, Wiedemann S, Einspanier R, Mayer J, Albrecht C (2005) Degradation of Cry1Ab protein from genetically modified maize in the bovine gastrointestinal tract. J Agric Food Chem 53:1453–1456. doi:10.1021/jf049222x
McClintock JT, Schaffer CR, Kough JL, Sjoblad RD (1995a) Relevant taxonomic considerations for regulation of Bacillus thuringiensis based pesticides by the US Environmental Protection Agency. In: Feng TY (ed) Bacillus thuringiensis biotechnology and environmental benefits. Hua Shiang Yuan Publishing, Taiwan, pp 313–325
McClintock JT, Schaffer CR, Sjoblad RD (1995b) A comparative review of the mammalian toxicity of Bacillus thuringiensis based pesticides. Pestic Sci 45:95–105. doi:10.1002/ps.2780450202
Mendelsohn M, Kough J, Vaituzis Z, Mathews K (2003) Are Bt crops safe? Nat Biotechnol 21:1003–1009. doi:10.1038/nbt0903-1003
Mizuki E, Ohba M, Akao T, Yamahita S, Saitoh H, Park YS (1999) Unique activity associated with non-insecticidal Bacillus thringiensis parasporal inclusions: in vitro cell-killing action on human cancer cells. J Appl Microbiol 86:477–486. doi:10.1046/j.1365-2672.1999.00692.x
Monnerat RG, Silva S, Dias D, Martins E, Praça L, Jones G et al (2004) Screening of high toxic Brazilian Bacillus sphaericus strains against Culex quinquefasciatus, and Aedes aegypti. J Appl Entomol 128:469–473. doi:10.1111/j.1439-0418.2004.00874.x
Monnerat RG, Dias D, Silva S, Martins E, Berry C, Falcão R et al (2005) Screening of Bacillus thuringiensis strains effective against mosquitoes. PAB 40:103–106
Monnerat RG, Batista AC, Medeiros P, Martins E, Melatti VM, Praça L (2007) Screening of Brazilian Bacillus thuringiensis isolates active against Spodoptera frugiperda, Plutella xylostella, and Anticarsia gemmatalis. Biol Control 41:291–295. doi:10.1016/j.biocontrol.2006.11.008
Müllauer L, Gruber P, Sebinger D, Buch J, Wohlfart S, Chott A (2001) Mutation in apoptosis genes: as pathogenic factor for human disease. Mutat Res 488:211–231. doi:10.1016/S1383-5742(01)00057-6
Noteborn HPJ, Bienenmann-Ploum ME, van den Berg JHJ, Alink GM, Zolla L, Reynaerts A et al (1995) Safety assessment of the Bacillus thuringiensis insecticidal crystal protein CRYIA(b) expressed in tomato. In: Engel KH, Takeoka GR, Teranishi R (eds) Genetically modified foods: safety issues. American Chemical Society, Washington, pp 134–147
OECD, Organisation for Economic Co-operation and Development (1997) Mammalian bone marrow chromosome aberration test. Test no. 475, OECD, Italy.
OECD, Organisation for Economic Co-operation and Development (2007) Consensus document on safety information on transgenic plants expressing Bacillus thuringiensis-derived insect control proteins. Series on harmonization of regulatory oversight in biotechnology number 42. ENV/JM/MONO(2007)14.
Oliveira-Filho EC (2007) Avaliação da periculosidade ambiental de bioinseticidas como uma nova perspectiva para a ecotoxicologia no Brasil. J Braz Soc Ecotoxicol 2: 289–295.
Prieto-Samsonov DL, Vazquez-Padron RI, Ayra-Pardo C, Gonzalez-Cabrera J, de la Riva GA (1997) Bacillus thuringiensis: from biodiversity to biotechnology. J Ind Microbiol Biotechnol 19:202–219. doi:10.1038/sj.jim.2900460
Puttonen KA, Lehtonen S, Lampela P, Männistö PT, Raasmaja A (2008) Different viabilities and toxicity types after 6-OHDA and Ara-C exposure evaluation by four assays in five cell lines. Toxicol In Vitro 22:182–189. doi:10.1016/j.tiv.2007.07.005
Romeis J, Meissle M, Bigler F (2006) Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat Biotechnol 24:63–71. doi:10.1038/nbt1180
Salamone MF, Heddle JA (1983) The bone marrow micronucleus assay: rationale for a revised protocol. In: de Serres FJ (ed) Chemical mutagens––principles and methods for their detection. Plenum Press, New York, pp 111–143
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Silva SF, Dias JMCS, Monnerat RG (2002) Comparação entre três métodos de isolamento de bacilos entomopatogênicos. Circular Técnica no. 14, Embrapa Recursos Genéticos e Biotecnologia, Brasília
USEPA, United States Environmental Protection Agency (1996) Microbial pesticide test guidelines OPPTS 885.4200 Freswater fish testing, Tier I. USEPA, Washington. EPA 712-C-96–332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grisolia, C.K., Oliveira-Filho, E.C., Ramos, F.R. et al. Acute toxicity and cytotoxicity of Bacillus thuringiensis and Bacillus sphaericus strains on fish and mouse bone marrow. Ecotoxicology 18, 22–26 (2009). https://doi.org/10.1007/s10646-008-0252-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0252-7