Abstract
To better understand the fate of metals in the environment, numerous parameters must be studied, such as the soil properties and the different sources of contamination for the organisms. Among bioindicators of soil quality, the garden snail (Cantareus aspersus) integrates multiple sources (e.g. soil, plant) and routes (e.g. digestive, cutaneous) of contamination. However, the contribution of each source on metal bioavailability and how soil properties influence these contributions have never been studied when considering the dynamic process of bioavailability. Using accumulation kinetics, this study showed that the main assimilation source of Cd was lettuce (68 %), whereas the main source of Pb was the soil (90 %). The plant contribution increased in response to a 2-unit soil pH decrease. Unexpectedly, an increase in the soil contribution to metal assimilation accompanied an increase in the organic matter (OM) content of the soil. For both metals, no significant excretion and influence of source on excretion have been modelled either during exposure or depuration. This study highlights how the contribution of different sources to metal bioavailability changes based on changes in soil parameters, such as pH and OM, and the complexity of the processes that modulate metal bioavailability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increased contamination of the environment with metals since the onset of the industrial revolution, the soil has been monitored with bioindicators (Pérès et al. 2011; Pauget et al. 2013a). Assessing the bioavailability of contaminants is a valuable tool for the decision-making process for contaminated site management, especially when the soil or water is contaminated (Moreno-Jiménez et al. 2011). During an ecological risk assessment (ERA), single-species tests that cover single and identified exposure source (e.g. soil, food) or different routes (e.g. digestive, dermal) associated with a single compartment (e.g. sediment, water) are typically performed (Tarazona and Vega 2002). However, when undertaking passive, i.e. naturally exposed sentinel species, or active, i.e. in situ caging of sentinels species (Beeby 2001), biomonitoring, organisms can be exposed to multiple sources of contamination, such as soil, vegetation and air. Thus, knowing the influence of these exposure sources on how the bioindicator accumulates metal is necessary to accurately characterise the metal bioavailability to this organism (Fairbrother et al. 2007; Burger 2008).
Among soil fauna, the land snail Cantareus aspersus has been used for biomonitoring using snails caged in microcosms (Scheifler et al. 2003a; Gimbert et al. 2008a; Pauget et al. 2013b). Active biomonitoring allows all contamination sources to be considered (Scheifler et al. 2003a; Gimbert et al. 2008a). For this purpose, active biomonitoring coupled with accumulation kinetics is relevant to evaluate the metal bioavailability to organisms via the modelling of assimilation fluxes (Gimbert et al. 2008a). After assimilation, snails can sequester metals in various compartments (Gimbert et al. 2008b). Even if sequestration and elimination of metals have been studied (Vijver et al. 2005; Notten et al. 2006; Gimbert et al. 2008b), the influence of the sources of the metals and soil parameters on excretion remains unknown. Previous studies on the contribution of contamination sources on metal accumulation (Scheifler et al. 2006; Fritsch et al. 2008) considered neither the dynamic processes of bioavailability nor the influence of soil properties on the metal transfer, even though these parameters are key factors. Thus, data on the contribution of each contamination source (e.g. soil, plant) on metal assimilation by snails and on the influence of soil properties on this contribution remain lacking.
Therefore, based on an experimental design using spiked and artificially modified soil, the present study aimed (i) to assess the relative contribution of soil and vegetation on the Cd and Pb assimilation and the influence of these sources on metal excretion by snails and (ii) to evaluate the influence of pH and organic matter (OM) on this contribution using kinetic modelling of accumulation.
Materials and methods
Animals
Juvenile brown garden snails (C. aspersus Müller, 1774) were reared under controlled conditions as described previously (Gomot-de Vaufleury 2000; ISO 15952 2006). The individuals used for the test were subadults, reared for 7–9 weeks and weighing 5.11 ± 0.75 g (±SD; n = 415) at the beginning of the experiment.
Exposition sources
Soil
An uncontaminated agricultural field soil (Chambornay-les-Pin, Eastern France) was used for further soil parameter modifications (Table 1). Sandy loam was taken from the top layer (depth, 0–15 cm) of a maize field and transferred to the laboratory, where it was air-dried and then sieved through a 4-mm mesh.
The soil characteristics (Table 1) were artificially modified by adding to the dried soil of Chambornay-les-Pins: (i) dried ground peat (OECD 1984) to raise the organic matter content (soil P) from 1.4 to 8 % and/or (ii) CaCO3 powder to obtain two nominal pH values (5 and 7) (OECD 1984).
The soils were spiked by spraying Cd (CdCl2, 99.9 % purity, Aldrich Chemical) and Pb (PbSO4, 99.9 % purity, Aldrich Chemical) in aqueous solution to reach nominal concentrations of 20 and 2000 mg kg−1 soil DW (dry weight), respectively. Deionised water was added to reach 50 % of the water holding capacities of the different substrates. The soils were left to stabilise in the dark for 1 month (Pauget et al. 2011) before snail exposure.
Lettuce
Lettuce was cultivated on polystyrene containers on each soil of the experiment under controlled conditions (20 ± 2 °C; photoperiod 18 h L/6 h D; relative humidity 80 ± 90 %). Three times per week, the lettuce was watered with uncontaminated water. During the experiment, snails were fed with lettuce that had grown for 2 months on the soil on which snails are exposed. Thus, contaminated lettuce was obtained for each contaminated soil to provide better environmental relevance.
Experimental design
Accumulation kinetics were determined based on a 10-day experiment, in which snails were exposed to (i) contaminated soil with contaminated lettuce (S*L*), (ii) contaminated soil with uncontaminated lettuce (S*L), (iii) uncontaminated soil with contaminated lettuce (SL*), (iv) contaminated soil without lettuce (S*) and (v) contaminated lettuce without soil (L*).
For each treatment, ten snails were housed in each of three replicate polystyrene containers (24 × 21 × 8 cm) containing a 1-cm layer (100 g dry mass) of soil prepared as described above (except for treatment L*, in which no substrate was added). The photoperiod was 18 L/6 D, and the temperature was 20 ± 2 °C. The relative humidity was kept at 80 to 95 %. The soil moisture content was maintained at its initial level by regular spraying with demineralised water. Three times per week, the containers were cleaned, any remaining food was replaced and demineralised water was sprayed to prevent the soil from drying out.
After 2, 4, 6, 8 and 10 days of exposure, one snail per treatment was randomly sampled. After 10 days of exposure, the organisms were transferred onto the corresponding uncontaminated soil and fed with uncontaminated lettuce to assess the metal depuration, and one snail per treatment was randomly sampled after 2, 5, 7 and 10 days of depuration.
Analytical procedures
Soil
Total Cd and Pb was measured by inductively coupled plasma atomic emission spectrophotometry (ICP-AES) after digestion of the soil samples (250 mg) with hydrofluoric and perchloric acid, as described in AFNOR (1996). Analyses were performed by the Laboratoire d’Analyse des Sols of Arras (France), which benefits from the COFRAC (French accreditation committee) accreditation n°1-1380 for its analytical quality for metal measurements in soils. Soil characteristics were assessed/described by the same laboratory.
Lettuce
Lettuce was freeze-dried until reaching a constant weight (0.08 ± 0.07 mg) and digested in a solution of 6 mL nitric acid and 2 mL hydrogen peroxide (HNO3 65 %, H2O2 30 %, Carlo-Erba analytical quality). After digestion, the samples were diluted by adding ultra-pure water (18.2 MΩ/cm2) as previously described by Fritsch et al. (2011) and analysed by ICP OES (iCAP 6000, Thermo Fisher Scientific).
Snails
Snails for analysis were placed in clean containers, fasted for 48 h (the faeces were removed after 24 h) and then weighed. The snails were sacrificed by freezing at −80 °C. After thawing, the whole soft body was removed from the shell, and the foot and viscera were separated (Gomot-de Vaufleury and Pihan 2002). Only the viscera were studied in this work because they contain the digestive gland (hepatopancreas), which is the main site of metal accumulation and storage in snails (Hopkin 1989). The viscera were oven-dried at 60 °C until reaching a constant weight (0.228 ± 0.049 g, n = 415) and digested in nitric acid (HNO3 65 %, Carlo-Erba analytical quality), as previously described (Gomot-de Vaufleury and Pihan 2002). After digestion, samples were diluted adding ultra-pure water (18.2 MΩ/cm2), filtered with ash-free filter paper and analysed by ICP OES (iCAP 6000, Thermo Fisher Scientific).
The analysis reliability was assessed with standard reference materials (ICHTJ-cta-VTL-2, Institute of Nuclear Chemistry and Technology, Poland, for plants and TORT-2 lobster hepatopancreas; National Research Council of Canada–Institute for National Measurement Standard, Ottawa, ON, Canada, for snails) and was within 10 % of the certified values.
Statistical analyses
Accumulation modelling
Bioavailability results from a dynamic interaction between the metal concentration in the soil (environmental availability) and the physiology of the target species. To assess bioavailability, a one-compartment model was used to fit the accumulation kinetic data (Gimbert et al. 2006; Pauget et al. 2011). This model expresses the dynamic change of metal concentration in the snail viscera (C sn in mgmetal kg DWsn −1) over time, based on the following equation (Eq. 1):
where a is the assimilation flux constant (mgmetal kg DWsn −1 day−1), which is considered as an indicator of the metal bioavailability to snails (Gimbert et al. 2008a; Pauget et al. 2011), k 2 is the excretion rate constant (day−1) and t is time (days). C sn(0) is the average metal concentration measured in ten snails at the beginning of the experiment (mgmetal kg DWsn −1).
When negative value estimates of excretion rate k 2 (which do not make biological sense) were modelled by Eq. 1, a linear model was substituted into Eq. 1 according to Eq. 2 to accurately assess the assimilation flux (a) in these particular cases:
Depuration modelling
During the depuration phase, excretion can occur and lead to variations of metal concentrations in snails (C sn) with time, according to the following equation (Eq. 3):
where C mu is the metal concentration at the end of the exposure period (mg kg DWsn −1 (Gimbert et al. 2006)), k 2d is the excretion of metal (day−1), t c is the time (days) at which animals were transferred to the clean soil and t is the time (days) since the beginning of the experiment. All negative estimates of excretion (k 2d) were considered as not biologically relevant and therefore are not presented in the tables of results.
The accumulation and excretion parameters were estimated by fitting the models with a mixed-effect procedure (non-linear mixed-effect (nlme) or linear mixed-effect (lme)) (Lindstrom and Bates 1990), allowing for nested random effects. The within-group errors were allowed to be correlated and/or have unequal variances. The nlme integrated the soil as a fixed factor and the container as a random effect. The lme integrated the metal concentration in snails as the dependent variable and the soil as the explanatory variable. For all models (lme and nlme), variance functions (power and exponential) were applied when residuals were skewed, and the best model was selected according to Akaike’s information criterion (AIC, pgirmess package) (Burnham and Anderson 2004). Significant differences in parameter estimates between treatments were judged from the absence of overlap of their 95 % confidence intervals (95 % CI). All statistical analyses were performed with the free statistics software package R (version 2.10.1, R Development Core Team 2011).
Determination of the contribution of soil and lettuce in the Cd and Pb bioavailability
To quantify the relative contribution of soil and lettuce to the metal bioavailability to snails, we compared the mean assimilation flux percentage of snails exposed to one contamination source to the sum of the assimilation fluxes of snails exposed to (i) the soil and (ii) the lettuce according to the following:
Results
Accumulation and depuration kinetics
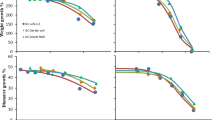
At the beginning of the exposure, the internal concentrations in snails were 0.73 ± 0.10 mg kg−1 for Cd and 0.59 ± 0.26 mg kg−1 for Pb (n = 10). After 10 days of exposure, Cd and Pb accumulated in the snails, as indicated by the significant assimilation fluxes, which ranged from 0.604 to 4.34 mg Cd kgsn −1 day−1 and from 0.954 to 66.7 mg Pb kgsn −1 day−1. Only 7P S* (p value = 0.267) for Cd and 7P SL* (p value = 0.169) for Pb yielded positive but non-significant assimilation fluxes, which have been modelled (Table 2).
Overall, the highest assimilation flux values for Cd were observed when the contaminated lettuce was offered, whereas for Pb, snails exposed to the contaminated soil presented with the highest assimilation fluxes.
During the exposure phase, no significant (p value >0.05) excretion rate (k 2) was modelled for Cd. For Pb, few excretion rates were modelled. Only one excretion rate was significant for 5P S*L*.
During the depuration phase, Cd did not appear to be excreted by the snails. Only three positive k 2d values were modelled (two are significant and one is not significant) (Table 2). In contrast to Cd, the Pb seems to be excreted, as indicated by the ten modelled positive k 2d values (four significant and six not significant).
Influence of soil properties on metal bioavailability to snails
Influence of organic matter content
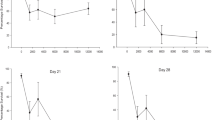
An increase of organic matter content appears to reduce Cd bioavailability to snails when they are exposed to the treatments presenting contaminated lettuce (L*) decreasing from 3.93 to 2.94 mgCd kgsn −1 day−1 (S*L*), from 3.15 to 1.36 mgCd kgsn −1 day−1 (SL*) and from 3.33 to 1.68 mgCd kgsn −1 day−1 (L*) at 1.4 and 8 % of OM content (respectively). In the treatments with contaminated soil (S*), a slight increase is observed (Table 2 and Figs. 1 and 3). For Pb, an increase of OM content seems to increase the Pb bioavailability to snails under the S*L*, S*L and S* treatments, as shown by the assimilation fluxes, which respectively increased from 19.5 to 23.8 mgPb kgsn −1 day−1 (S*L*), from 16.3 to 21.3 mgPb kgsn −1 day−1 (S*L) and from 7.3 to 20.4 mgPb kgsn −1 day−1 (S*) (Table 2 and Figs. 2 and 3).
Influence of pH
A decrease of 2.6 units of pH tended to increase Cd bioavailability in all treatments (Table 2 and Figs. 1 and 3) with assimilation fluxes values going from 2.94 to 4.34 mgCd kgsn −1 day−1 (S*L*), from 1.07 to 1.95 mgCd kgsn −1 day−1 (S*L), from 1.36 to 2.83 mgCd kgsn −1 day−1 (SL*) and from 1.68 to 3.69 mgCd kgsn −1 day−1 (L*). For Pb, a significant increase of bioavailability was observed (Table 2 and Figs. 2 and 3) for the S*L* treatment at the lower pH, with the assimilation flux going from 23.8 to 66.8 mgPb kgsn −1 day−1, and the L* treatment, with the assimilation going from 0.579 to 4.15 mgPb kgsn −1 day−1, but no difference was observed between the S*L and S* treatments.
Determination of the contribution of soil and plants to metal bioavailability to snails
We wanted to first determine whether the sum of the assimilation fluxes of snails exposed to (i) soil and (ii) lettuce corresponded to the assimilation flux of snails exposed to the both sources. For Cd, the sum of the assimilation fluxes (a S*L + a SL* and a S* + a L*) corresponded to the measured assimilation fluxes on the S*L* treatments for the three soils (Table 3). For Pb, the sum of the assimilation fluxes of the two sources corresponded to the bioavailability to snails of both sources on soils 7P and 7C with the S*L and SL* treatments and on soil 7P with the S* and L* treatment. For the other cases, the assimilation flux with both contamination sources was higher than the sum of the assimilation fluxes for the separate contamination sources. However, on soil 5P, the Pb bioavailability to snails exposed to the two contamination sources may have been overestimated; the sums of a S*L + a SL* and a S* + a L* were the same, and the internal concentrations of Pb after 10 days of exposure were quite similar among the S*L*, S*L and S* treatments.
The relative contribution of each contamination source is presented in Table 4. Without considering the influence of the soil parameters, the contribution of soil to Pb assimilation by snails is approximately three times higher than for Cd (90 vs 32 %, respectively), whereas the contribution of lettuce was more than six time higher for Cd than for Pb (68 vs 10 %, respectively). When considering the influence of soil characteristics, we found that raising the OM content increased the soil contribution by 29 % for Cd and by 6 % for Pb, thereby decreasing the lettuce contribution. A decrease of two pH units decreased the soil contribution by 13 % for Cd and 11 % for Pb, thereby increasing the lettuce contribution.
Discussion
Accumulation and excretion of metals
In this 10-day exposure study, we characterised relevant indicators of the bioavailability of Cd and Pb that are present in soil and lettuce to snails by modelling assimilation fluxes for both contamination sources. Soil metal bioavailability to snails appears higher for Cd than for Pb, as shown by the maximum factor of ten between the assimilation fluxes of Cd and Pb, whereas the total concentration in soil is 100 times lower for Cd than for Pb. This high Cd bioavailability can be explained by the ability of the snail to assimilate the non-labile fraction (Scheifler et al. 2003b). Moreover, the use of kinetic studies allows the excretion rates to be modelled, which could be particularly interesting to highlight the internal management of these contaminants (Gimbert et al. 2008a). In our study, significant excretion rates was not reached, probably due to the short exposure duration and inter-individual variability of metal accumulation, the latter of which has already been observed. Moreover, Cd accumulation by snail tends to be linear, with Cd binding to metallothioneins (MT) that are very slowly excreted (Vaufleury 2015). Under our experimental conditions, the sequestration of Cd, which mainly binds to MT in the cytosolic fraction (Vaufleury 2015), may not be efficient enough to be overloaded by the elevated assimilation of Cd (Höckner et al. 2011). The Cd concentration in snails after the exposure duration was greatly lower than that found in snails in the study of Dragos et al. (Nica et al. 2015). For Pb, its sequestration within metal-rich granules allows all assimilated Pb to be stored, limiting its excretion during our short exposure phase (Gimbert et al. 2008b).
The depuration period allowed the identification of positive Cd excretion values in only three treatments and Pb excretion values in eight treatments. In accordance with the literature (Hopkin 1989; Spurgeon and Hopkin 1999; Dallinger et al. 2004), our results highlight the differences in Cd and Pb excretion by snails. The low turnover of Cd-MT may explain the low excretion, and the internal concentrations remained elevated at the end of the depuration phase, whereas the Pb bounded to granules is more easily excreted (Gimbert et al. 2008b). The absence of modelled Cd excretion values during the depuration phase is due to a slight increase in the Cd concentration in snails even though they were exposed to uncontaminated soil and lettuce. During this study, the metal concentration was not measured in the foot. The slight increase in the Cd concentration during the depuration phase may be due to a metal transfer from the foot to the viscera. Indeed, in the absence of an external metal source during the depuration phase, only the foot can be involved because the shell is not a metal sink (Laskowski and Hopkin 1996). Because the dermal route via the foot is a significant route for metal assimilation (Coeurdassier et al. 2002), Cd may continue to come from the foot during the depuration phase and/or the snails can relocate metal in different parts of their body (Gimbert et al. 2006), thus obscuring metal depuration by a metal flux coming from the foot.
Soil and plant contributions to metal bioavailability to the snails
Our experimental design permitted us to assess the contribution of both soil and lettuce on metal bioavailability to snail. Soil characteristics, such as pH and OM, and plant contributions are important explanatory variables to model Cd and Pb accumulation in snails, as demonstrated in situ (Pauget et al. 2015). However, understanding the complex interactions between soil-plant and soil-plant-snails transfer remains a great challenge. The use of accumulation kinetics allows the influence of soil parameter, such as OM content and pH, on metal fluxes in organisms (assimilation and excretion) to be assessed, whereas these influences are not identified when comparing metal concentrations in the snail viscera after 10 days of exposure.
We observed different influences of contamination sources on the amount and fluxes of metal assimilated by the snails. Cd was mainly assimilated from lettuce (68.1 %), whereas assimilated Pb came mainly from the soil (89.9 %). These results are in accordance with a previous study based on two durations of exposure; this study estimated that the soil contribution to metal bioaccumulation by snails can be higher than 80 % for Pb and from 2 to 40 % for Cd, depending on the stage of development of the plant (Scheifler et al. 2006). The use of kinetic studies to identify the contamination sources allows the fluxes of metal in snails from each source of exposure to be determined and highlights difference even if the same concentrations in metal are measured in the viscera at the end of exposure. The determination of the fluxes, the sources and the speciation of the metal assimilated are important during bioavailability assessments because they condition the adverse effects of metals (Notten et al. 2005; Peijnenburg et al. 2007; Calhôa et al. 2011). The difference between the Cd and Pb in the plant contribution may be due to differences in the metal locations within the plant. Indeed, Cd may be mainly present as free metal ions in the leaves (Leita et al. 1991; Mendoza-Cozatl et al. 2011) and thus be easily bioavailable to snails (Li et al. 2015), whereas Pb is located in the cell walls (Qureshi et al. 1986). The sequestration form of the metal in the contamination source is important because Cd that has bound to metallothioneins is less bioavailable to isopod than Cd bound to heat-denatured proteins (Monteiro et al. 2008).
The similar assimilation fluxes of the snails exposed to both contamination sources and the addition of assimilation fluxes of snails exposed only to one contamination source (S* + L* or S*L + SL*) suggest that the feeding behaviour of snails is not modified by the presence of metal in the food (soil or plant), as previously observed (Noret et al. 2005; Sinnett et al. 2009). When we focus on soil parameters that influence the contribution of the sources on metal bioavailability to snails, we observe an influence of OM content. Unexpectedly, the addition of organic matter increases the contribution of soil for Cd and Pb bioavailability, possibly due to the increase of the OM content in the soil solution, leading to greater metal accessibility (Girard et al. 2005). Another hypothesis is an increase in soil ingestion by the snails. When deriving the uptake rates (k 1 = a/metal concentration in soil or lettuce, representing the exposure (Pauget et al. 2011)) for the S*L treatment with soils 7C and 7P, we observed an increase of soil exposure, indicating increased soil ingestion. Snails must eat soil to obtain nutrients (Gomot et al. 1989). Thus, the addition of OM in soil 7P may decrease the concentration of nutrients in soil and may force the snails to eat more soil to satisfy their physiological needs. Moreover, a soil with a high OM content may be more palatable, and the OM consumed by snails contributes to their growth (Elmslie 1998).
When focusing on the influence of a decrease in pH, we observed an increase in the contribution of the lettuce to Cd and Pb assimilation related to the greater lettuce contamination. Alternatively, this increased lettuce contribution may be due to the decreased soil contribution. Even if the soil contribution has decreased, assimilation fluxes from the soil have increased but to a lesser extent than the increased assimilation fluxes in snail exposed to lettuce. This difference may be due to differences in the bioavailability of metal coming from soil and plants. An increase of Cd and Pb bioavailability has already been shown with a 2-pH-unit decrease by Pauget et al. (2011) when soil is the only source of contamination. This increase occurs because at neutral pH, the Cd and Pb adsorption and precipitation increase (Boshoff et al. 2015). This increase is mainly due to the modification of metal mobility in the soil, as observed in previous studies (Sterckeman et al. 2004; Van Gestel and Koolhaas 2004) that have shown that Cd and Pb speciation partially depend on soil acidity. Indeed, acidic soils might modify colloid effects on proton activity, increasing their solubility and their bioavailability to snails and lettuce. Moreover, Sauvé et al. (2000) reported that the pH explained 47 % of the Pb and Cd partitioning coefficients.
Conclusion
This study provides new information that is necessary to better understand metal bioavailability and transfer to snails. Indeed, the complex evaluation of contamination sources and the respective influences of environmental parameters such as OM and pH on both phyto- and zooavailability is needed to understand metal transfer during in situ metal bioavailability assessment because organisms are exposed to multiple contamination sources. The great contribution of lettuce in Cd assimilation by snails reinforces the fact that only measuring the total soil concentration is insufficient to accurately assess Cd bioavailability to snails. These data on source contributions will improve bioavailability modelling under complex exposure patterns (e.g. active biomonitoring) and biodynamic modelling to assess metal bioavailability to snails for risk-assessment purposes.
References
AFNOR (1996) Qualité des sols - Méthodes chimiques - sols sédiments, mise en solution totale par attaque acide - NF X31-147. Association Française de Normalisation, Paris
Beeby A (2001) What do sentinels stand for? Environ Pollut 112:285–298
Boshoff M, Jordaens K, Baguet S, Bervoets L (2015) Trace metal transfer in a soil–plant–snail microcosm field experiment and biomarker responses in snails. Ecol Indic 48:636–648. doi:10.1016/j.ecolind.2014.08.037
Burger J (2008) Assessment and management of risk to wildlife from cadmium. Sci Total Environ 389:37–45
Burnham KP, Anderson DR (2004) Multimodel inference understanding AIC and BIC in model selection. Sociol Methods Res 32:261–304
Calhôa CF, Monteiro MS, Soares AMVM, Mann RM (2011) The influence of metal speciation on the bioavailability and sub-cellular distribution of cadmium to the terrestrial isopod, Porcellio dilatatus
Coeurdassier M, Gomot-de Vaufleury A, Lovy C, Badot P-M (2002) Is the cadmium uptake from soil important in bioaccumulation and toxic effects for snails? Ecotoxicol Environ Saf 53:425–431
Dallinger R, Lagg B, Egg M et al (2004) Cd accumulation and Cd-metallothionein as a biomarker in Cepaea hortensis (Helicidae, Pulmonata) from laboratory exposure and metal-polluted habitats. Ecotoxicology 13:757–772
Elmslie LJ (1998) Humic acid: a growth factor for Helix asersa Muller (gasteropoda: Pulmonata). J Molluscan Stud 64:400–401
Fairbrother A, Wenstel R, Sappington K, Wood W (2007) Framework for metals risk assessment. Ecotoxicol Environ Saf 68:145–227
Fritsch C, Scheifler R, Beaugelin-Seiller K et al (2008) Biotic interactions modify the transfer of cesium-137 in a soil-earthworm-plant-snail food web. Environ Toxicol Chem 27:1698–1707. doi:10.1897/07-416.1
Fritsch C, Coeurdassier M, Gimbert F et al (2011) Investigations of responses to metal pollution in land snail populations (Cantareus aspersus and Cepaea nemoralis) from a smelter-impacted area. Ecotoxicology 20:739–759
Gimbert F, de Vaufleury A, Douay F et al (2006) Modelling chronic exposure to contaminated soil: a toxicokinetic approach with the terrestrial snail Helix aspersa. Environ Int 32:866–875
Gimbert F, Mench M, Coeurdassier M et al (2008a) Kinetic and dynamic aspects of soil-plant-snail transfer of cadmium in the field. Environ Pollut 152:736–745
Gimbert F, Vijver MG, Coeurdassier M et al (2008b) How subcellular partitioning can help to understand heavy metal accumulation and elimination kinetics in snails. Environ Toxicol Chem 27:1284–1292
Girard MC, Walter C, Rémy JC, et al (2005) Sols et Environnement. Sciences Sup, Dunod, Paris
Gomot A, Gomot L, Boukraa S, Bruckert S (1989) Influence of soil on the growth of the land snail Helix aspersa - An experimental study of the absorption route for the simulating factors. J Molluscan Stud 55:1–7
Gomot-de Vaufleury A (2000) Standardized growth toxicity testing (Cu, Zn, Pb, and Pentachlorophenol) with Helix aspersa. Ecotoxicol Environ Saf 46:41–50
Gomot-de Vaufleury A, Pihan F (2002) Methods for toxicity assessment of contaminated soil by oral or dermal uptake in land snails: metal bioavailability and bioaccumulation. Environ Toxicol Chem 21:820–827
Höckner M, Stefanon K, de Vaufleury A et al (2011) Physiological relevance and contribution to metal balance of specific and non-specific Metallothionein isoforms in the garden snail, Cantareus aspersus. BioMetals 24:1079–1092. doi:10.1007/s10534-011-9466-x
Hopkin SP (1989) Ecophysiology of metals in terrestrial invertebrates. Elsevier, New York
ISO 15952 (2006) Soil quality - effects of pollutants on juvenile land snails (Helicidae) -- determination of the effects on growth by soil contamination. International Organization for Standardization, Geneva, Switzerland
Laskowski R, Hopkin SP (1996) Effect of Zn, Cu, Pb, and Cd on fitness in snails (Helix aspersa). Ecotoxicol Environ Saf 34:59–69
Leita L, Contin M, Maggioni A (1991) Distribution of cadmium and induced Cd-binding proteins in roots, stems and leaves of Phaseolus vulgaris. Plant Sci 77:139–147
Li C-C, Dang F, Cang L et al (2015) Internal distribution of Cd in lettuce and resulting effects on Cd trophic transfer to the snail: Achatina fulica. Chemosphere 135:123–128. doi:10.1016/j.chemosphere.2015.03.096
Lindstrom MJ, Bates DM (1990) Nonlinear mixed effects models for repeated measures data. Biometrics 46:673–687
Mendoza-Cozatl DG, Jobe TO, Hauser F, Schroeder JI (2011) Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14:554–562
Monteiro MS, Santos C, Soares AMVM, Mann RM (2008) Does subcellular distribution in plants dictate the trophic bioavailability of cadmium to Porcellio dilatatus (Crustacea, Isopoda)? Environ Toxicol Chem 27:2548–2556. doi:10.1897/08-154.1
Moreno-Jiménez E, Garcia-Gomez C, Oropesa AL et al (2011) Screening risk assessment tools for assessing the environmental impact in an abandoned pyritic mine in Spain. Sci Total Environ 409:692–703
Nica DV, Filimon MN, Bordean D-M et al (2015) Impact of soil cadmium on land snails: a two-stage exposure approach under semi-field conditions using bioaccumulative and conchological end-points of exposure. PLoS ONE 10:e0116397. doi:10.1371/journal.pone.0116397
Noret N, Meerts P, Tolra R et al (2005) Palatability of Thlaspi caerulescens for snails: influence of zinc and glucosinolates. New Phytol 165:763–772. doi:10.1111/j.1469-8137.2004.01286.x
Notten MJM, Oosthoek AJP, Rozema J, Aerts R (2005) Heavy metal concentrations in a soil-plant-snail food chain along a terrestrial soil pollution gradient. Environ Pollut 138:178–190
Notten MJM, Oosthoek A, Rozema J, Aerts R (2006) The landsnail Cepaea nemoralis regulates internal Cd levels when fed on Cd-enriched stinging nettle (Urtica dioica) leaves at low, field-relevant concentrations. Environ Pollut 139:296–305. doi:10.1016/j.envpol.2005.05.007
OECD (1984) Test N° 207: Earthworm acute toxicity test. Guidel Test Chem Paris 1–9
Pauget B, Gimbert F, Coeurdassier M et al (2011) Use of chemical methods to assess Cd and Pb bioavailability to the snail Cantareus aspersus: a first attempt taking into account soil characteristics. J Hazard Mater 192:1804–1811
Pauget B, Gimbert F, Coeurdassier M et al (2013a) Ranking field site management priorities according to their metal transfer to snails. Ecol Indic 29:445–454
Pauget B, Gimbert F, Coeurdassier M et al (2013b) Assessing the in situ bioavailability of trace elements to snails using accumulation kinetics. Ecol Indic 34:126–135. doi:10.1016/j.ecolind.2013.04.018
Pauget B, Faure O, Conord C et al (2015) In situ assessment of phyto and zooavailability of trace elements: a complementary approach to chemical extraction procedures. Sci Total Environ 521–522:400–410. doi:10.1016/j.scitotenv.2015.03.075
Peijnenburg W, Zablotskaja M, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol Environ Saf 67:163–179. doi:10.1016/j.ecoenv.2007.02.008
Pérès G, Vandenbulcke F, Guernion M et al (2011) Earthworm indicators as tools for soil monitoring, characterization and risk assessment. An example from the national Bioindicator programme (France). Pedobiologia 54:S77–S87
Qureshi JA, Hardwick K, Collin HA (1986) Intracellular localization of lead in a lead tolerant and sensitive clone of Anthoxanthum odoratum. J Plant Physiol 122:357–364
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Sauvé S, Hendershot W, Allen HE (2000) Solid-solution partitioning of metals in contaminated soils: dependence on pH, total metal burden, and organic matter. Environ Sci Technol 34:1125–1131
Scheifler R, Brahim MB, Gomot-de Vaufleury A et al (2003a) A field method using microcosms to evaluate transfer of Cd, Cu, Ni, Pb and Zn from sewage sludge amended forest soils to Helix aspersa snails. Environ Pollut 122:343–350
Scheifler R, Schwartz C, Echevarria G et al (2003b) “Nonavailable” soil cadmium is bioavailable to snails: evidence from isotopic dilution experiments. Environ Sci Technol 37:81–86. doi:10.1021/es025677w
Scheifler R, de Vaufleury A, Cœurdassier M et al (2006) Transfer of Cd, Cu, Ni, Pb and Zn in a “soil–plant–invertebrate” food chain: a microcosm study. Environ Toxicol Chem 25:815–822
Sinnett D, Hutchings TR, Hodson ME (2009) Food-chain transfer of zinc from contaminated Urtica dioica and Acer pseudoplatanus L. to the aphids Microlophium carnosum and Drepanosiphum platanoidis Schrank. Environ Pollut 158:267–271
Spurgeon DJ, Hopkin SP (1999) Comparisons of metal accumulation and excretion kinetics in earthworms (Eisenia fetida) exposed to contaminated field and laboratory soils. Appl Soil Ecol 11:227–243
Sterckeman T, Perriguey J, Cael M et al (2004) Applying a mechanistic model to cadmium uptake by Zea mays and Thlaspi caerulescens: consequences for the assessment of the soil quantity and capacity factors. Plant Soil 262:289–302
Tarazona JV, Vega MM (2002) Hazard and risk assessment of chemicals for terrestrial ecosystems. Toxicology 181–182:187–191
Van Gestel CAM, Koolhaas JE (2004) Water-extractability, free ion activity, and pH explain cadmium sorption and toxicity to Folsomia candida (collembola) in seven soil-pH combinations. Environ Toxicol Chem 23:1822–1833
Vaufleury A de (2015) Landsnail for ecotoxicological assessment of chemicals and soil contamination—ecotoxicological assessment of chemicals and contaminated soils using the terrestrial snail, Helix aspersa, at various stage of its life cycle: a review. In: Armon RH, Hänninen O (eds) Environmental Indicators. Springer Netherlands, pp 365–391
Vijver MG, Wolterbeek HT, Vink JPM, van Gestel CAM (2005) Surface adsorption of metals onto the earthworm Lumbricus rubellus and the isopod Porcellio scaber is negligible compared to absorption in the body. Sci Total Environ 340:271–280. doi:10.1016/j.scitotenv.2004.12.018
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Pauget, B., Gimbert, F., Coeurdassier, M. et al. How contamination sources and soil properties can influence the Cd and Pb bioavailability to snails. Environ Sci Pollut Res 23, 2987–2996 (2016). https://doi.org/10.1007/s11356-015-5765-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5765-z