Abstract
The pine processionary moth Thaumetopoea wilkinsoni (Tams, 1926) (Lepidoptera: Notodontidae) is one of the most harmful species that causes destruction in pine ecosystems and also causes critical skin reactions in humans and animals due to its urticating hairs. This study aimed to evaluate the efficacy of different strains [Bti ATCC 35646 (wild-type strain), Bti pHT315, and Bti pHTppk (mutant strain)] of Diptera-targeted Bacillus thuringiensis subsp. israelensis (Bti) and toxins (spore/crystal mixtures) obtained from these strains against the 4th larval stage of T. wilkinsoni under laboratory conditions. T. wilkinsoni eggs were collected from pine trees at Ondokuz Mayıs University in Samsun, Turkey, in 2022. Pine needles were contaminated with bacterial strains of different concentrations (1 × 107, 1 × 108, 1 × 109 cfu/mL) in 5 mL and toxins in different concentrations (0.15, 0.3, 0.6, 1.25, 2.5, 5 mg/mL) in 2 mL. Larvae were placed in containers with 30 larvae in each group and were observed for 25 days. As a result of the study, Bti ATCC 35646 with 63.4% mortality and the lowest LC50 value of 1.2 × 109 cfu/mL among the three strains of Bti and Bti pHTppk with 76.7% mortality and the lowest LC50 value of 1.2 mL among the toxins from the Bti strains had found to be virulent for T. wilkinsoni. The results obtained from our study suggest that Diptera-targeted Bti is virulent to T. wilkinsoni and that this strain and spore/crystal mixture can be used in the biological control of this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pinaceae is the largest family of conifers including more than 200 species common throughout the Northern Hemisphere (Farjon, 2017). One of the most important genera belonging to this family is Pinus. Distinct parts of trees belonging to the Pinus genus are rich in Mg, P, Na, Fe, Cu, and Zn minerals as well as high oil content (Dziedziński et al., 2021). Additionally, Pinus species have essential oils that exhibit antioxidant (Abbou et al., 2019; Bouyahya et al., 2019), anticoagulant (Abbou et al., 2019), antibacterial (Demirtaş, 2021), anticancer (Khatamian et al., 2022), antifungal (Karličić et al., 2021), anti-inflammatory (Abbou et al., 2019), anti-hemolytic (Meziti et al., 2019), larvicide (Koutsaviti et al., 2015; Mitić et al., 2019), and herbicide (Hamrouni et al., 2015) properties. Essential oils from these species and the leaves of the trees are used in traditional practices for treating various diseases, including diarrhea, wounds, rheumatism, cough, gastrointestinal diseases, hypertension, and hemorrhoids (El Omari et al., 2021). Besides, the bark, needle, and other parts of these species have been used as excellent raw materials for many years (Li et al., 2015).
The pine processionary moth Thaumetopoea wilkinsoni (Tams, 1926) (Lepidoptera: Notodontidae) is a pest invading the Pinus species, which is important medically, economically and ecologically. This insect is an important defoliator of pine forests. Pine processionary moth larvae can cause deformations in saplings, stunting, and loss of yield in pine nuts (Aydın et al., 2018), and even indirectly cause tree death by facilitating the attacks of other pests, mostly bark beetles (Barta et al., 2020). Additionally, the larvae of this species also cause health problems by causing painful skin irritation, rashes, and allergic reactions in humans and animals because their hair contains the urticating protein thaumetopoein (Barta et al., 2020).
In addition to being a major forest pest, it has extremely harmful effects in terms of public health, so it is inevitable to control this insect. Chemical and mechanical-physical methods are generally used in controlling this pest (Cebeci et al., 2010). Considering both the difficulties in mechanical-physical control and the adverse effects of insecticides used in chemical control on human health and other organisms, biological control methods should be preferred in the control of this species (Onaran and Kati, 2010). Various studies have been conducted in this context on nematodes (Karabörklü et al., 2015) and fungi (Güven et al., 2021; Topkara et al., 2022; Yanar et al., 2023), as well as essential oils and leaf extracts (Semiz, 2017; Faria, 2021). Bacteria are the most commonly used species in the control of this species within the scope of biological control (Gindin et al., 2007a, 2008; Yılmaz et al., 2013). Bacillus thuringiensis (Bt), an entomopathogenic microorganism, is a ubiquitous Gram-positive bacterium (Rabha et al., 2023). Bt produces various proteins toxic to different invertebrates (Santos et al., 2022). Protoxins of Bt produced in an inactive form are proteolytically activated by the midgut protease of the target insect. Activated toxins interact with midgut receptors in target insects, and then migrate to the cell membrane to form pores, destroying the midgut epithelium and insect death (Bravo et al., 2007; Vachon et al., 2012; Pardo-López et al., 2013). Bacillus thuringiensis subsp. israelensis (Bti) is the first subspecies of Bt used as an effective biological control agent against the larvae of many Diptera species in the world (Ben-Dov, 2014). The effectiveness of Bti in other insects (Federici and Bauer, 1998; Porcar et al., 2009; Bordalo et al., 2020; Tudoran et al., 2021; Yanar et al., 2022) as well as in flies and mosquitoes (Abuldahab et al., 2019; Nasser et al., 2021; Poulin et al., 2022) has also been examined.
Many studies have been conducted to obtain a hypertoxic mutant strain and increase the efficacy of Bti (Doruk et al., 2013; Bahareth et al., 2018; Zorzetti et al., 2018; Valtierra-de-Luis et al., 2020; Ioannou et al., 2021). In one of these studies, Doruk et al. (2013) found that increased polyphosphate (polyP) levels in the cells affect bioinsecticide biosynthesis by Bti. They overexpressed the polyphosphate kinase gene in Bti, which is responsible for polyP synthesis, and demonstrated that this recombinant strain (Bti pHTppk) is approximately eight times more toxic than the wild type against late the 2nd instar laboratory-reared Culex quinquefasciatus (Say, 1823) (Diptera: Culicidae) larvae.
This study aimed to determine the effectiveness of different strains of Bti [Bti ATCC 35646 (wild-type strain), Bti pHT315, and Bti pHTppk (mutant strain)] and the toxins (spore/crystal mixtures) obtained from these strains against the 4th instar larvae of T. wilkinsoni. Most of the studies have shown the effects of bacterial strains on the early stages of insects (Gindin et al., 2007b; Cebeci et al., 2010). In this study, our purpose in using the 4th instar larvae was to determine the effects of the bacterial strains used in the study on the late instar of T. wilkinsoni larvae.
MATERIALS AND METHODS
Obtaining Larvae
Thaumetopoea wilkinsoni eggs were collected from the needles of Pinus sylvestris L. at Ondokuz Mayıs University Kurupelit Campus in Samsun, Turkey (41°22′26.5116′′ N 36°13′17.6340′′ E) in 2022 and brought to the laboratory. The eggs were disinfected with 10% sodium hypochlorite (Code: 105614, Merck, Darmstadt, Germany) for about 7 min, and then washed with distilled water for about 7 min and rinsed. The disinfected eggs were taken to the air-conditioning room at a relative humidity of 70% and 24°C (12 h dark/12 h light). The hatched larvae were given pine needles to feed on.
Strains
Bti ATCC 35646 was kindly provided by Gwo-Chyuan Shaw (National Yang-Ming University, Taiwan), control strain harboring pHT315 (Bti pHT315) and ppk overexpressing strain (Bti pHTppk) were from our previous study (Doruk et al., 2013). Briefly, the polyphosphate kinase gene (ppk) of Bti (GenBank: EAO55476.1) with its own promoter was amplified from the genomic DNA of Bti using forward (5′ GTGAACAGTCTGCATTAGCAG 3′) and reverse (5′ GCCGCCAGCACCTTATCCTTG 3′) primers and was cloned into pHT315 plasmid (Arantes and Lereclus, 1991). The resultant plasmid (pHTppk) was introduced into Bti via electroporation. Bti cells carrying empty pHT315 plasmid were used as control.
Protein Extraction for Toxicity Measurements
Proteins were extracted according to the procedure of Donovan et al. (1988). 10 mL of cells (spore/crystal mixture) from 72 h grown cultures were harvested, and washed once with 5 mL of 1 M NaCl (Code: 106404, Merck, Darmstadt, Germany) and twice with 5 mL of deionized water. Then, the cells were suspended in deionized water at 100 mg wet cell weight per mL. Equal amounts of lysis buffer [TE buffer (100 mM Tris-HCl, 20 mM EDTA pH 7.4) containing 10 mg/mL lysozyme] were added to the cell suspension and incubated at 37°C for 30 min. After incubation, 10 µL of 2% SDS was added and the suspension was vortexed for 30 s, centrifuged for 5 min in a microfuge and then the pellet (spore/crystal mixture) was suspended in 50 µL of 0.2% SDS. Protein concentrations were determined by the BCA Protein Assay Method with bovine serum albumin as the standard (Smith et al., 1985).
Larvicidal Activity
Larvae were exposed to spore/crystal mixtures isolated from equal volumes of each bacterial strain. 2 mL of serially diluted spore/crystal mixtures ranging from 5 to 0.15 mg/mL were applied to P. sylvestris needles on the boxes. Thirty of the fourth instar larvae were then placed in the boxes and the viability of the larvae was checked daily. On the first day of the experiment, the needles given to the bacteria/toxin applied groups were consumed by the larvae in a short time. Thus, it was ensured that the larvae received the bacteria/toxins into their bodies. After the first needles were consumed by the larvae, fresh needles were given every day to all experimental groups, including the control groups. LC50 values were determined at the end of the 25th day by taking the average results of three independent experiments.
The Experimental Setup
The hatched larvae were fed with the needles of the P. sylvestris (disinfected with 50% ethyl alcohol, and then washed with distilled water), and the fourth instar larvae were used for the experiment. The experiment was carried out in two stages. In the first stage, different concentrations (1 × 107, 1 × 108, and 1 × 109 cfu/mL) of Bti ATCC 35646, Bti pHT315, and Bti pHTppk strains were poured into separate containers with 5 mL for each dose and spread all over the plant needles. After plant needles infested with bacteria were placed in containers, the 4th instar larvae were placed in containers with 30 larvae per container and three replicates per group. A total of 900 larvae, including the control group, were used at this stage.
In the second stage, different concentrations (0.15, 0.3, 0.6, 1.25, 2.5, and 5 mg/mL) of toxin (spore/crystal mixture) of Bti ATCC 35646, Bti pHT315, and Bti pHTppk strains of 2 mL were applied to P. sylvestris needles for each group, and then the 4th instar larvae were placed in containers with 30 larvae per container and three replicates per group. The experiment was carried out in three repetitions and a total of 1710 larvae, including the control group, were used for the experiment. In both stages of the experiment, disinfected P. sylvestris needles were given to the larvae in the control group. In both stages, the larvae were observed for 25 days.
Statistical Analyses
The Kaplan–Meier test was used to calculate the survival rates of T. wilkinsoni larvae. The survival rates of the T. wilkinsoni larvae infected with different concentrations of three different Bti strains and toxins from these strains were compared with the control group with the Log-Rank test. The Cox-Regression analysis was used to compare the mortality risk of larvae exposed to three different Bti strains and toxins from these strains. The lethal dose (LC50) was calculated using Probit analysis. SPSS version 22.0 was used for these tests.
RESULTS
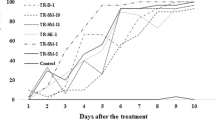
The survival rates of T. wilkinsoni larvae infected with Bti ATCC 35646, Bti pHT315, and Bti pHTppk strains and toxins from these strains are shown in Table 1. Among the groups infected with different Bti strains, the lowest survival rate was found in the group infected with the highest concentration (1 × 109 cfu/mL) of Bti ATCC 35646 (36.6%). The survival rates in larvae exposed to different concentrations of Bti ATCC 35646 were between 36.6–61.1%, in those exposed to Bti pHT315 were between 43.3–63.3%, and in those exposed to Bti pHTppk were 47.7–64.4. The highest survival rate was in the control group (95.5%). Among the survival rates of T. wilkinsoni larvae exposed to toxins from Bti strains, the lowest survival rate was in larvae exposed to the highest concentration (5 mg/mL) of Bti pHTppk (23.3%). The survival rates in larvae exposed to different concentrations of Bti ATCC 35646 toxin were between 44.4–94.4%, in those exposed to Bti pHT315 toxin were between 46.7–93.3%, and in those exposed to Bti pHTppk toxin were between 23.3–83.3%. It was determined that the highest survival rate was in the control group (97.8%) (Fig. 1).
According to the Log-Rank test results, it was found that the control group was statistically different from the infected larvae exposed to Bti strains, but there was no statistical difference between the infected larvae (Table 2). In the groups infected with toxins, it was determined that the control group was statistically different from the larvae of the groups infected with toxins. It was noted that there was no difference between the groups in which the Bti ATCC 35646 and Bti pHT315 toxins were applied, but the group in which Bti pHTppk was applied was statistically different from the other two groups.
According to the results of Cox regression analysis, it was determined that the Bti ATCC 35646 strain increased the risk of death 14 times, and the Bti pHTppk strain increased the risk of death 11 times. While the Bti ATCC 35646 toxin increased the risk of death 14 times, the Bti pHTppk toxin increased the risk of death 25 times (Table 3).
LC50 values for the 4th instar T. wilkinsoni larvae fed with P. slyvestris needles exposed to different Bti strains and toxins from these strains were shown in Table 4. Accordingly, the Bti ATCC 35646 strain had the lowest LC50 value (1.2 × 109 cfu/mL), while the Bti pHT315 strain had the highest LC50 value (1.8 × 109 cfu/mL). Among the larvae exposed to different Bti toxins, the lowest LC50 value was in the Bti pHTppk group (1.2 mL), while the highest LC50 value was in the Bti pHT315 group (5.3 mL).
Mean survival times for different Bti strains and toxins from these strains applied to T. wilkinsoni larvae were shown in Table 5. Among the groups exposed to Bti strains, the shortest survival time (12.9 days) was found in the group exposed to the highest concentration of Bti ATCC 35646. The survival times of larvae exposed to different concentrations of Bti ATCC 35646 were between 12.9–17.7 days, those exposed to Bti pHT315 were between 14.7–18.0 days, and those exposed to Bti pHTppk were between 16.1–18.0 days. The longest survival time was found in the control group (24.2 days). In the groups exposed to different Bti toxins, it was noted that the shortest survival time (10.8 days) was in the group exposed to the highest concentration of Bti pHTppk. The survival times of larvae exposed to different concentrations of Bti ATCC 35646 toxin were between 15.7–23.9 days, those exposed to Bti pHT315 toxin between 16.2–23.8 days, whereas those exposed to Bti pHTppk were between 10.8–22.0 days. The longest survival time was in the control group (24.6 days).
DISCUSSION
Bt strains are thought to have insecticidal activity for target pests belonging to one or more orders, but the contrary has been proven in studies (Redmond et al., 2020). In this study, the effectiveness of different strains of Diptera-targeted Bti (Bti ATCC 35646, Bti pHT315, and Bti pHTppk) and the toxins (spore/crystal mixtures) from these strains against the 4th instar larvae of T. wilkinsoni were determined.
It was determined that the highest survival rates of T. wilkinsoni larvae infected with different Bti strains and toxins from these strains were in the control groups. The lowest survival rate of larvae infected with different strains of Bti was found in the group exposed to the highest concentration (1 × 109 cfu/mL) of Bti ATCC 35646. The lowest survival rate of larvae infected with toxins from Bti strains was noted in the group exposed to the highest concentration (5 mg/mL) of Bti pHTppk. There are two interesting results here. The first is that the survival rate of larvae exposed to toxins from strain Bti pHTppk is about half that of those exposed to this strain. In fact, the lowest survival rate among the groups exposed to both the Bti strains and the toxins from these strains was in the group exposed to the toxin obtained from the Bti pHTppk strain. Crystal toxins or δ-endotoxins are considered the main factor conferring insecticidal properties to Bt (Bouslama et al., 2020). This idea is in line the result obtained from our study.
The second interesting result is that the Bti ATCC 35646 strain at the highest concentration had the lowest survival rate, while the larvae exposed to the spore/crystal mixture from this strain increased in survival rate. This did not meet our expectations because we expected that those exposed to Bti pHTppk toxin would have the lowest survival rate in larvae infected with the Bti pHTppk strain. The mutant strain we used in our study was obtained from the study conducted by Doruk et al. (2013). The related authors determined that the mutation created a stress situation in the bacteria and the mutant strain produced more sigma factor (σE), which enables the adaptation of bacteria to stress conditions, in all samples studied. We think that the toxicity of the mutant strain, which is currently under stress, may have decreased because it has a disadvantage compared to the wild strain in adapting to field conditions and maintaining its viability.
In addition, the survival rates of the groups exposed to both Bti strains and toxins from these strains decreased with concentration increases. Guo et al. (2020) reported that the larval mortality rates increased with the concentration increase of the Bt Cry1Ac toxin applied to Plutella xylostella larvae. Ramasubramanian et al. (2022), on the other hand, noted that the larval mortality rates increased with the concentration increases of Bacillus applied to the 3rd and the 4th instar Cnaphalocrocis medinalis. These findings coincide with our results.
In previous studies, the effectiveness of commercial Bt products was evaluated against the larvae of pine processionary moths T. wilkinsoni and T. pityocampa. Cebeci et al. (2010) applied Bacillus thuringiensis subsp. kurstaki (Btk) bioinsecticides Foray 76B and VBC 60074, which were based on a mixture of spores and parasporal crystals of Btk strain ABTS-351, to T. pityocampa and determined that these insecticides cause 97–99% mortality. Zamoum et al. (2016) in their study, in which they applied Foray 48B (which was based on a mixture of spores and parasporal crystals of Btk strain ABTS-351) containing Btk 3a and 3b serotypes to pine processionary moth, noted that the mortality rate seven days after the application varied between 41–75% and 14 days after this rate ranged between 73–93%. The effects of Bt applied to pine processionary moth on mortality in the EU and some Mediterranean countries have been shown in various studies. Osuna et al. (1994) recorded a mortality rate of 100% for serotypes of H7 (B. thuringiensis subsp. aizawai), H27 (B. thuringiensis subsp. mexicanensis), and 3a3b3c (Btk), while Ghent (2003) 95–98% for Btk strains and Martin et al. (2003) 75–98% for Foray 96 B, Foray 76 SI, and Foray 48 SI (which were based on a mixture of spores and parasporal crystals of Btk strain ABTS-351). In Turkey, 100% mortality for Bt strain (Besceli, 1969) and 94% mortality for MVP, which was Cry 1AC from Btk, (Ozcankaya and Can, 2004) were noted in various studies conducted within the scope of combating pine processionary moth. Gindin et al. (2007b) determined that the larvae were sensitive to Bt application in the study they applied Delfin WG (Btk, serotype 3a 3b, strain SA-11), Dipel DF, and Foray 48B (both were based on a mixture of spores and parasporal crystals of Btk strain ABTS-351) to T. wilkinsoni. The results of our study are parallel to the results of all these studies in terms of lethality.
In various studies, the effectiveness of different Bt strains against pine processionary moths was also evaluated. Yılmaz et al. (2013) in their study, in which they applied different Bt isolates against T. wilkinsoni, determined that the isolates could be used as environmentally safe insecticides to control this pest species. Rausell et al. (1999) applied Cry1B, Cry1C, and Cry1E toxins and B. thuringiensis-based bioinsecticides (Cordalene, Dipel, Foray 48B, and Foray 76B) to T. pityocampa and determined that Cry1B was toxic to larvae. The results of these studies are consistent with the findings obtained from our study in terms of lethality of Bt.
When LC50 values were compared, the highest LC50 values among all groups were in the groups exposed to the Bti pHT315 strain and toxin obtained from this strain. The lowest LC50 value for larvae exposed to different Bti strains was found in the group exposed to the Bti ATCC 35646, and the lowest value for toxins was determined in the group exposed to the Bti pHTppk. These findings are in parallel with the survival data. It can be said that the Bti ATCC 35646 strain and the Bti pHTppk toxin are the most effective because the low LC50 value means that the applied strain is effective even in small amounts. In our previous study (Yanar et al., 2022), where we applied different Bti pHTppk concentrations to the 2nd instar T. wilkinsoni larvae, we recorded the LC50 value as 3.4 × 107 cfu/mL for the group exposed to the highest concentration of Bti pHTppk. Although we used the same strain, the reason why we found the LC50 value higher compared to our previous study is that we used the 4th instar larvae in our study. As the larval age increases, the LC50 value also increases.
CONCLUSIONS
Bti ATCC 35646 with 63.4% mortality and the lowest LC50 value of 1.2 × 109 cfu/mL among the three strains of Bti and Bti pHTppk with 76.7% mortality and the lowest LC50 value of 1.2 mL among the toxins from the Bti strains had found to be virulent for T. wilkinsoni. Results from our study suggest that Diptera-targeted Bti is also virulent against one insect from a different order, T. wilkinsoni, and that testing different strains of Bt with toxins can be developed prospectively as a management strategy for pests. In addition, it emphasizes that the effectiveness of mutant strains in living organisms may be more ineffective than wild-type strains.
REFERENCES
Abbou, A., Kadri, N., Debbache, N., Dairi, S., Remini, H., Dahmoune, F., Berkani, F., Adel, K., Belbahi, A., and Madani, K., Effect of precipitation solvent on some biological activities of polysaccharides from Pinus halepensis Mill. seeds, Int. J. Biol. Macromol., 2019, vol. 141, pp. 663–670. https://doi.org/10.1016/j.ijbiomac.2019.08.266
Abuldahab, F.F., Salama, E.M., Abdel-Meguid, A.D., and Henen, M.M., Insecticidal activity of some phytochemical groups of Calotropis procera plant and Bacillus thuringiensis isrealensis (HD-1), on the morphology of different developmental stages of Musca domestica vicina (Diptera: Muscadae), J. Bas. Environ. Sci., 2019, vol. 6, pp. 267–272.
Arantes, O. and Lereclus, D., Construction of cloning vectors for Bacillus thuringiensis, Gene, 1991, vol. 108, no. 1, pp. 115–119. https://doi.org/10.1016/0378-1119(91)90495-w
Aydın, T., Branco, M., Güven, Ö., Gonçalves, H., Lima, A., Karaca, İ., and Butt, T., Significant mortality of eggs and young larvae of two pine processionary moth species due to the entomopathogenic fungus Metarhizium brunneum, Biocontrol Sci. Technol., 2018, vol. 28, no. 4, pp. 317–331. https://doi.org/10.1080/09583157.2018.1447084
Bahareth, O., Alsahhaf, Z., Saleh, A., Hijji, A., and Osman, G., The effect of Bacillus thuringiensis israelensis (Bti) as a microbial control agent against Musca domestica in Makkah Region, J. Pure Appl. Microbiol., 2018, vol. 12, no. 4, pp. 2077–2085. https://doi.org/10.22207/JPAM.12.4.44
Barta, M., Horáková, M.K., Georgieva, M., Mirchev, P., Zaemdzhikova, G., Pilarska, D., Takov, D., Todorov, M., Hubenov, Z., Pilarski, P., and Georgiev, G., Entomopathogenic fungi (Ascomycota: Hypocreales) as natural antagonists of the Pine processionary moth Thaumetopoea pityocampa (Denis & Schiffermüller, 1775) (Lepidoptera: Notodontidae) in Bulgaria, Acta Zool. Bulg. Suppl., 2020, vol. 15, pp. 89–96.
Ben-Dov, E., Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins, Toxins, 2014, vol. 6, no. 4, pp. 1222–1243. https://doi.org/10.3390/toxins6041222
Besceli, O., Le controle et la biologie de Thaumetopoea pityocampa Schiff. (The biology and control of Thaumetopoea pityocampa Schiff.), For. Res. Inst. Tech. Bull., 1969, vol. 35, p. 65.
Bordalo, M.D., Gravato, C., Beleza, S., Campos, D., Lopes, I., and Pestana, J.L.T., Lethal and sublethal toxicity assessment of Bacillus thuringiensis var. israelensis and Beauveria bassiana based bioinsecticides to the aquatic insect Chironomus riparius, Sci. Total Environ., 2020, vol. 698, p. 134155. https://doi.org/10.1016/j.scitotenv.2019.134155
Bouslama, T., Chaieb, I., Rhouma, A., and Laarif, A., Evaluation of a Bacillus thuringiensis isolate based formulation against the pod borer, Helicoverpa armigera Hübner (Lepidoptera: Noctuidae), Egypt. J. Biol. Pest Control, 2020, vol. 30, p. 16. https://doi.org/10.1186/s41938-020-00218-z
Bravo, A., Gill, S.S., and Soberón, M., Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control, Toxicon, 2007, vol. 49, pp. 423–435. https://doi.org/10.1016/j.toxicon.2006.11.022
Bouyahya, A., Belmehdi, O., Abrini, J., Dakka, N., and Bakri, Y., Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities, Asian Pac. J. Trop. Med., 2019, vol. 12, no. 3, pp. 117–122. https://doi.org/10.4103/1995-7645.254937
Cebeci, H.H., Oymen, R.T., and Acer, S., Control of pine processionary moth, Thaumetopoea pityocampa with Bacillus thuringiensis in Antalya, Turkey, J. Environ. Biol., 2010, vol. 31, no. 3, pp. 357–361.
Demirtaş, A., Assessing the susceptibility of some gut bacteria to the extract from needles of Turkish pine, MAKU J. Health Sci. Inst., 2021, vol. 9, no. 1, pp. 1–6. https://doi.org/10.24998/maeusabed.869812
Donovan, W.P., Dankocsik, C.C., Gilbert, M.P., Gawron-Burke, M.C., Groat, R.G., and Carlton, B.C., Amino acid sequence and entomocidal activity of the P2 crystal protein: an insect toxin from Bacillus thuringiensis var. kurstaki, J. Biol. Chem., 1988, vol. 263, no. 1, pp. 561–567.
Doruk, T., Avican, U., Camci, I.Y., and Gedik, S.T., Overexpression of polyphosphate kinase gene (ppk) increases bioinsecticide production by Bacillus thuringiensis, Microbiol. Res., 2013, vol. 168, no. 4, pp. 199–203. https://doi.org/10.1016/j.micres.2012.11.009
Dziedziński, M., Kobus-Cisowska, J., and Stachowiak, B., Pinus species as prospective reserves of bioactive compounds with potential use in functional food-current state of knowledge, Plants, 2021, vol. 10, no. 7, p. 1306. https://doi.org/10.3390/plants10071306
El Omari, N., Ezzahrae Guaouguaou, F., El Menyiy, N., Benali, T., Aanniz, T., Chamkhi, I., Balahbib, A., Taha, D., Shariati, M.A., Zengin, G., El-Shazly, M., and Bouyahya, A., Phytochemical and biological activities of Pinus halepensis Mill., and their ethnomedicinal use, J. Ethnopharmacol., 2021, vol. 268, p. 113661. https://doi.org/10.1016/j.jep.2020.113661
Faria, J.M., Bioactivity of essential oils and respective volatile monoterpenoids against Thaumetopoea pityocampa and T. wilkinsoni, Biol. Life Sci. Forum, 2021, vol. 3, no. 1, p. 36. https://doi.org/10.3390/IECAG2021-09692
Farjon, A., A Handbook of the World’s Conifers, Brill, Leiden, Netherlands, 2017.
Federici, B.A. and Bauer, L.S., Cyt1Aa protein of Bacillus thuringiensis is toxic to the cottonwood leaf beetle, Chrysomela scripta, and suppresses high levels of resistance to Cry3Aa, Appl. Environ. Microbiol., 1998, vol. 64, pp. 4368–4371. https://doi.org/10.1128/AEM.64.11.4368-4371.1998
Ghent, J.H., Ecological risk assessment of Bacillus thuringiensis var. kurstaki, in Proceedings of Integrated Pest Management for Pine Processionary Caterpillar in Cyprus, Ciesla, W.M., and Gulensoy, N., Eds., Technical Workshop, 2003, pp. 65–72.
Gindin, G., Kuznetsova, T., Protasov, A., Saphir, N., Madar, Z., and Mendel, Z., Susceptibility of the pistachio processionary moth Thaumetopoea solitaria to three Bacillus thuringiensis products, Phytoparasitica, 2008, vol. 36, pp. 472–482. https://doi.org/10.1007/BF03020293
Gindin, G., Navon, A., Saphir, N., Protasov, A., and Mendel, Z., Environmental persistence of Bacillus thuringiensis products tested under natural conditions against Thaumetopoea wilkinsoni, Phytoparasitica, 2007a, vol. 35, pp. 255–263. https://doi.org/10.1007/BF02981159
Gindin, G., Navon, A., Saphir, N., Protasov, A., and Mendel, Z., Differing susceptibility to Bacillus thuringiensis formulations of Thaumetopoea wilkinsoni populations between forests with different Bt management in Israel, Phytoparasitica, 2007b, vol. 35, pp. 179–190. https://doi.org/10.1007/BF02981112
Guo, Z., Kang, S., Sun, D., Gong, L., Zhou, J., Qin, J., Guo, L., Zhu, L., Bai, Y., Ye, F., Wu, Q., Wang, S., Crickmore, N., Zhou, X., and Zhang, Y., MAPK-dependent hormonal signaling plasticity contributes to overcoming Bacillus thuringiensis toxin action in an insect host, Nat. Commun., 2020, vol. 11, p. 3003. https://doi.org/10.1038/s41467-020-16608-8
Güven, Ö., Aydin, T., Karaca, I., and Butt, T., Biopesticides offer an environmentally friendly solution for control of pine processionary moth (Thaumetopoea wilkinsoni Tams) larvae and pupae in urban areas, Biocontrol Sci. Technol., 2021, vol. 31, no. 1, pp. 35–52. https://doi.org/10.1080/09583157.2020.1826905
Hamrouni, L., Hanana M., Amri I., Romane A.E., Gargouri, S., and Jamoussi, B., Allelopathic effects of essential oils of Pinus halepensis Miller: chemical composition and study of their antifungal and herbicidal activities, Arch. Phytopathol. Pflanzenschutz, 2015, vol. 48, no. 2, pp. 145–158. https://doi.org/10.1080/03235408.2014.884667
Ioannou, C.S., Hadjichristodoulou, C., Kyritsi, M.A., and Papadopoulos, N.T., Short-term selection to diflubenzuron and Bacillus thuringiensis var. israelensis differentially affects the winter survival of Culex pipiens f. pipiens and Culex pipiens f. molestus (Diptera: Culicidae), Insects, 2021, vol. 12, no. 6, p. 527. https://doi.org/10.3390/insects12060527
Karabörklü, S., Ayvaz, A., Yilmaz, S., Azizoglu, U., and Akbulut, M., Native entomopathogenic nematodes isolated from Turkey and their effectiveness on pine processionary moth, Thaumetopoea wilkinsoni Tams, Int. J. Pest Manage., 2015, vol. 61, no. 1, pp. 3–8. https://doi.org/10.1080/09670874.2014.984256
Karličić, V., Zlatković, M., Jovičić-Petrović, J., Nikolić, M. P., Orlović, S., and Raičević, V., Trichoderma spp. from pine bark and pine bark extracts: potent biocontrol agents against Botryosphaeriaceae, Forests, 2021, vol. 12, p. 1731. https://doi.org/10.3390/f12121731
Khatamian, N., Soltani, M., Shadan, B., Neamati, A., Tabrizi, M.H., and Hormozi, B., Pinus morrisonicola needles essential oil nanoemulsions as a novel strong antioxidant and anticancer agent, Inorg. Nano-Met. Chem., 2022, vol. 52, no. 2, pp. 253–261. https://doi.org/10.1080/24701556.2021.1892760
Koutsaviti, K., Giatropoulos, A., Pitarokili, D., Papachristos, D., Michaelakis, A., and Tzakou, O., Greek Pinus essential oils: larvicidal activity and repellency against Aedes albopictus (Diptera: Culicidae), Parasitol. Res., 2015, vol. 114, no. 2, pp. 583–592. https://doi.org/10.1007/s00436-014-4220-2
Li, Y.Y., Feng, J., Zhang, X.L., and Cui, Y.Y., Pine bark extracts: nutraceutical, pharmacological, and toxicological evaluation, J. Pharmacol. Exp. Ther., 2015, vol. 353, pp. 9–16. https://doi.org/10.1124/jpet.114.220277
Martin, J.C., Mazet, R., Jean, F., and Xavier, B., Essais comparatifs d’efficacité du Foray 96 B, du Foray 76 SI, et du Foray 48 SI contre la processionnaire du pin. Au cours de l’automne 2002. Unite Experimentale Forestiére Mediterranéenne, INRA, Rapport de fin de prestation pour la société Valent Biosciences, 2003, p. 9.
Meziti, H., Bouriche, H., Kada, S., Demirtas, I., Kizil, M., and Senator, A., Phytochemical analysis, and antioxidant, anti-hemolytic and genoprotective effects of Quercus ilex and Pinus halepensis Mill. methanolic extracts, J. Pharm. Pharmacogn. Res., 2019, vol. 7, no. 4, pp. 260–272.
Mitić, Z.S., Jovanović, B., Jovanović, S.Č., Stojanović-Radić, Z.Z., Mihajilov-Krstev, T., Jovanović, N.M., Nikolić, B.M., Marin, P.D., Zlatković, B.K., and Stojanović, G.S., Essential oils of Pinus halepensis and P. heldreichii: chemical composition, antimicrobial and insect larvicidal activity, Ind. Crops Prod., 2019, vol. 140, p. 111702. https://doi.org/10.1016/j.indcrop.2019.111702
Nasser, S., da Costa, M.P.M., de Mello Ferreira, I.L., and Lima, J.B.P., k-Carrageenan-Bacillus thuringiensis israelensis hydrogels: a promising material to combat larvae of the Aedes aegypti mosquito, Carbohydr. Polym. Technol. Appl., 2021, vol. 2, p. 100125. https://doi.org/10.1016/j.carpta.2021.100125
Onaran, M.A. and Kati, M., Biological control with the pine processionary beetle (Thaumetopoeo pityocampa Shiff), Balikesir Univ. Fen Bilim. Enst. Derg., 2010, vol. 12, pp. 21–27.
Osuna, E.V., Ledesma, J.M., Aldebis, H.K., and Santiago-Alvarez, C., Patogenos y parasitos para el control de la procesionaria del pino, Thaumetopoea pityocampa (D. y Schiff.) (Lep., Notodontidae). (Pathogens and parasites for control of Thaumetopoea pityocampa (D. and Schiff.) (Lep., Notodontidae), Bol. San. Veg. Plagas, 1994, vol. 20, pp. 511–515.
Ozcankaya, I.M. and Can, P., Research on improvement of possibilities of mechanical and biological control of pine processionary caterpillar (Thaumetopoea pityocampa Den. and Schiff.) (Lep., Thaumetopoeidae)) in young Turkish red pine plantations in Muğla, Ege For. Res. Inst. Tech. Bull., 2004, vol. 26, p. 77.
Pardo-López, L., Soberón, M., and Bravo, A., Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection, FEMS Microbiol. Rev., 2013, vol. 37, pp. 3–22. https://doi.org/10.1111/j.1574-6976.2012.00341.x
Porcar, M., Grenier, A.M., Federici, B., and Rahbé, Y., Effects of Bacillus thuringiensis δ-endotoxins on the Pea aphid (Acyrthosiphon pisum), Appl. Environ. Microbiol., 2009, vol. 75, pp. 4897–4900. https://doi.org/10.1128/AEM.00686-09
Poulin, B., Lefebvre, G., Hilaire, S., and Després, L., Long-term persistence and recycling of Bacillus thuringiensis israelensis spores in wetlands sprayed for mosquito control, Ecotoxicol. Environ. Saf., 2022, vol. 243, p. 114004. https://doi.org/10.1016/j.ecoenv.2022.114004
Rabha, M., Das, D., Konwar, T., Acharjee, S., and Kumar Sarmah, B., Whole genome sequencing of a novel Bacillus thuringiensis isolated from Assam soil, BMC Microbiol., 2023, vol. 23, p. 91. https://doi.org/10.1186/s12866-023-02821-0
Ramasubramanian, R., Karthi, S., Senthil-Nathan, S., Sivanesh, H., Sundar, N.S., Stanley-Raja, V., Ramkumar, G., Muthu-Pandian, C.K., Vasantha-Srinivasan, P., Alarjani, K.M., Elshikh, M.S., Abdel-Megeed, A., and Krutmuang, P., Effe-ct of bacterial toxin identified from the Bacillus subtilis against the Cnaphalocrocis medinalis Guenée (Lepidoptera: Crambidae), Toxin Rev., 2022, vol. 42, pp. 264–274. https://doi.org/10.1080/15569543.2022.2111444
Rausell, C., Martínez-Ramírez, A.C., García-Robles, I., and Real, M.D., The toxicity and physiological effects of Bacillus thuringiensis toxins and formulations on Thaumetopoea pityocampa, the pine processionary caterpillar, Pestic. Biochem. Phys., 1999, vol. 65, no. 1, pp. 44–54. https://doi.org/10.1006/pest.1999.2426
Redmond, C.T., Wallis, L., Geis, M., Williamson, R.C., and Potter, D.A., Strengths and limitations of Bacillus thuringiensis galleriae for managing Japanese beetle (Popillia japonica) adults and grubs with caveats for cross-order activity to monarch butterfly (Danaus plexippus) larvae, Pest Manage. Sci., 2020, vol. 76, pp. 472–479. https://doi.org/10.1002/ps.5532
Santos, E.N., Menezes, L.P., Dolabella, S.S., Santini, A., Severino, P., Capasso, R., Zielinska, A., Souto, E.B., and Jain, S., Bacillus thuringiensis: from biopesticides to anticancer agents, Biochimie, 2022, vol. 192, pp. 83–90. https://doi.org/10.1016/j.biochi.2021.10.003
Semiz, G., Larvicidal activity of Nerium oleander L. leaf extract against pine processionary moth (Thaumetopoea wilkinsoni Tams.), J. Entomol. Zool. Stud., 2017, vol. 5, no. 6, pp. 79–81.
Smith, P.K., Krohn, R.I., Hermanson, G.T., Mallia, A.K., Gartner, F.H., Provenzano, M.D., Fujimoto, E.K., Goeke, N.M., Olson, B.J., and Klenk, D.C., Measurement of protein using bicinchoninic acid, Anal. Biochem., 1985, vol. 150, no. 1, pp. 76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Topkara, E.F., Yanar, O., Tuncer, C., Ozdemir, I.O., and Yildirim, E., Efficacy of Beauveria bassiana and Beauveria pseudobassiana isolates against the pine processionary moth, Thaumetopoea wilkinsoni Tams, 1926 (Lepidoptera: Notodontidae), Egypt. J. Biol. Pest Control, 2022, vol. 32, p. 3. https://doi.org/10.1186/s41938-021-00501-7
Tudoran, A., Nordlander, G., Karlberg, A., and Puentes, A., A major forest insect pest, the pine weevil Hylobius abietis, is more susceptible to Diptera-than Coleoptera-targeted Bacillus thuringiensis strains, Pest Manage. Sci., 2021, vol. 77, no. 3, pp. 1303–1315. https://doi.org/10.1002/ps.6144
Vachon, V., Laprade, R., and Schwartz, J.L., Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review, J. Invertebr. Pathol., 2012, vol. 111, no. 1, pp. 1–12. https://doi.org/10.1016/j.jip.2012.05.001
Valtierra-de-Luis, D., Villanueva, M., Lai, L., Williams, T., and Caballero, P., Potential of Cry10Aa and Cyt2Ba, two minority δ-endotoxins produced by Bacillus thuringiensis ser. israelensis, for the control of Aedes aegypti larvae, Toxins, 2020, vol. 12, no. 6, p. 355. https://doi.org/10.3390/toxins12060355
Yanar, O., Topkara E.F., and Doruk, T., Efficacy of Diptera-targeted Bacillus thuringiensis var. israelensis against the pine processionary moth, Thaumetopoea wilkinsoni (Lepidoptera: Notodontidae), Arch. Phytopathol. Pflanzenschutz, 2022, vol. 55, no. 13, pp. 1530–1541. https://doi.org/10.1080/03235408.2022.2105133
Yanar, O., Topkara, E.F., Şahin, F., Yanar, Y., Yanar, D., and Terzi, Y., Efficacy of Beauveria bassiana and Metarhizium brunneum isolates against the pine processionary moth, Thaumetopoea wilkinsoni Tams, 1926 (Lepidoptera: Notodontidae), Egypt. J. Biol. Pest Control, 2023, vol. 33, p. 32. https://doi.org/10.1186/s41938-023-00679-y
Yılmaz, S., Karabörklü, S., Azizoğlu, U., Ayvaz, A., Akbulut, M., and Yildiz, M., Toxicity of native Bacillus thuringiensis isolates on the larval stages of pine processionary moth Thaumetopoea wilkinsoni at different temperatures, Turk. J. Agric. For., 2013, vol. 37, no. 2, pp. 163–172. https://doi.org/10.3906/tar-1201-21
Zamoum, M., Martin, J.C., Bensidi, A., and Bahmane, R., Immediate and delayed mortality of the pine processionary moth treated by Bacillus thuringiensis var. kurstaki 3a 3b in the sub-Saharian pine reforestations, Turk. J. For., 2016, vol. 17, pp. 76–79. https://doi.org/10.18182/tjf.44293
Zorzetti, J., Ricietto, A.P.S., Fazion, F.A.P., Meneghin, A.M., Neves, P.M.O.J., Vilas-Boas, L.A., and Vilas-Boas, G.T., Isolation, morphological and molecular characterization of Bacillus thuringiensis strains against Hypothenemus hampei Ferrari (Coleoptera: Curculionidae: Scolytinae), Rev. Bras. Entomol., 2018, vol. 62, no. 3, pp. 198–204. https://doi.org/10.1016/j.rbe.2018.07.002
ACKNOWLEDGMENTS
Not applicable.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct thisparticular research were obtained.
Author information
Authors and Affiliations
Contributions
EFT, OY, and TD designed the experiment; EFT, OY and TD performed bioassays; OY and YT performed the statistical analysis. EFT and OY wrote the manuscript; EFT, OY, TD and YT revised the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and vertebrate animal subjects. No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elif-Fatma Topkara, Yanar, O., Doruk, T. et al. Effects of Bacillus thuringiensis var. israelensis Strains and Toxins on the Pine Processionary Moth Thaumetopoea wilkinsoni (Lepidoptera: Notodontidae). Biol Bull Russ Acad Sci 51, 1301–1311 (2024). https://doi.org/10.1134/S1062359024607250
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359024607250