Abstract
Five bacterial isolates were screened for resistance to organotin compound, i.e. tributyltin chloride (TBTC) up to 2 mM. The optimum pH, temperature and salinity for the growth of the isolates were found to be 7, 28°C and 2.5%, respectively. The isolates were tested for survival tolerance to heavy metals (mercury, cadmium and zinc) and co-resistance to antibiotics viz. ampicillin, kanamycin, rifampicin, streptomycin, penicillin, chloramphenicol, tetracycline, nalidixic acid and neomycin. Although our earlier study reported that these five bacterial strains are of different species of Pseudomonas, our present 16S rRNA gene sequence analysis revealed that all the strains are Pseudomonas aeruginosa. One of five isolates P. aeruginosa strain 25W could grow in mineral salt medium with 2 mM of TBTC as a sole source of carbon and survive up to 5 mM of TBTC. In presence of 2 mM of TBTC there was comparable up-regulation of 45 kDa protein in the cell extract of the 25W isolate was found indicating involvement of certain enzymes in TBTC resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of organotin biocides in the aquatic environment have been reported from different parts of the world due to its extensive use in antifouling paints (Fent 2003; Konstantinou and Albanis 2004). In general, organotin toxicity to microbes decreases in the following order: R3SnX > R2SnX2 > RSnX3 > R4Sn. Microorganisms accumulate organotin in the cell wall envelope by a non energy requiring process, organotins such as tripropyl, tributyl and triphenyltin seem to be highly toxic to bacteria and fungi (Cooney and Wuertz 1989; Laurence et al. 1989; Cooney 1995). Several reports have been documented on isolation and characterization of tributyltin (TBT) resistant bacteria from soil, marine and estuarine environment (Hallas and Cooney 1981; McDonald and Trevors 1988; Wuertz et al. 1991; Fukagawa et al. 1992; Suzuki et al. 1992; Pain and Cooney 1998; Stasinakis et al. 2005). The isolation and characterization of TBT resistant marine bacterium, Alteromonas sp. M-l was first record of its kind (Suzuki et al. 1992; Fukagawa et al. 1994). These resistant bacteria could tolerate high levels of TBT biocides due to their inherent capability to (a) transform them into less toxic compounds viz. di- and mono-butyltin by dealkylation mechanism or (b) exclusion/efflux of these toxicants outside the cell mediated by membrane proteins or (c) degradation/metabolic utilization of them as carbon sources mediated by enzymes or (d) bioaccumulation of the biocide without breakdown, using metallothionein like proteins (Blair et al. 1982; Fukagawa et al. 1994). Though many authors have reviewed many proposed hypothesis, very little is known about the actual resistance mechanism of microorganisms to tolerate this biocide (Wuertz et al. 1991; Dubey and Roy 2003). In this process of investigation, many reports are available which deals with cross-tolerance of metals and antibiotics of tributyltin chloride (TBTC) resistant bacteria from many parts of the world (Wuertz et al. 1991; Pain and Cooney 1998; Suzuki et al. 1992) suggesting that resistant of heavy metals may be associated to organotin resistance. These sporadic reports did not give any clear idea about the organotin resistance mechanisms or phylogenetic affiliation of organotin resistance bacteria. Recent reports of extensive organotin contamination of west coast of India, due to enormous ship traffic in different shipyard and oil fields (Kanthak et al. 1999; Bhosle et al. 2004), have raised the concern of existence and diversity of organotin tolerant bacteria in this area. Our earlier study reported the presence of organotin resistance Pseudomonas sp. from west coast (Roy et al. 2004), so the present work grew the need for sound taxonomic framework for particularly organotin resistance Pseudomonas strain. The diversity of Pseudomonas was repeatedly discussed because, the frequency of recombination among different P. aeruginosa genotype was high leading to the random association of alleles (Kiewitz and Tummler 2000), thus diversity within the species is also possible (Hummerjohann et al. 1998; Spiers et al. 2000). The present study includes the comprehensive comparative study of TBTC resistant P. aeruginosa with respect to its biochemical and genetic diversity as well as we have investigated the regulation of cellular protein involved in organotin resistance.
Materials and methods
Culture
Five TBTC resistance bacterial isolates were obtained from marine surface water sample of west coast of India (Roy et al. 2004). The five strains were designated as 3(4Sub), 25B, 5Y2, 25W and 9(3A) and identified as P. aeruginosa with 16S rRNA sequencing with primer 16F27N (CCAGAGTTTGATCMTGGCTCAG), 530F (GTC CCAGCMGCCGCGG) and 16F704A (GTAGCGGTGAAATGCGTAGA) and submitted to GenBank to get the accession no. DQ014539 (5Y2), DQ014538 (25W), DQ082859 [9(3A)], DQ082857 [3(4 Sub)] and DQ082858 (25B), respectively. The same cultures were deposited in NCIM, National Chemical Laboratory, Pune, India and obtain the accession no. NCIM 5223 (5Y2), NCIM 5224 (25W), NCIM 5225 [9(3A)], NCIM 5226 [3(4 Sub)] and NCIM 5227 (25B), respectively. These isolates were purified and maintained on mineral salt medium (MSM) supplemented with 2 mM TBTC (MSM + 2 mM TBTC).
Media and compound
Mineral salt medium (Mahtani and Mavinkurve 1979) and Luria Bertani (LB) broth (Gerhardt et al. 1989) as per the company provided and literature cited. Standard TBTC [1461-22-9] (MERCK, Germany) stock solution was prepared in filter sterilized ethyl alcohol and kept in dark at 4°C.
Antibiotics and metal salt solution
Antibiotic (SIGMA) stock solution (1 mg/ml) was prepared in sterile de-ionized double distilled water (D.W) and filter sterilized (0.2-μm syringe filter, Nalgene, Rochester, NY, USA). The stock solution was stored in dark at 4°C. Antibiotics used were ampicillin, kanamycin, rifampicin, streptomycin, penicillin, chloramphenicol, tetracyclin, nalidixic acid, neomycin, spectinomycin, antimycin and amikacin. Stock solution (1 M) of mercuric chloride (HgCl2), cadmium chloride (CdCl2) and zinc sulphate (ZnSO4) were prepared by dissolving the metal salt in 25 ml of D.W. The solution was filter sterilized and stored at 4°C.
Determination of heavy metal tolerance limit of bacterial isolate
Three heavy metals, i.e. Hg, Cd and Zn were chosen for metal tolerance study. Metal tolerance of the five strains was determined by growing the five isolates with different increasing concentration of metal salts in LB medium. After incubation at 28°C for 24 h, the growth was measured as optical density of the culture at 600 nm. The survival graph was plotted based on percent growth of the strains at different concentrations of metal salts. Minimum inhibitory concentration (MIC) values of different metal salt viz. CdCl2, HgCl2 and ZnSO4 for five TBTC tolerant isolates were determined from percent survival curve.
Determination of antibiotic resistance of bacterial isolates
Antibiotic sensitivity of natural isolates was determined using different antibiotics such as ampicillin, kanamycin, rifampicin, streptomycin, penicillin, chloramphenicol, tetracyclin, nalidixic acid, neomycin, spectinomycin, antimycin and amikacin. Different antibiotic plates were prepared using LB agar and five bacterial isolates were spot inoculated on the plates and incubated at 28°C for 24 h. After incubation, the sensitivity of the isolates was detected based on their inhibition of their growth on the respective antibiotic plates.
Molecular identification and phylogenetic analysis
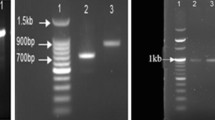
A single isolated colony of the selected bacterial cultures were taken from agar plate and was suspended in 50 μl of colony lysis solution (10 mM, Tris–HCl, pH 7.5, 10 mM EDTA and 50 μg/ml of proteinase K). The reaction mixture was incubated at 55°C for 15 min followed by proteinase K inactivation at 80°C for 10 min. The reaction mixture was centrifuged at 15,000 rpm at 4°C for 15 min. The supernatant containing genomic DNA was directly used as template in PCR reaction. PCR amplification of almost full-length 16S rRNA gene was carried out with eubacteria specific primer set 16F27N (5′-CCAGAGTTTGATCMTGGCTCAG-3′) and 16R1525XP (5′-TTCTGCAGTCTAGAAGGAGGTGWTCCAGGC-3′) (Pidiyar et al. 2002), in a 25-μl final reaction volume, containing about 10 ng of genomic DNA, 1 × reaction buffer (10 mM Tris–HCl, pH 8.8 at 25°C, 1.5 mM MgCl2, 50 mM KCl and 0.1% Triton X-100), 0.4 mM (each) deoxynucleoside triphosphates (Invitrogen), 0.5 U of DNA Polymerase (New England Labs, UK) and the final volume was made 25 μl by adding sterile nuclease free water. The PCR was performed in an automated Gene Amp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) under the following conditions. The amplification conditions were as follows 94°C for 1 min (denaturation), 55°C for 1 min (annealing), 72°C for 1.30 min (elongation), 72°C for 10 min final elongation. Expected PCR product of around 1.5 kb was checked by electrophoresis of 5 μl of the PCR product on 1% agarose gel in 1 × TBE buffer and stained with ethidium bromide 0.5 μg/ml. The PCR product was precipitated by PEG–NaCl (20% PEG in 2.5 M NaCl) precipitation at 37°C for 30 min. The reaction mixture was centrifuged at 12,000 rpm for 30 min at room temperature. The supernatant was discarded and the pellet was washed twice with 70% ethanol. After drying the pellet it was resuspended in 5 μl of sterile nuclease free water. One microlitre (50 ng) of purified 16S rRNA PCR product was sequenced by 16S rRNA specific primer, i.e. 16F27N, 530F (GTCCCAGCMGCCGCGG) and 16R1525XP. The samples were sequenced by using BIG DYE Terminator cycle sequencing ready reaction kit (v3.1) in ABI PRISM 3730 Genetic Analyzer (Applied Biosystems). The 16S sequence database was used to create phylogenetic tree of the studied isolates. Primarily, the analysis of sequences was done at NCBI server (http://www.ncbi.nlm.nih.gov/BLAST). Sequence of the isolates representing different name but having identical sequences is considered as single representative. The incomplete sequences, sequences containing large gap or mistakes, undetermined nucleotides, which were lacking any alleles, were excluded from the study. The complete sequences were aligned using multiprocessor of CLUSTALW programmed at European Bioinformatics site (http://www.ebi.eic.uk/clustalw). Aligned sequences were used for un-rooted phylogenetic tree with neighbour joining methods subjected to a bootstrapping analysis of 1,000 simulations to assess topology. The analysis was performed in programmed package (MEGA, Version-2.1) with default parameters of Kimura-2 model (Kumar 2001). During analysis of alignments with maximum evaluation methods, gaps were considered missing data points, genetic distances, estimated using nucleotide/Jukes–Cantor models, where all substitution were included in pair-wise distances were estimated using 1,000 permutations. In addition, the original tested isolates were highlighted and numbered in tree. The sequences obtained from BLAST search were named as the accession number. The classification of P. aeruginosa genotype was performed by cluster analysis.
Determination of optimum concentration of TBTC
Pseudomonas aeruginosa strain 25W was grown on MSM with different concentration of TBTC ranging from 1 to 10 mM. After 48-h incubation, the growth was observed with respect to protein content and graphical representation was done as protein (μg/ml) versus TBTC concentration. From same culture broth, 0.1 ml of each sample was surface layered on the MSM agar medium with different concentration ranging from 1 to 10 mM TBTC separately. Respective plates were incubated at 28°C and after incubation total viable count was determined as colony forming unit (cfu)/ml. In both the cases, a control experiment was set up with isolate in MSM + 0.5% glucose medium. A comparable graphical analysis was done with protein content and viable count of the isolate with respect to different concentration of TBTC.
Protein profile (SDS-PAGE)
Cells were grown in different concentration of TBTC, i.e. 0.1, 0.5, 1 and 2 mM, in MSM. The culture was also grown in MSM + (0.5%) glucose and MSM + 0.5% glucose + 2 mM of TBTC as a positive control for same period. The culture was harvested during exponential growth phase. Protease inhibitor cocktail (SIGMA) was added to both cell and supernatant (1 mg/ml final concentration) to inhibit the protease activity. Cell pellet suspended in saline, was sonicated to rupture the cells (65W, 30 S, repeated three times). Sonicated samples were again centrifuged and cell supernatant was separated from cell pellet. Required amount of sample was boiled with 30 μl of sample buffer for 10 min. The sample was loaded with loading dye (2 μl). The SDS-PAGE was performed as per Laemlli (1970) method at 30 V for 2 h and gel was subsequently stained and de-stained to observe the protein bands. Result was documented in gel documentation system.
Result
In our earlier study, five bacterial isolates were primarily screened on the basis of TBTC utilization (Roy et al. 2004). Most of the isolates which initially grew on MSM agar supplemented with 2 mM TBTC, lost their viability on the same medium after repeated sub-culturing, because organotin compounds inhibit or kill most of the aquatic microorganisms (Pettibone and Cooney 1986), but five of the present isolates could survive at higher concentration of TBTC, i.e. 2 mM. The selected five isolates consistently showed good growth on MSM agar supplemented with 2 mM TBTC with zone of clearance within 7 days of incubation (Fig. 1). With subsequent higher concentration, i.e. 3, 4 mM, etc., isolates did not grow in MSM broth or on agar plate.
Growth pattern of TBTC resistant isolates (as identified biochemically) on MSM agar supplemented with 2 mM TBTC. 1 Pseudomonas aeruginosa strain 25B, 2 Pseudomonas aeruginosa strain 5Y2, 3 Pseudomonas fluorescens strain 3(4Sub), 4 Pseudomonas stutzeri strain 9(3A), 5 Pseudomonas aeruginosa strain 25W
Cross tolerance to heavy metals, i.e. Hg, Cd, Zn
Metal resistance was observed in the range of μM to mM concentration of heavy metals. The result was summarized with respect to MIC values of three different metal ions, i.e. Hg, Cd and Zn. The five isolates showed comparatively higher level of Zn resistance with maximum resistance of 11.6 mM for 3(4Sub) and lowest for 9(3A) isolates, which were found sensitive as the MIC values obtained was 4 mM (Table 1). In case of cadmium, all the five strains showed equal level of resistance with MIC values ranging from 4 to 5 mM (Table 1). The mercury resistance of five Pseudomonas sp. were varied from 8 to 9.5 mM of Hg. Unlikely the 9(3A) strain showed markedly high MIC values, i.e. 10.7 mM for Hg, whereas the same isolate was comparatively sensitive to zinc than other isolates.
Antibiotic resistance
A broad range of antibiotics were tested ranging from 1 to 900 μg/ml. The list of concentrations compiled in Table 2 was the threshold concentration for the five isolates found resistant to those antibiotics. All the five isolates were found more resistant to penicillin, ampicillin, rifampicin, kanamycin, neomycin, spectinomycin, antimycin and streptomycin including some other antibiotics viz. amikacin, chloramphenicol, tetracyclin and nalidixic acid (Table 2). Only strain 5Y2 was sensitive to amikacin, whereas strain 9(3A) was sensitive to both kanamycin and spectinomycin at 400 and 100 μg/ml, respectively. Both the strains were found sensitive even at lower concentration of kanamycin and spectinomycin.
Biodiversity study
The initial BLAST analysis of all five isolates showed that they are very close to P. aeruginosa. The phylogenetic tree of 16S rRNA gene revealed five cluster based genome diversity of the studied sequences as well as other species of Pseudomonas sp. (Fig. 2). The strain 25W and 3(4Sub) were fallen in one cluster with AY360347 and DQ95913 sequences of the NCBI database and both the sequences showed high homology to each other. The sequences of strain 25B showed genetically closer to AY631058 and AF531099, like 9(3A), which showed 99% similarity to AY162139. The strain 5Y2 remains out of the any cluster but fairly closer to AY268175 and AY738268. The total phylogenetic representation explained genetic uniqueness of the strain though they fall in monophyletic group.
Selection of potent strain for TBTC degradation study
Among the five isolates, P. aeruginosa strain 25W showed rapid growth within 48 h in MSM broth with 2 mM TBTC and prominent degradation ability (Roy and Bhosle 2006). Thus this isolate was selected for further characterization. The strain 25W was further characterized to detect its organotin tolerance limit. The comparative graphical analysis in Fig. 3, showed that the protein content of the culture broth was markedly decreased from the control when it was exposed to 1 mM of TBTC, though it increased with 2 mM TBTC, the subsequent increase in concentration decreases the protein content up to 5 mM of TBTC. At higher concentration of TBTC a consistent amount of protein was observed which was insignificant. The trace amount of protein was from inoculums as it was compared with protein content of the inoculums. In case of bacterial viable count highest count was observed in presence of 2 mM of TBTC although it was less than control and till 5 mM of TBTC the viable count was 55 cfu/μl. At higher concentration no viable count was observed. Thus in this study, protein content and viable count of the culture inferred that it can tolerate up to 5 mM of TBTC, although the optimum growth is at 2 mM of TBTC.
Analysis of TBTC induced protein
SDS-PAGE analysis of P. aeruginosa strain 25W clearly revealed that cells exposed to TBTC at 0.1 and 0.5 mM levels inhibit synthesis of certain proteins (Fig. 4; lanes 2 and 3). The protein profile of the extract obtained from cells grown in MSM + glucose (0.5%) and MSM + glucose (0.5%) + 2 mM TBTC showed almost similar profile (lanes 4 and 5). The up-regulation protein of the cell extract obtained from cells grown in MSM + 1 mM TBTC also revealed a protein band of 27 kDa (Fig. 4; lane 6) which was consistent in control lane (Fig. 4; lane 4). Unlike other lanes, MSM + 2 mM TBTC grown cells clearly expressed a 45-kDa protein (Fig. 4; lane 7).
Protein profile of Pseudomonas aeruginosa strain USS25 (SDS-PAGE). 1 Broad range protein marker (PMW-B), 2 protein sample of cells grown in MSM + 0.1 mM TBTC, 3 protein sample of cells grown in MSM + 0.5 mM TBTC, 4 protein sample of cells grown in MSM + 0.5% glucose, 5 protein sample of cells grown in MSM + 0.5% glucose + 2 mM TBTC, 6 protein sample of cells grown in MSM + 1 mM TBTC, 7 protein sample of cells grown in MSM + 2 mM TBTC
Discussion
A number of approaches have been adopted time to time to investigate the organotin resistance mechanisms in microorganisms, study them and implement it in organotin bioremediation. After isolating five organotin resistant Pseudomonas strains (Roy et al. 2004), our study was more focused on parameters of characterization and classification of these five natural isolates. Initially, during the growth parameters study, cultures were incubated at three different temperatures viz. 28, 37 and 42°C, but optimum growth was found at 28°C only, whereas, all five isolates showed highest growth yield at stationary phase at pH 7 in terms of protein content as compared to cultures growth at pH 5. Therefore, natural growth conditions were comparatively more favourable than acidic conditions, for all the strains. The present observation also revealed that TBTC is cytotoxic at acidic pH of growth medium (i.e. pH 5), which correlates the findings by Gadd (2000). As in aquatic system, both pH and salinity determine organotin speciation and therefore bioavailability and reactivity. These environmental factors also alter selectively for TBT resistant microorganisms in polluted system as it was evident from selective enrichment of TBTC resistant bacterial strains in TBTC contaminated marine environment (Fukagawa et al. 1994; White et al. 1999). Growth of five isolates showed a similar pattern of growth in MSM broth as growth increased proportionately with increase in salt (sodium chloride) concentration up to 2.5% and that was found to be optimum condition for growth. Since, the aqueous solubility of organotin compound such as TBTC was decreased with increased salinity (Inaba et al. 1995). The observation revealed that TBTC toxicity is reduced at higher salinity levels, as the availability of TBTC is high at low concentration of NaCl.
Bacterial based bioremediation needs that the microorganisms should function in presence of target contaminant as well as other contaminant. The probable reason of high zinc resistance (5 mM) of the five isolates must be involving some mechanisms to remove this metal from cell or to prevent their entry into the cell. In case of P. aeruginosa, the resistance patterns to different heavy metals were studied earlier (de Vicente et al. 1990; Barkay et al. 2003). Fukagawa et al. (1994) have reported that out of the 55 bacterial strains, which are TBT resistant (250 nM), 11 of them showed cross resistance to methyl mercury (20 nM). In the present study, the high mercury tolerant bacterial strain 9(3A) might possess one of these mechanisms and according to our knowledge that is highest resistance by Pseudomonas sp. The study made it evident that TBT tolerant bacteria may possess common mechanisms. As it is known that most of the antifouling paints contains many biocides including heavy metals, mainly zinc (Sanchez-Bayo and Goka 2005), it was expected that these organotin tolerant bacteria would develop a mechanism of resistance to heavy metals that is commonly present in these paints.
The resistance patterns of P. aeruginosa strains to different antimicrobial agents were studied. Wuertz et al. (1991) reported TBT resistant bacterial (8.2 μM) isolates from Boston harbour, which were resistant to cephalothin, ampicillin, novobiocin, carbenicillin, erythromycin and penicillin. Many workers have reported earlier about multi-drug resistant mechanism Pseudomonas sp. (de Vicente et al. 1990; Esiobu et al. 2002; Bruins et al. 2003) and P. aeruginosa (Sader et al. 2002). These facts also satisfy the findings of Esiobu et al. (2002) which reported that Pseudomonas sp. has plasmid mediated multiple drug resistance, such as ampicillin, penicillin, tetracycline, streptomycin, kanamycin, etc. These reports clearly confirm that organotin resistant natural bacterial communities invariably demonstrate resistance to commonly used antibiotics, although sensitivity of strain 9(3A) and 5Y2 to kanamycin, spectinomycin and amikacin, respectively, was an exceptional phenomenon. Though present study reconfirms previous reported fact, it is still yet to answer how these bacteria develops resistance to antibiotics which is not commonly present even in organotin contaminated water.
Our earlier study on biochemical analysis revealed that strain 25W, 25B and 5Y2 are P. aeruginosa and biochemically they were 93.7–97.5% similar to each other (Roy et al. 2004) (Fig. 5), which reflects the diversity of phenotype. At the genetic level, sequences analysis of housekeeping loci of P. aeruginosa has low sequence diversity (Kiewitz and Tummler 2000). The biochemical characteristics of bacterial isolate 3(4Sub) and 9(3A) were led to identification as Pseudomonas fluorescens and Pseudomonas stutzeri (Roy et al. 2004) and they have 79.4 and 89.2% similarity with rest of the strains, but 16S rRNA analysis confirmed it as P. aeruginosa. Although the sequence analysis of 16S rRNA gene confirmed its identity, the possible reason of difference in biochemical characteristic could be environmental stress. Pseudomonas is considered more evolvable than other bacteria and evolution arises by variation, which causes due to mutation or recombination process in bacterial population (Spiers et al. 2000). These changes in genetic makeup show the biochemical difference in the same strain.
The study gives a comparative analysis of genetic and biochemical evidence based classification of the five natural P. aeruginosa, obtained from the marine surface water. It also confirmed the fact that analysis of conserved sequences are tool for bacterial identification although the biochemical method supports the detection of extraordinary diversification within Pseudomonas sp. Finally, the ecological forces are the ultimate determinants of patterns of diversity, although genetic factors are equally significant to locate the root of divergence.
Diversification is a solely ecological process. Without variation there is no evolution and no divergence. Variation arises by mutation and recombination and both processes operate in Pseudomonas (like other bacteria) because of its ubiquitous distribution and remarkable adaptability (Spiers et al. 2000). This might be the reason why out of the five studied bacterial strains only strain 25W showed tolerance limit up to 5 mM of TBTC with optimum growth and degradation ability at 2 mM TBTC. This indicated that this strain might possess enzymes to metabolize high levels of TBTC, certainly up to 2 mM. Above this level, cells get killed due to cytotoxic effects on cell metabolism, which involves Ca2+ overload and cytoskeletal damage (Stridh et al. 1999). Interestingly this isolate certainly exhibits much higher TBTC tolerance as compared to other reported TBTC tolerant bacterial strains (Boopathy and Daniels 1991; Suzuki et al. 1992, 1994; Fukagawa et al. 1992), so the strain might have adapted some biochemical or genetic factor to get through the ecological force.
This comparable up-regulation of protein have been mentioned earlier in TBTC resistant Vibrio sp., which exhibited enhanced synthesis of two polypeptides of 30 and 12 kDa when cells are grown in presence of 125 μM TBTC (Fukagawa et al. 1992). The 45-kDa protein in P. aeruginosa strain 25W was different from earlier observations, which is expressed in presence of 2 mM of TBTC. The possible reason could be that the level of TBTC which is optimum for growth and induction of metallothionein like proteins. So at that concentration only the 45-kDa protein gets induced, while the 27-kDa protein, which is present in all the conditions, gets up-regulated, which might be constitutive. Certain heavy metal tolerant bacteria such as Pseudomonas putida and Vibrio alginolyticus commonly show induced synthesis of cysteine rich polypeptides like proteins which bind with specific metals such as cadmium and copper making them unavailable to the bacterial cells (Pazirandeh et al. 1995, 1998). There is evidence that the site of toxic action of organotins may be both at the cytoplasmic membrane and intracellular level. Studies on the effect of TBT on certain microbial enzymes indicated that in some bacteria TBT can interact with cytosolic enzymes (White et al. 1999). TBT acts on mitochondria and chloroplast by causing ion exchange through membranes and inhibiting phosphorylation and ATPase. Some of the enzymes like glucose dehydrogenase, glucose-6-phosphate dehydrogenase, β-galactoside galactohydrase and alkaline phosphatase are affected by TBT except for ATPase and NADH oxidase. On the contrary, Bacillus sp. ATPase gets activated in presence of TBTC and the NADH oxidase activity was stimulated as the concentration of TBT was increased in relatively resistant strain of P. putida TBT-6 and Pseudomonas sp. BP-4 (Tsing and Cooney 1995). The identified proteins suggested that there must be one constitutive and one inducer protein present in organotin resistant Pseudomonas sp. As P. aeruginosa was proven to be harbouring wide range of enzymes which it utilizes for growth (Hummerjohann et al. 1998), so under the growth condition tested with organotin, the up-regulation of 27 kDa protein and induction of 45 kDa protein represent some uncharacterized enzymes involved in organotin resistance. More work is required both at the biochemical level and at the level of gene organization to characterize the function of protein in TBTC resistance.
Abbreviations
- TBTC:
-
Tributyltin chloride
- MSM:
-
Mineral Salt Medium
- 16SrRNA:
-
16S ribosomal RNA
- TBT:
-
Tributyltin
References
Barkay T, Miller SM, Summers AO (2003) Bacterial mercury resistance from atoms to ecosystem. FEMS Microbiol Rev 27(2–3):355–348
Bhosle NB, Garg A, Jadhav S, Harjee R, Sawant SS, Venkat K, Anil AC (2004) Butyltins in water, biofilm, animals and sediments of the west coast of India. Chemosphere 57:897–907
Blair WR, Olson GJ, Brinckman FE, Iverson WP (1982) Accumulation and fate of tri-n-butyltin cation in estuarine bacteria. Microb Ecol 8:241–251
Boopathy R, Daniels L (1991) Pattern of organotin inhibition of methanogenic bacteria. Appl Environ Microbiol 57:1189–1193
Bruins MR, Kapil S, Oehme FW (2003) Characterization of a small plasmid (pMBCP) from bovine Pseudomonas pickettii that confers cadmium resistance. Ecotoxicol Environ Saf 54:241–248
Cooney JJ (1995) Organotin compounds and aquatic bacteria—a review. Helgoland Meeresunter 49:663–677
Cooney JJ, Wuertz S (1989) Toxic effect of tin compounds on microorganisms. J Ind Microbiol 4:375–402
Dubey SK, Roy U (2003) Biodegradation of tributyltins (organotins) by marine bacteria. Appl Organometallic Chem 17:1–6
Esiobu N, Armenta L, Ike J, Esiobu JN (2002) Antibiotic resistance in soil and water environments. Int J Environ Health Res 12:133–144
Fent K (2003) Ecotoxicological problems associated with contaminated sites. Toxicol Lett 141:353–365
Fukagawa T, Suzuki S, Fukagawa K, Suzuki T, Takama K (1992) Isolation and characterization of tributyltin chloride resistant marine Vibrio. FEMS Microbiol Lett 93:83–86
Fukagawa T, Konno S, Takama K, Suzuki S (1994) Occurrence of tributyltin (TBT) and methyl mercury tolerant bacteria in natural seawater to which TBT was added. J Mar Biotechnol 1:211–214
Gadd GM (2000) Microbial interaction with tributyltin compounds, detoxification, accumulation and environmental fate. Sci Total Environ 258:119–127
Gerhardt P Murray RGE Wood WA Krug NR (1989) Gene transfer in gram positive bacteria. In: Methods for general and molecular bacteriology, chap 15. American Society for Microbiology, pp 353–402
Hallas LE, Cooney JJ (1981) Tin and tin resistant microorganisms in Chesapeake bay. Appl Environ Microbiol 41:466–471
Hummerjohann J, Kuttel E, Quadroni M, Ragaller J, Leisinger T, Kertesz MA (1998) Regulation of the sulfate starvation response in Pseudomonas aeruginosa: role of cysteine biosynthesis intermediates. Microbiology 144:1375–1386
Inaba K, Shiraishi H, Soma Y (1995) Effect of salinity, pH and temperature on aqueous solubility of four organotin compounds. Water Res 29:1415–1417
Kanthak DIJ, Bernstorff A, Jayaraman N (1999) Ship for scrap: steel and toxic wastes for Asia. Green Peace 5:26
Kiewitz C, Tummler B (2000) Sequence diversity of Pseudomonas aeruginosa: impact on population structure and genome evolution. J Bacteriol 182:3125–3135
Konstantinou IK, Albanis TA (2004) Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: a review. Environ Int 30:235–248
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1224–1245
Laemlli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laurence OS, Cooney JJ, Gadd GM (1989) Toxicity of organotins towards the marine yeast, Debaryomyces hansenii. Microb Ecol 17:275–285
Mahtani S, Mavinkurve S (1979) Microbial purification of longifolene—a sesquiterpene. J Ferment Technol 57:529–533
McDonald L, Trevors JT (1988) Review of tin resistance, accumulation and transformation by microorganisms. Water Air Soil Pollut 40:215–221
Pain A, Cooney JJ (1998) Characterization of organotin resistant bacteria from Boston harbour sediment. Arch Environ Contam Toxicol 35:412–416
Pazirandeh M, Chrisey LA, Mauro JM, Campbell JR, Gaber BP (1995) Expression of the Neurospora crassa metallothionein gene in Escherichia coli and its effect on heavy metal uptake. Appl Microbiol Biotechnol 43:1112–1117
Pazirandeh M Welles BM Ryan RL (1998) Development of bacterium-based heavy metal biosorbents: enhanced uptake of cadmium and mercury by Escherichia coli expressing a metal binding motif. Appl Environ Microbiol 64:4068–4072
Pettibone GW, Cooney JJ (1986) Effect of organotins in fecal pollution indicator organisms. Appl Environ Microbiol 52:562–566
Pidiyar VJ, Jangid K, Dayanand KM, Patole MS, Gonzales JM, Kaznowski A, Shouche YS (2002) Phylogenetic affiliation of Aeromonas culicicola MTCC 3249T based on gyrB gene sequence and PCR-amplicon sequence analysis of cytolytic enterotoxin gene. Syst Appl Microbiol 26:197–202
Roy U, Bhosle S (2006) Microbial transformation of tributyltin chloride by Pseudomonas aeruginosa strain USS25 NCIM5224. Appl Organometallic Chem 20:5–11
Roy U, Dubey SK, Bhosle S (2004) Tributyltin chloride utilizing bacteria from marine ecosystem of west coast of India. Curr Sci 85:702–705
Sader HS, Jones RN, Silve JB, Sader HS (2002) Skin and soft tissue infection in Latin American medical center: four year assessment of the pathogen frequency and antimicrobial susceptibility patterns. Diagn Microbiol Infect Dis 44:281–288
Sanchez-Bayo F, Goka K (2005) Unexpected effects of zinc pyrithione and imidacloprid on Japanese medaka fish (Oryzias latipes). Aquat Toxicol 74:285–293
Spiers AJ, Buckling A, Rainey P (2000) The case of Pseudomonas diversity. Microbiology 146:2345–2350
Stasinakis AS, Thomaidis NS, Nikolaou A, Kantifes A (2005) Aerobic biodegradation of organotin compounds in activated sludge batch reactors. Environ Pollut 134:431–438
Stridh H, Fava E, Single B, Nicotera P, Orrenius S, Leist M (1999) Tributyltin-induced apoptosis requires glycolytic adenosine triphosphate production. Chem Res Toxicol 12:874–882
Suzuki S, Fuagawa T, Takma K (1992) Occurrence of tributyltin tolerant bacteria in tributyltin or cadmium containing sea water. Appl Environ Microbiol 58:3410–3412
Suzuki S, Tsukamoto KK, Fukagawa T (1994) The 16S rRNA sequence and genome sizing of tributyltin resistant marine bacterium, strain M-1. Microbiology 101:101–109
Tsing RK, Cooney JJ (1995) Action of tributyltin on enzymes on four bacteria. Environ Toxicol Chem 14:1113–1121
de Vicente A, Aviles M, Codina JC, Borrego JJ, Romero P (1990) Resistance to antibiotics and heavy metals of Pseudomonas aeruginosa isolated from natural waters. J Appl Bacteriol 68:625–632
White JS, Tobin JM, Cooney JJ (1999) Organotin compounds and their interaction with microorganisms. Can J Microbiol 45:541–554
Wuertz S, Miller CE, Pfister RM, Cooney JJ (1991) Tributyltin-resistant bacteria from estuarine and freshwater sediments. Appl Environ Microbiol 57:2783–2789
Acknowledgements
Authors are grateful to Dr S.K. Dubey, Goa University, for his suggestion and support. We are thankful to Dr Y.S. Shouche, NCCS, Pune, India for 16S rRNA analysis of the bacterial isolates and Dr K. Kannan, Wordsworth Center, N.Y. State Department of Health, USA for his valuable comment on this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, U., Nair, D. Biodiversity of organotin resistant Pseudomonas from west coast of India. Ecotoxicology 16, 253–261 (2007). https://doi.org/10.1007/s10646-006-0125-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-006-0125-x