Abstract
The present study characterized copper (Cu) uptake and depuration by juvenile and adult Florida apple snails (Pomacea paludosa) from water, soil, and diet. During a 28-day uptake period, juvenile apple snails were exposed to aqueous Cu and adult apple snails were exposed to Cu-contaminated soil, water, and food. In the follow-up 14-day depuration period, both juvenile and adult apple snails were held in laboratory freshwater with background Cu concentrations <4 μg/l. For juvenile apple snails, whole body Cu concentrations increased with time and reached a plateau after 14 days. The data followed Michaelis–Menten kinetics rather than a one compartment first order kinetics model. The mean Cu bioconcentration factor (BCF) for juvenile apple snails was 1493 and the depuration half-life was 10.5–13.8 days. For adult snails, dietary uptake of Cu resulted in higher bioaccumulation factors (BAFs) compared to uptake from soil. Most of the accumulated Cu was located in soft tissue (about 60% in the viscera and 40% in the foot). The shell contained <1% of the total accumulated copper. Soft tissue is usually consumed by predators of the apple snail. Therefore, the results of the present study show that Cu transfer through the food chain to the apple snail may lead to potential risk to its predators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The long history of copper (Cu) use in Florida citrus agriculture (as fertilizers and fungicides) has elevated Cu concentrations in South Florida ecosystems (Alva et al. 1995). According to soil sampling performed by the South Florida Water Management District (SFWMD) from 2001 to 2006, Cu concentrations in soils were as high as 1200 mg/kg in St. Lucie County, 924 mg/kg in Dade County, 406 mg/kg in Hendry County, 180 mg/kg in Martin County, 110 mg/kg in Palm Beach County, 72 mg/kg in Highlands County and 47 mg/kg in Broward County (SFWMD 2001–2006). The Implementation of the Comprehensive Everglades Restoration Plan (CERP) under the Water Resources Development Act of 2000 requires acquisition of thousands of acres of land in these counties for maintaining hydrologic buffer areas and for the creation of storm water treatment areas, water storage reservoirs and wetlands (Everglades National Park 2001). A recent study conducted by Hoang et al. (2008) found that dissolved Cu concentrations in over-lying water collected from flooded soils originating from these counties were as high as 300 μg/l, depending on initial Cu concentrations in soils. However, concentrations of dissolved organic carbon (DOC) were also high (up to 51 mg/l DOC) in overlying water. The presence of DOC will decrease Cu bioavailability (Ryan et al. 2004; Sciera et al. 2004; Rogevich et al. 2008). Using the biotic ligand model (BLM) to estimate Cu bioavailability indicated that the bioavailable portion of Cu in the overlying water was low (DiToro et al. 2000, Notten et al. 2005).
A number of studies indicate that freshwater snails accumulate metals, including Cu, from three routes of exposures: water, soil, and diet (Laskowski and Hopkin 1996; Gomot and Pihan 1997; Gomot-de Vaufleury and Pihan 2002; Heng et al. 2004; Notten et al. 2005). However, there are no studies with the Florida apple snail (Pomacea paludosa), a periphyton-grazing, freshwater mollusk and a key species in the Everglades ecosystem since it is the main food source of the federally endangered Florida (formerly Everglades) snail kite (Rostrhamus sociabilis plumbeus) and a prey species for other birds, fish, reptiles and mammals (Sharfstein and Steinman 2001). Copper may be transferred from sediment and/or periphyton to the apple snail and finally to predators of the apple snail. Recently, the U.S. Fish and Wildlife Service reported that Cu concentrations in field-collected P. paludosa were as high as 336 mg/kg and were correlated primarily with Cu concentrations in sediments, followed by periphyton (Frakes et al. 2008). However, Cu concentrations in water were below detection limits. This suggests that Cu uptake by P. paludosa may be from sediment and/or periphyton.
The present study characterizes Cu uptake and depuration by juvenile P. paludosa from Cu-contaminated water and by adult P. paludosa from three exposure routes: water, soil, and diet. The distribution of accumulated Cu in the body of adult P. paludosa is also characterized. Bioconcentration factors (BCFs) were determined for Cu uptake from the aqueous exposures and bioaccumulation factors (BAFs) were determined for Cu uptake from dietary and soil exposures.
Materials and methods
Organism
Pomacea paludosa eggs were collected from Water Conservation Areas 3A and 2B, Dade County, Florida, and hatched under laboratory conditions at a temperature of 26 ± 1°C, 16-h light, 8-h dark photoperiod in the Ecotoxicology Laboratory at Florida International University. Pomacea paludosa used in the juvenile uptake study were hatched from field- collected eggs and were ≤96-h-old, while P. paludosa in the adult uptake study were cultured in laboratory freshwater under flow-through conditions for 3 months prior to testing. Laboratory freshwater was carbon-filtered, UV-sterilized water from the city of North Miami, FL. Pomacea paludosa were fed romaine lettuce daily.
Juvenile uptake study
To examine Cu uptake and depuration and to determine a bioconcentration factor, P. paludosa were exposed to three concentrations of aqueous Cu (3, 6, and 12 μg/l) and a control for 28 days followed by a depuration period of 14 days. Snails were held under static conditions in 18-l glass tanks with three replicates per treatment and thirty-five juvenile P. paludosa per replicate. Copper treatments were made by adding Cu stock solution to laboratory freshwater and equilibrated for at least 24 h prior to use. Copper stock solution was made from copper sulfate pentahydrate (Fisher Scientific, Fairlawn, NJ, USA). Water was renewed weekly. Snails were fed Cu-free lettuce every 2 days.
Water samples and snails were collected at the beginning (day 0), days 1, 3.5, 7, 14, 21, and 28 during the uptake phase and days 1, 4, 7, and 14 during the depuration phase for Cu analysis. The sampling points and exposure concentrations were used to determine the relationship of Cu uptake with exposure time and concentration. The exposure tanks were continuously aerated during the experiment. Temperature, pH, and dissolved oxygen (DO) were measured daily. Water hardness and alkalinity, ammonia, and concentration of DOC were measured weekly for each exposure tank before and after renewal. Before and after renewal, water was also collected for Cu analysis to ensure stable exposure concentrations.
Water samples were passed through 0.45 μm filter, placed in 15-ml graduated polyproplene tubes, and acidified with concentrated nitric acid to pH 2 to measure dissolved Cu. Three snails were collected from each replicate tank at each sampling time, rinsed with EDTA solution and de-ionized water (DI) to wash Cu bound on the snail shell surface and frozen at −20°C until analysis. Additional lettuce was placed in the exposure tanks and collected for Cu analysis to determine if Cu in the water adsorbed to the lettuce, introducing a dietary exposure route. Snails and lettuce were digested with HNO3 based on U.S. EPA Method 3050B for analyzing whole body Cu (including shell) and total Cu in lettuce, respectively (U.S. EPA 1996). Copper was analyzed using Inductively Couple Plasma Atomic Emission Spectrometer (ICPAES).
Michaelis–Menten kinetics was used to analyze Cu uptake data.
where C is whole body Cu concentration at time t. C sat is the whole body Cu concentration at saturated state (maximal concentration). K M is the Michaelis–Menten constant, which is the exposure time needed to reach a half of whole body Cu concentration at saturated state. K M was determined by fitting the uptake Cu data to a linear relationship by rearranging Eq. (1) as follows:
where the ratio of K M/C sat is the slope and 1/C sat is the intercept of the relationship. The unit of the slope is (days)(g snail)/(μg Cu). Using Michaelis–Menten kinetics, the exposure time needed for accumulated Cu to reach 50% (t u0.5) and 90% (t u0.9) of saturation was determined.
The copper depuration rate constant (k d) was determined using first order kinetics. Time needed to depurate 50% (t d0.5) and 90% (t d0.9) of the whole body Cu was also determined. Copper bioconcentration factor (BCF) for P. paludosa was the ratio of whole body Cu concentration to water Cu concentration. For comparison, one compartment first-order kinetics model (1CFOK) also was used to analyze the uptake and depuration data.
where C is the whole body Cu concentration (μgCu/g snail) at time t (d). C w is the Cu concentration in water (mg/l Cu). k u is the uptake rate constant (l/kg/d) and k d is the depuration rate constant (1/day).
Adult uptake study
To characterize Cu uptake of adult P. paludosa from water, soil, and food, P. paludosa was exposed for 28 days to three treatments and a control (Fig. 1). Copper contaminated soil in treatments 1 and 2 was collected from the Agler property, a citrus agriculture site in St. Lucie County, Florida. Soil in treatment 1 was covered with a nitex sheet, which allowed for Cu exchange between the soil and water but did not allow the P. paludosa to come into direct contact with the soil. The soil in treatment 2 was the same as treatment 1 but there was no nitex sheet separating the snails from the soil. Therefore, Cu accumulated in P. paludosa in treatment 1 resulted from aqueous Cu exposure only, while Cu accumulated in P. paludosa in treatment 2 resulted from both water and soil exposures. Pomacea paludosa in treatments 1 and 2 were fed with Cu-free lettuce. Hence, the difference between the concentrations of accumulated Cu in P. paludosa from treatments 1 and 2 can be used to determine Cu uptake from soil alone. Pomacea paludosa in treatments 3 and 4 were exposed to reference soil; however, snails in treatment 3 were fed Cu-free lettuce while snails in treatment 4 were fed Cu-treated lettuce (as described below). The difference between the concentrations of accumulated Cu in P. paludosa from treatments 3 and 4 can be used to determine Cu uptake from dietary exposure. Reference soil in treatments 3 and 4 were collected from the Sunrise Boys property, a citrus agriculture site in Palm Beach County, Florida. Physical and chemical characteristics of all soils are presented in Hoang et al. (2008). Following exposure, P. paludosa in all treatments were allowed to depurate for 14 days in carbon filtered, UV-sterilized freshwater (laboratory freshwater).
Four 492-l polyethylene tanks containing 76 l of soil (20 gallons) were flooded with 152 l of laboratory freshwater and held for 2 weeks prior to the addition of P. paludosa. According to the study by Hoang et al. (2008), the soil/water system reached Cu equilibrium in overlying water after 2 weeks. The soil Cu bioaccumulation factor was the ratio of the Cu uptake concentration from soil to the soil Cu concentration. Copper uptake concentration from soil was the difference between the concentrations of accumulated Cu in P. paludosa from treatments 1 and 2. The dietary Cu bioaccumulation factor was the ratio of the Cu uptake concentration from diet to the concentration of Cu in the diet. Copper uptake concentration from diet was the difference between the concentrations of accumulated Cu in P. paludosa from treatments 3 and 4.
Overlying water was collected from each treatment on day 0 (prior to addition of organisms), days 1, 4, 7, 14, 21, and 28 during the uptake phase and days 1, 4, 7, and 14 during the depuration phase for Cu and minerals analysis. At each sampling period, three snails were also collected from each treatment, rinsed with EDTA solution and DI water to wash Cu bound to the shell surface and frozen at −20°C until analysis of tissue Cu. The procedure for water sampling and water Cu analysis were similar to the procedure described in the juvenile uptake study. For determining tissue Cu concentrations, each snail was thawed and dissected into three parts (shell, foot, and viscera) following an adaptation of a method published by Gomot-de Vaufleury and Pihan (2002). Each part of the snail was digested separately with HNO3 based on U.S. EPA Method 3050B for tissue Cu analysis (U.S. EPA 1996). Average Cu concentrations of three snails for each treatment are reported for each sampling time.
For the dietary exposure with Cu treated lettuce, lettuce was grown for 45 days in control soil spiked with Cu solution, resulting in a concentration of 65 mg Cu/kg soil. The Cu concentration of 65 mg/kg was selected based on preliminary lettuce seed germination and lettuce studies. At six soil Cu concentrations (31.25, 62.5, 125, 250, 500, 1000 mg/kg Cu) no seed germination occurred at concentrations greater than 125 mg/kg Cu but at 31.25 and 62.5 mg/kg, there was 30% germination. At 31.25 and 62.5 mg/kg Cu in soil, 8.26–11 mg/kg dw Cu was found in lettuce leaves grown in these soils, respectively. From the initial study, one soil Cu treatment (62.5 mg/kg dw) was chosen to grow Cu-treated lettuce to feed to adult snails, resulting in 8.70 mg/kg dw Cu in lettuce tissue. Snails in the control were fed lettuce grown in soil containing only background levels of Cu, resulting in 4.15 mg/kg dw Cu in lettuce tissue.
Data analysis
Linear regression was used for determining Michaelis–Menten constants. The T-test method was used for single treatment comparisons for data from the adult uptake experiment. An effect with a P < 0.05 was considered significant. All statistical analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). All figures were constructed using Microsoft Excel 2000 (Microsoft, Redmond, WA, USA).
Results and discussion
Copper and water chemistry of exposure media
Results of measured copper concentrations in soils and water are shown in Table 1. In the juvenile uptake study, Cu concentrations in water ranged from 6.0–7.2, 8.2–9.6, and 12.2–14.4 μg/l for treatments 1, 2, and 3, respectively. Measured Cu concentrations were higher than the nominal concentrations due to background Cu in laboratory freshwater (3–4 μg/l). In the juvenile uptake study, the average temperature, pH, DO, and ammonia were 25.2 ± 1.2, 8.4 ± 0.2, 7.7 ± 0.4, and 0.23 ± 0.08 mg/l, respectively. The average hardness and alkalinity were 54 ± 13 and 48 ± 11 mg/l as CaCO3, respectively. The DOC in laboratory freshwater was 0.26 ± 0.1 mg/l.
In the adult uptake study, Cu concentration in reference soil and contaminated soil were 30.5 ± 4.9 and 119.8 ± 26.4 μg/g, respectively. The Cu concentrations in overlying water of reference and contaminated soils ranged from 19.5 to 46.7 μg/l and from 51.9 to 90.3 μg/l, respectively. The Cu concentrations in overlying water from reference and contaminated soils increased with time even though soils were flooded and held for 2 weeks prior to the addition of snails. According to Koster et al. (2006) and Hoang et al. (2008), Cu concentrations in overlying water reached saturation state after 2 weeks but there were no snails in these studies. Movement of apple snails at the soil/water interface in the present study may have disturbed the soil surface, resulting in increased Cu concentrations in overlying water with time. In the adult uptake study, the average temperature and DO were 24.9 ± 1.1 and 7.75 ± 0.32 mg/l, respectively. The average hardness, alkalinity, pH, ammonia, and DOC for treatments 1 and 2 were 144 ± 41, 138 ± 30 mg/l CaCO3, 8.34 ± 0.20, 0.15 ± 0.02, and 23.5 ± 0.4 mg/l, respectively, and for treatments 3 and 4 were 244 ± 58, 239 ± 61 mg/l CaCO3, 8.41 ± 0.21, 0.26 ± 0.03, and 16.5 ± 0.4 mg/l, respectively.
Juvenile uptake study
Uptake and depuration
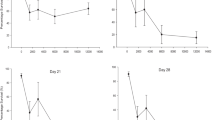
This study found that juvenile apple snails accumulated Cu during the exposure phase and eliminated Cu during the depuration phase. Whole body Cu concentrations of snails increased with exposure time and reached a plateau (saturation) after 14 days of exposure (Fig. 2). During the depuration phase, whole body Cu decreased with time and followed first order kinetics. The values of t d0.5 and t d0.9 (time to depurate 50% and 90% accumulated Cu) ranged from 10 to 14 days and 15 to 34 days, respectively. In general, uptake and depuration kinetics are similar to the results found in the literature for aquatic organisms (Grosell et al. 1996; Lorenzo et al. 2005; Schultz et al. 1980; Yu and Wang 2002; Morgan et al. 2004; Blackmore and Wang 2004). However, the exposure time needed for whole body Cu to reach the saturation level (related to k u) and the depuration rate constant (k d) may vary depending on the organisms and metals. For example, it took about 2 days for accumulated Cu in gill filaments of the European eel (Anguilla anguilla) to reach saturated level (Grosell et al. 1996). Whole body silver in rainbow trout reached saturated level after 10 hours of aqueous exposure (Morgan et al. 2004). Accumulated Se in Daphnia magna reached saturated level after 4 days of exposure (Schultz et al. 1980).
Copper uptake and depuration in juvenile Pomacea paludosa. (Triangle, open cycle, and start are average measured concentrations in wet weight. Error bars are standard deviations (n = 3). Smooth lines are Michaelis–Menten model predictions. Dash lines are one compartment first order kinetics model predictions)
The whole body Cu concentrations at saturation in juvenile apple snails found in the present study (average of days 14, 21, 28) were 29.57 ± 2.30, 48.41 ± 4.29, and 61.87 ± 5.01 μg/g dw at exposure water Cu concentrations of 6.0, 8.2, and 12.2 μg/l, respectively (Table 2). The uptake data found in the present study followed Michaelis–Menten kinetics rather than the 1-CFOK model. The saturated kinetics for metal uptake by aquatic organisms has been documented (Simkiss and Taylor 1989; Newman 1995; McKim 1994; McDonald and Wood 1993). The 1-CFOK model is more applicable for accumulation of organic compounds using K ow for predicting BCF (Spacie and Hamelink 1995; Meylan et al. 1999). The exposure time needed for the accumulated Cu to reach 50% and 90% of saturated level (K M) based on Michaelis–Menten kinetics was ≤1.6 days and ≤14.3 days, respectively (Table 2). This K M is equal t u0.5 (Table 2).
Bioconcentration factor and dependence of Cu uptake on exposure time and concentration
Whole body Cu concentrations reached a plateau after 14 days in the juvenile apple snail study. From this, it can be assumed that Cu partitioning between water and apple snails reached equilibrium after 14 days. Therefore, average whole body Cu concentrations on day 14, 21, and 28 were used to calculate BCFs for the apple snail. The Cu BCFs found in the present study were 1497, 1518, and 1464 at water Cu concentrations of 6.0, 8.2, and 12.2 μg/l, respectively (Table 2). BCFs found in the present study are within the range of BCFs found in the literature for Cu (1144–1854) (McGeer et al. 2003). McGeer et al. (2003) reported that there was an inverse relationship between BCFs and metal exposure concentration but this was not observed here. The values of BCF for Cu found in the present study are lower than the value of BCF for Zn (>1800) and Hg (>4900) (McGeer et al. 2003).
The time needed for P. paludosa to accumulate Cu to a half of the saturated Cu concentration (K M) was ≤1.6 days while the time needed to depurate a half of the saturated Cu concentration (t d0.5) was 10.5–13.8 days. This indicates that the uptake process was faster than the depuration process. During the water uptake phase, metals enter organisms via passive diffusion at cell membranes (e.g., gill membrane) due to the concentration gradient (Hoar and Randall 1984; Grosell et al. 2002). Therefore, the magnitude of accumulation is dependant on exposure time and concentration of the substance in the environment. When exposure time was ≤14 days, whole body Cu was not significantly different for the two exposure scenarios; (2X concentration and 1Y exposure time) and (1X concentration and 2Y exposure time) (Table 3). However, when exposure time ≥14 days, whole body Cu was significantly higher in the exposure scenario of 2X concentration and 1Y exposure time than the exposure scenario of 1X concentration and 2Y exposure time (P = 0.002). This suggests that chronic exposure of apple snails to low Cu concentrations does not result in high Cu accumulation in apple snails. However, apple snails can accumulate high concentrations of Cu from acute exposure to high Cu concentrations. This result also supports the movement of Cu along the concentration gradient from water to apple snails.
Adult uptake study
Water uptake versus uptake from soil ingestion and/or dermal contact
During the uptake phase, whole body Cu concentrations of adult apple snails in the treatment without nitex increased with time (Fig. 3a). This result is similar to the results found in the juvenile uptake study. However, whole body Cu concentrations were stable during exposure when nitex was present and lower than the whole body Cu concentrations of snails with direct contact with soil. The major route of Cu exposure in the latter treatment was water only, with Cu concentrations ranging from 53.6 to 89.3 μg/l. These Cu concentrations were 9- to 14-fold higher than the Cu concentrations in water of the juvenile uptake study. However, the whole body Cu concentrations did increase with exposure time in the juvenile uptake study while they did not in the water only exposure (treatment 1) in the adult uptake study. Lorenzo et al. (2005) demonstrated that the presence of humic acids (≤10 mg/l DOC) significantly reduced Cu uptake by blue mussel gills. This would be due to the complexation of Cu with DOC, resulting in a decrease in the bioavailability of Cu. The presence of DOC was shown to affect acute toxicity of Cu in the Florida apple snail (Rogevich et al. 2008).
To determine bioavailability of Cu in the two exposure studies of the present study (juvenile and adult uptake studies), Windermere Humic-Aqueous Model (WHAM) incorporated in the biotic ligand model (BLM) was used to determine Cu speciation (DiToro et al. 2000). The free Cu portion was about 20-fold higher in the water of the juvenile uptake study (0.0277 μg/l Cu2+) than in the adult uptake study (0.0012 μg/l Cu2+). Most Cu in the water of the adult uptake study was bound to DOC (Cu-DOC). The Cu-DOC is not a bioavailable form of copper since it has been shown that only free Cu is bioavailable (Campbell 1995; Simkiss and Taylor 1995; DiToro et al. 2000). Therefore, although the total Cu concentrations in water of the juvenile uptake study was 9–14-fold lower than the total Cu concentrations in the water of the adult uptake study, Cu bioavailability was 20-fold higher in the juvenile uptake study than in adult uptake study. This would explain why the whole body Cu concentrations of the snails in the adult uptake experiment (treatment with nitex) did not increase over time. It should also be noted that during 28 day exposure, the aperture of the adult apple snails in treatment with nitex were closed and their movement was limited as compared to the movement of the apple snails in the treatment without nitex. This behavioral response may explain the stability of whole body Cu concentrations over time in the adults in the treatment with nitex. The whole body Cu concentration was, on average, 47.19 mg/kg higher in the treatment without nitex than treatment with nitex.
The difference of the whole body Cu concentrations of the adult apple snails in treatments with and without nitex indicates that apple snails can accumulate Cu from soil via soil ingestion and/or dermal contact with soil. This result is in agreement with the results in the literature. A study conducted by USFWS found that whole body Cu concentrations in apple snails were correlated with Cu in sediment (Frakes et al. 2008). A similar result also was demonstrated by Heng et al. (2004) for the freshwater snail (Turitella sp.). Another study conducted by Gomot-de Vaufleury and Pihan (2002) found that Cr uptake by land snails (Helix aspersa and Helix aspersa maxima) via dermal contact was significant compared to the uptake from water. Gomot-de Vaufleury and Pihan (2002) also demonstrated that soil ingestion was the main route of Zn, Cd, and Pb uptake by land snails. If dermal contact is a significant route for Cu accumulation by the apple snail, the potential for Cu accumulation in apple snail tissue would be high because the apple snail lives at the soil/water interface and occasionally burrows under soil surface (Darby et al. 2002). Copper accumulation in snail tissue would also pose a risk to apple snail predators, such as snail kite.
During the depuration phase, whole body Cu concentrations did not decrease in adult snails. In fact, whole body Cu concentrations of the snails from the treatment with nitex appeared to be higher during the depuration phase than the uptake phase (Fig. 3a). During the depuration phase, the snails were living in laboratory freshwater that had a Cu concentration about 3–4 μg/l and DOC about 0.26 mg/l The free Cu in laboratory water was 0.0037 μg/l, which was higher than the free Cu in the water of the uptake phase (0.0012 μg/l). Therefore, the snails would likely accumulate more Cu, which resulted in higher whole body Cu concentrations in the depuration phase than in the uptake phase. This indicates that DOC is an important factor that influences Cu uptake and Cu toxicity to the apple snail. A study conducted by Rogevich et al. (2008) with apple snails found that the 96 h-LC50 for Cu increased with increasing DOC concentration. The influence of DOC on Cu toxicity was also found for other trace metals with other organisms (Hoang et al. 2004; Sciera et al. 2004; VanGenderen et al. 2003; Ryan et al. 2004; Schwartz et al. 2004). Similarly, in the treatment with no nitex, whole body Cu concentrations did not decrease with depuration time (Fig. 3a). Copper uptake by apple snails from this treatment may primarily be from dermal contact and/or soil ingestion during the uptake phase, it was likely from aqueous Cu in the laboratory water during the depuration phase. Therefore, the stability of whole body Cu during the depuration phase suggests that the depuration of Cu was negated by the uptake of aqueous Cu from the depuration water.
Dietary uptake and potential for trophic transfer
Whole body Cu concentrations appeared to increase with time for both the Cu-free and Cu-treated food treatments (Fig. 3b). The reference soil contained 30.5 mg/kg Cu; therefore, the snails fed Cu-free food obtained some Cu from the reference soil, resulting in increase whole body Cu concentrations with time. However, the snails fed Cu-treated lettuce contained higher whole body Cu concentrations than the snails fed Cu-free lettuce, indicating that apple snails can accumulate Cu via diet. The difference between the whole body Cu concentrations was, on average, 18.83 mg/kg higher in adult snails from the Cu-treated food treatment than in the Cu-free food treatment. Copper accumulation via diet also was demonstrated for different snail species and trace metals (Laskowski and Hopkin 1996; Gomot and Pihan 1997; Swaileh and Ezzughayyar 2000; Notten et al. 2005). Recently, a field study conducted by Notten et al. (2005) suggested that dietary metal uptake was the most important exposure route for the landsnail (Cepaea nemoralis) compared to other routes of uptake.
During the depuration phase, whole body Cu concentrations of the apple snails did not decrease in either dietary treatment (Fig. 3b). For the Cu-free dietary treatment, whole body Cu concentrations were higher during the depuration phase than the uptake phase. This may be due to the higher Cu bioavailability in the depuration water than the uptake water, as described previously.
Body distribution of accumulated Cu and bioaccumulation factors
For all potential exposure routes in both the uptake and the depuration phases, the distribution of accumulated Cu in adult apple snails was similar (Fig. 4). The highest Cu concentration was found in the viscera (about 60%) and the lowest Cu concentration was in the shell (<4%). The copper burden in the foot was about 40%. Similar results were found by Laskowski and Hopkin (1996) for the garden snail: trace metals (Cu, Zn, Cd, Pb) concentrations in shell of snails exposed to either individual or mixtures of Cu, Zn, Cd, and Pb for three months did not exceed 5% of the whole body concentration. Most of the accumulated metals were in soft tissues (foot and viscera). Recently, Desouky (2006) also reported that up to 80% of accumulated metals (Al, Cd, Zn) were found in the viscera (kidney and digestive gland) of the pond snail (Lymnaea stagnalis).
When exposed to metals, aquatic organisms have developed strategies for regulating internal copper concentrations. Some species store excess copper by sequestering the metals in forms that are either metabolically available (e.g., as metallothionein) or unavailable (e.g., as phosphate granules) (Phillips and Rainbow 1989; Simkiss 1981; Viarengo 1989; Depledge and Rainbow 1990; Mason and Nott 1981). Desouky (2006) found that the number of granules in the digestive gland of the garden snail increased after 10 days of exposure to metals (Al, Zn, Cd). In granule cells, metals can bind to ligands such as P and S and become insoluble and immobile. This mechanism may account for the high Cu concentration found in viscera in the apple snails. The high Cu burden found in the soft tissues of the apple snails (foot and viscera) reveals potential for Cu transfer through the food chain, since most apple snail predators (e.g., snail kite) consume the soft tissues and not the shell.
Bioaccumulation factors were calculated for the foot, the viscera and the shell. The results of BAFs are shown in Table 4. For both the dietary uptake and the uptake from soil, Cu BAFs were highest for the viscera and lowest for the shell. Gomot and Pihan (1997) found that dietary Cu BAFs for Helix aspersa maxima and Helix aspersa aspersa were higher for the foot than for the viscera. Based on the data reported by Laskowski and Hopkin (1996) for Helix aspersa, the Cu BAFs for soft tissues (foot and viscera) would range from 0.45 to 10.96 when dietary Cu concentrations ranged from 2 to 1250 μg/g. The dietary Cu BAFs found in the present study were within this range of BAFs.
BAFs may vary, however, depending on specific metals and routes of uptake. The dietary Cu BAFs found in the present study were higher than the dietary Zn BCFs found by Gomot and Pihan (1997) and Gomot-De Vaufleury and Pihan (2002). Results found by Laskowski and Hopkin (1996) support the present results. The present study found that dietary Cu BAFs were from 3- to 8-fold higher than the soil Cu BAFs. This indicates that dietary Cu uptake is more important than uptake through soil ingestion and/or dermal soil contact. This conclusion is supported by Notten et al. (2005) who found that the relationship between metal concentrations in snails and plant leaves was stronger than the relationship between metal concentrations in snails and soil.
Summary and conclusions
This study demonstrated that the Florida apple snail accumulated Cu from the environment via different routes. Aqueous Cu uptake was strongly dependent on DOC in the water. Elevated DOC concentration decreased Cu bioavailability and uptake. The distribution of the measured total organic carbon and Cu concentrations in surface water in Everglades since 1971 shows that 90% of the measurements had TOC concentrations ≥7.7 mg/l and 10% of the measurements had Cu concentrations ≥4.2 μg/l (SFWMD, DBHYDRO database, 1971–1985). With these TOC and Cu concentrations, bioavailability of Cu would be <0.0001 μg/l. Therefore, aqueous Cu uptake will not be of concern for apple snails in the Everglades ecosystem. However, soil ingestion and/or soil dermal contact appear to also be important Cu uptake routes for apple snails. In addition, dietary uptake resulted in high BAFs. This indicates that Cu transfer through the food chain from food to the apple snail was the most important uptake route compared to water or soil exposure and therefore, may pose a threat to apple snail predators, such as the Florida snail kite. For all exposure routes, most of the Cu in apple snails accumulated in the soft tissues, which is typically consumed by predators. More studies should be conducted to examine the potential of Cu transfer from the apple snail to organisms at higher trophic levels.
References
Alva AK, Graham JH, Anderson CA (1995) Soil-pH and copper effects on young Hamlin Orange Trees. Soil Sci Soc Am J 59:481–487
Blackmore G, Wang WX (2004) The transfer of cadmium, mercury, methylmercury, and zinc in an intertidal rocky shore food chain. J Exp Mar Biol Ecol 307:91–110. doi:10.1016/j.jembe.2004.01.021
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, New York, pp 45–102
Darby PC, Bennetts RE, Miller SJ, Percival HF (2002) Movements of Florida apple snails in relation to water levels and drying events. Wetlands 22:489–498. doi:10.1672/0277-5212(2002)022[0489:MOFASI]2.0.CO;2
Depledge MH, Rainbow PS (1990) Models of regulation and accumulation of trace metals in marine invertebrates. Comp Biochem Physiol 97:1–7
Desouky MMA (2006) Tissue distribution and subcellular localization of trace metals in the pond snail Lymnaea stagnalis with special reference to the role of lysosomal granules in metal sequestration. Aquat Toxicol 77:143–152. doi:10.1016/j.aquatox.2005.11.009
DiToro DM, Allen HE, Bergman HL, Meyer JS, Santore RC, Paquin P (2000) The biotic ligand model: a computational approach for assessing the ecological effects of copper and other metals in aquatic systems. International Copper Association, Ltd., New York
Everglades National Park (2001) Everglades National Park Strategic Plan 2001–2005. National Parks Service, U.S. Department of the Interior, Homestead, FL
Frakes RA, Bargar TA, Boughner EA (2008) Sediment copper bioavailability to freshwater snails in South Florida: risk implications for the Everglade snail kite (Rostrhamus sociabilis plumbeus). Ecotoxicology. doi:10.1007/s10646-008-0233-x
Gomot A, Pihan F (1997) Comparison of the bioaccumulation capacities of copper and zinc in two snail subspecies (Helix). Ecotoxicol Environ Saf 38:85–94. doi:10.1006/eesa.1997.1566
Gomot-de Vaufleury A, Pihan F (2002) Methods for toxicity assessment of contamined soil by oral or dermal uptake in land snails: metal bioavailability and bioaccumulation. Environ Toxicol Chem 21:820–827. doi:10.1897/1551-5028(2002)021 ≤ 0820:MFTAOC ≥ 2.0.CO;2
Grosell M, Boetius I, Hansen HJM, Rosenkilde P (1996) Influence of preexposure to sublethal levels of copper on 64Cu uptake and distribution among tissues of the europeaan eal (Anguilla anguilla). Comp Biochem Physiol 114:229–235
Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol 133:287–303
Heng LY, Mokhtar MB, Rusin S (2004) The bioaccumulation of trace essential metals by the freshwater snail, Turritella sp. found in the rivers of Borneo East Malaysia. J Biol Sci 4:441–444
Hoang TC, Tomasso JR, Klaine SJ (2004) Influence of water quality and age on nickel toxicity to fathead minnows (Pimephales promelas). Environ Toxicol Chem 23:86–92. doi:10.1897/03-11
Hoang TC, Rogevich EC, Rand GM, Gardinali PR, Frakes RA, Bargar TA (2008) Copper desorption in flooded agricultural soils and toxicity to the Florida apple snail (Pomacea paludosa): implications in Everglades restoration. Environ Pollut 154:338–347
Hoar WS, Randall DJ (1984) Fish Physiology: ion and water transfer, part B, vol 10. Academic Press, Inc, Orlando, FL
Koster M, de Groot A, Vijver M, Peijnenburg W (2006) Copper in the terrestrial environment: verification of a laboratory-derived terrestrial biotic ligand model to predict earthworm mortality with toxicity observed in field soils. Soil Biol Biochem 38:1788–1796. doi:10.1016/j.soilbio.2005.11.033
Laskowski R, Hopkin SP (1996) Accumulation of Zn, Cu, Pb and Cd in the garden snail (Helix aspersa): implications for predators. Environ Pollut 91:289–297. doi:10.1016/0269-7491(95)00070-4
Lorenzo JI, Beiras R, Mubiana VK, Blust R (2005) Copper uptake by Mytilus edulis in the presence of humic acids. Environ Toxicol Chem 24:973–980. doi:10.1897/04-216r.1
Mason AZ, Nott JA (1981) The role of intracellular biomineralized granules in the regulation and detoxification of metals in gastropods with special reference to the marine prosobranch Littorina littorea. Aquat Toxicol 1:239–256. doi:10.1016/0166-445X(81)90018-7
McDonald DG, Wood CM (1993) Branchial mechanisms of acclimation to metals in freshwater fish. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman & Hall, London, pp 297–321
McGeer JC, Brix KV, Skeaff JM, DeForest DK, Brigham SI, Adams WJ et al (2003) Inverse relationship between bioconcentration factor and exposure concentration for metals: implications for hazard assessment of metals in the aquatic environment. Environ Toxicol Chem 22:1017–1037. doi:10.1897/1551-5028(2003)022<1017:IRBBFA>2.0.CO;2
McKim JM (1994) Physiological and biochemical mechanisms that regulate the accumulation and toxicity of environmental chemicals in fish. In: Hamelink JL, Landrum PF, Bergman HL, Bensen WH (eds) Bioavailability: physical, chemical and biological interactions. CRC Press, Boca Raton, FL, pp 179–201
Meylan WM, Howard PH, Boethling RS, Aronson D (1999) Improved method for estimating bioconcentration/bioaccumulation factor from octanol/water partition coefficient. Environ Toxicol Chem 18:664–672. doi:10.1897/1551-5028(1999)018<0664:IMFEBB>2.3.CO;2
Morgan TP, Grosell M, Playle RC, Wood CM (2004) The time course of silver accumulation in rainbow trout during static exposure to silver nitrite: physiological regulation or an artifact of the exposure conditions? Aquat Toxicol 66:55–72. doi:10.1016/j.aquatox.2003.07.003
Newman MC (1995) Bioaccumulation in quantitative methods in aquatic ecotoxicology. CRC Press, Boca Raton, FL
Notten MJM, Oosthoek AJP, Rozema J, Aerts R (2005) Heavy metal concentrations in a soil-plant-snail food chain along a terrestrial soil pollution gradient. Environ Pollut 138:178–190. doi:10.1016/j.envpol.2005.01.011
Phillips DJH, Rainbow PS (1989) Strategies of trace metal sequestration in aquatic organisms. Mar Environ Res 28:207–210. doi:10.1016/0141-1136(89)90226-2
Rogevich EC, Hoang TC, Rand GM (2008) Influence of water quality and organism age on copper toxicity to the Florida apple snail (Pomacea paludosa). Arch Environ Contam Toxicol 54:690–696
Ryan AC, VanGenderen EJ, Tomasso JR, Klaine SJ (2004) Influence of natural organic matter source on copper toxicity to larval fathead minnows (Pimephales promelas). Environ Toxicol Chem 23:1567–1574. doi:10.1897/02-476
Schultz TW, Freeman SR, Dumont JN (1980) Uptake, depuration, and distribution of selenium in Daphnia magna and its effects on survival and ultrastructure. Arch Environ Contam Toxicol 9:23–40. doi:10.1007/BF01055497
Schwartz ML, Curtis PJ, Playle RC (2004) Influence of natural organic matter source on acute copper, lead, and cadmium toxicity to rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 23:2889–2899. doi:10.1897/03-561.1
Sciera KL, Isley JJ, Tomasso JR, Klaine SJ (2004) Influence of multiple water-quality characteristics on copper toxicity to fathead minnows (Pimephales promelas). Environ Toxicol Chem 23:2900–2905. doi:10.1897/03-574.1
Sharfstein B, Steinman AD (2001) Growth and survival of the Florida apple snail (Pomacea paludosa) fed 3 naturally occurring macrophyte assemblages. J N Am Benthol Soc 20:84–95. doi:10.2307/1468190
Simkiss K (1981) Metal discriminating processes in metal accumulating cells. J Exp Biol 94:317–327
Simkiss K, Taylor MG (1989) Metals fluxes cross membranes of aquatic organisms. Rev Aquat Sci 1:173–188
Simkiss K, Taylor MG (1995) Transport of metals across membranes. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, Chichester
South Florida Water Management District (2001–2006) Reports (submitted to). South Florida Water Management District for Phase I/II Environmental Site Assessments. South Florida Water Management District, West Palm Beach, FL
Spacie A, Hamelink JL (1995) Bioaccumulation. In: Rand GM (ed) Fundamentals of aquatic toxicology: effects of environmental fate and risk assessment. Taylor & Francis, New York, pp 1052–1082
Swaileh KM, Ezzughayyar A (2000) Effects of dietary Cd and Cu on feeding rate and growth rates of the landsnail Helix engaddensis. Ecotoxicol Environ Saf 47:253–260. doi:10.1006/eesa.2000.1961
U.S. Environmental Protection Agency (1996) Acid digestion of sediment, sludges and soils: EPA method 3050B, SW–846 manual, Washington, DC
VanGenderen EJ, Ryan AC, Tomasso JR, Klaine SJ (2003) Influence of dissolved organic matter on silver toxicity to Pimephales promelas. Environ Toxicol Chem 22:2746–2751. doi:10.1897/02-501
Viarengo A (1989) Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. CRC Rev Aquat Sci 1:295–317
Yu RQ, Wang WX (2002) Kinetic uptake of bioavailable cadmium, selenium, and zinc by Daphnia magna. Environ Toxicol Chem 21:2348–2355. doi:10.1897/1551-5028(2002)021<2348:KUOBCS>2.0.CO;2
Acknowledgments
We thank Kathy Moore for her help with Cu analysis. This study was funded by U.S. Fish and Wildlife Service Cooperative Agreement No. 401816J034. This is SERC contribution no. 393.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoang, T.C., Rogevich, E.C., Rand, G.M. et al. Copper uptake and depuration by juvenile and adult Florida apple snails (Pomacea paludosa). Ecotoxicology 17, 605–615 (2008). https://doi.org/10.1007/s10646-008-0243-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0243-8