Abstract
The present study was aimed at applying condition factor (CF), brain acetylcholinesterase (AChE) and gill histology as biomarkers for detecting possible exposure/effect induced by pesticides in fish residing rice field associated waterbodies in Sri Lanka. Biomarkers of an indigenous fish, Rasbora caverii collected from five sampling sites including canals near rice fields, a river and a reservoir (the reference site) were evaluated at four sampling stages covering pesticide application periods during rice cultivation season in 2004. Results indicated that CF of the fish did not show significant alterations regardless of the sampling sites or sampling stages. Site specific differences in AChE activities of the fish were not evident either prior to application of pesticides or at 7 days after Paraquat application to the rice fields. Two days after the application of a mixture of Fenthion and Phenthoate to the rice fields, AChE activity of the fish collected from canals near rice fields was significantly depressed (65–75%) compared to the fish in the reference site. The activities remain depressed to 50–56% even at 65 days after the insecticides application. Laboratory studies showed that prior exposure of R. caverii to Paraquat (2 μg l−1, 7 days) enhanced the extent of inhibition of brain AChE activity induced by Fenthion (3 μg l−1) or a mixture of Fenthion (3 μg l−1) and Phenthoate (5 μg l−1). Gills of fish collected from canals near rice fields exhibited abnormal multiple divisions at the tips of some secondary lamellae in addition to hyperplasia, hypertrophy and club shaped deformities. Results indicate that application of pesticides in rice culture could manifest a threat to native fish populations residing rice field associated waterbodies. The response of brain AChE and histological changes in the gills of R. caverii allowed differentiating sampling sites after insecticide applications to the rice fields. Hence, R. caverii may be considered as a surrogate species in ecotoxicological risk evaluation of agrochemicals in the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomarkers are sublethal biological measures of the response to and effects of pollutants in living organisms. Biomarkers have been identified as a powerful and cost—effective approach to obtain state of the environment and the effects of pollution (McCarthy and Shugart 1990). Inhibition of Acetylcholinesterase activity (AChE; EC 3.1.1.7) in fish has been recognized as a biomarker for toxic effects of pollutants as well as to diagnose exposure to anticholinesterase compounds such as organophosphorous (OP) and carbamate (CM) pesticides (Coppage and Bradeich 1976; Gruber and Munn 1998; Fulton and Key 2001). AChE is a key enzyme of the nervous system. When it is inhibited, the neurotransmitter acetylcholine is accumulated in cholinergic synapses affecting the proper functioning of the nerves (Ecobichon 1992). In addition to AChE, Vertebrates may also contain the related enzyme, butyrylcholinesterase in several tissues. In most teleost fish brain contains mainly AChE (Kozlovskaya et al. 1993).
Use of herbicides to control weeds and OP and CM insecticides to control insect pests is a common practice in rice cultivation in Asian countries including Sri Lanka. In Sri Lanka, many of the rice cultivation areas contain intricate network of freshwater resources such as reservoirs, canals, rivers and their tributaries. When used in the vicinity of aquatic ecosystems, these pesticides may enter water resources as a result of spray drift, leaching from soil and surface runoff during raining in concentrations which may exert adverse effects on diverse non-target aquatic organisms especially fish. In Sri Lanka, biodiversity in freshwaters is high and over 100 fish species including endemic, indigenous and exotic species inhabit different freshwater resources (Pethiagoda 1991). However, information on effects of pesticides on Sri Lankan aquatic fauna is meager. Although effects of several pesticides on some exotic fish species cultured in Sri Lanka for human consumption have been studied under laboratory conditions (Pathiratne 1999; De Mel and Pathiratne 2005) no field investigations have been carried out to monitor the effects induced by chemical pollutants on native fish populations in the waterbodies.
The present study was aimed at applying biomarkers for detection of possible exposure/effects of pesticides induced in native fish populations in the rice-field associated waterbodies. Rasbora caverii (Order Cypriniformes; family Cyprinidae) is a common indigenous small fish widely distributed in freshwater environments in Sri Lanka. In the present study, condition factor (CF), brain AChE activity and histological structure of the gills of R. caverii inhabiting selected rice field associated waterbodies in Sri Lanka were evaluated during a rice cultivation season. In addition, the effects of low concentrations of the pesticides used in this rice cultivation season viz. Paraquat, Fenthion and Phenthoate on R. caverii were also assessed under controlled laboratory conditions.
Methods
Study area and sampling sites
The study area is located in the Western province in Sri Lanka in the Kaduwela Divisional Secretary’s division at 6°53′ N–6°56′ N and 79°57′ E–79°57′ E. Five sampling sites (A–E) were selected from the study area (Fig. 1). The distance between two consecutive sampling locations was at least 2 km. The sampling site A was a small perennial reservoir (Akuragoda Wewa), which was considered as the reference site. No rice fields are located in the watershed area of Akuragoda Wewa. As it is located at a higher elevation than that of the rice fields, the water draining from the rice fields is not likely to flow into this reservoir. The sampling site B was the main canal, which was located in an area with abandoned rice-fields where the pesticides have not been applied for the past 2 years. The sampling site C (a feeder canal) and the sampling site D (part of the main canal) were located in the area of cultivated rice-fields. The sampling site E was the area where the main canal joins the Kelani River. The rice fields in the area are cultivated using rainwater in the two cultivation seasons per year called “Yala” and “Maha”. The water draining from rice fields flows through the feeder canals and flows into the main canal running from the reservoir to the Kelani River. The main canal joins the Kelani River about 10 km east from Colombo.
The present study was carried out in the “Maha” cultivation season started in March 2004. During this cultivation season, a commercial weedicide formulation, Gramoxone® (Paraquat) was used by farmers in the study area on 23 April 2004 to control weeds prior to planting rice. During the rice plant growing period, two OP insecticide formulations, Lebaycid® (Fenthion) and Elsan® (Phenthoate) were used as a mixture on 21 June 2004 by the farmers for controlling the rice bug (Leptocorisa orotorius) and rice thrips (Stenchaetothrips biformis) which infested the rice plants. Paraquat, Fenthion and Phenthoate were applied to the rice fields as sprays by the farmers in the area and the application rates were 0.4, 0.6, 1.12 kg ha−1, respectively.
Fish sampling
Rasbora caverii were sampled from each of the five sampling locations in the study area at four stages during the period March–August in 2004. The first fish sample was taken 14 days before application of any pesticide (19 March 2004) and the second sample was taken 7 days after the application of Paraquat (30 April 2004). The third sample was taken 2 days after the application of a mixture of Fenthion and Phenthoate (23 June 2004). The Fourth sample was taken 65 days after Fenthion and Phenthoate application (27 August 2004), which corresponded to a week after the completion of culture cycle of rice.
Rasbora caverii were caught from randomly selected three sub-locations of each sampling location using a cast net of 1 cm stretched mesh. During three applications of cast net, 25–100 fish could be collected from each sampling location. Samples of fish were transported live to the laboratory in plastic bags containing oxygenated water. In the laboratory fish were sacrificed by pithing, their body weight and length were recorded. Their brain tissues were removed and immediately stored at −80°C until they were processed for the brain AChE assay. In each sampling stage, ten fishes from each sampling location were used for CF and AChE determinations. The gill tissues (second gill on the left side) of the fish collected at the sampling stage 3 were removed and preserved in neutral buffered formalin for histopathological studies.
Water sampling and analysis
Water samples were collected from each of the five sampling locations in the study area at the third sampling stage. They were transported immediately to the Industrial Technology Institute (ITI), Colombo in chilled condition. Analysis of pesticides in the water samples was carried out in the ITI laboratory. Water samples were extracted with dichloromethane and passed through a clean up Florosil column and analysed by Capillary Gas Chromatography coupled with nitrogen–phosphorus detector.
Exposure of fish to pesticides in the laboratory
Samples of R. caverii collected from the reference site, Akuragoda Wewa were allowed to acclimate to the laboratory conditions in fiberglass tanks filled with continuously aerated aged tap water under natural photoperiod for 7 days. During the acclimation period, fish were fed daily with commercial fish food pellets (Prima, Sri Lanka) at 1% of the body weight. The body size of the fish used in the laboratory experiments was 6.5–8.4 cm in total length and 2.1–3.1 g body weight.
Commercial preparations of the pesticides viz. Gramoxone (Paraquat, 200 g l−1, an emulsifiable concentrate (EC) from Hayleys Agro Products Ltd., Colombo, Sri Lanka), Lebaycid (Fenthion, 500 g l−1 EC from Hayleys Agro Products Ltd., Colombo, Sri Lanka) and Elsan (Phenthoate, 500 g l−1 EC from Lankem Ceylon Ltd., Colombo, Sri Lanka) were used for the exposure of fish. Prior to the exposure of fish, stock solutions of the pesticides (1 mg l−1) were prepared freshly by diluting the commercial formulation of the pesticides with aged tap water. The solutions were further diluted to obtain required concentrations of the pesticides in the glass aquaria (60 × 30 × 30 cm3), which contained 36 l of the test water.
The application rates of Paraquat, Fenthion and Phenthoate to the rice fields by the farmers during the cultivation season were 0.4, 0.6, 1.12 kg ha−1, respectively. Paraquat was applied once to the rice fields only during the field preparation period and subsequently, a mixture of the other two pesticides were applied once during rice growing period. If these pesticides were directly applied to water, expected highest concentration of Paraquat, Fenthion and Phenthoate in 10 cm depth of water just after the application would have been 0.4, 0.60 and 1.12 mg l−1, respectively. As environmentally relevant concentrations of active ingredient to which the fish may get exposed in the natural environment after their application in the field at the concentration recommended by the manufacturers were not available, 0.5% of the estimated highest concentration in 10 cm depth of water in the field which correspond to 2 μg l−1 Paraquat, 3 μg l−1 Fenthion and 5 μg l−1 Phenthoate were tested in the laboratory study.
Two experiments were designed to expose R. caverii to the pesticides, which were used in the rice cultivation season in the study area. In the first experiment, the fish were exposed to 2 μg l−1 Paraquat, or 3 μg l−1 Fenthion or 5 μg l−1 Phenthoate in 36 l glass aquaria for 48 h (n = 10 fish per aquarium, two aquaria for each pesticide). Daily renewal of exposure media was not performed in this experiment in order to study the response of fish to continuous exposure to the original level pesticide and/or degradation products for 48 h as the respective pesticide was applied only once to the rice fields. In rice cultivation seasons, the fish residing in waterbodies close to the rice fields are pre-exposed to Paraquat before being exposed to insecticides because Paraquat is applied to agricultural fields during the field preparation period. Hence, the second laboratory experiment was designed to study the influence of pre-exposure of R. caverii to Paraquat on the extent of inhibition of the brain AChE enzyme due to subsequent exposure to Fenthion and Phenthoate. In the second experiment, fish were exposed to 2 μg l−1 Paraquat for 7 days and samples of the fish pre-exposed to Paraquat were subsequently exposed to 2 μg l−1 Paraquat or 3 μg l−1 Fenthion or 5 μg l−1 Phenthoate or a combination of 3 μg l−1 Fenthion and 5 μg l−1 Phenthoate for another 48 h (n = 10 fish per aquarium, two aquaria for each treatment). Fish maintained in aged tap water served as controls. In this experiment, exposure media of control aquaria and experimental aquaria containing Paraquat were renewed every 2 days in order to maintain the required Paraquat concentration.
Temperature, pH, dissolved oxygen (DO) in water in the aquaria were measured once in every 2 days using water quality monitors (HACH company, Loveland, CO, USA) and were within the favourable limits for fish: temperature 26–28°C; pH 7.4–7.8; DO 5.3–5.9 mg l−1. In both experiments, the fish were sacrificed after the exposure by pithing, body weights and lengths were recorded and their brain tissues were immediately removed and stored at −80°C until they were used in brain AChE assay. Their gill tissues were also removed and preserved in neutral buffered formalin for histopathological studies.
Determination of condition factor and brain AChE activity
The condition factor of each fish was calculated using the equation, CF = (W/L 3) × 100 where W = body weight (g) and L = total length (cm). The CF was expressed in g cm−3. The brain tissues were used for the AChE assay within a period of 1 week from storage at −80°C. The brain tissues were homogenized in ice cold 0.1 M phosphate buffer (pH 8.0) (1:5 w/v) in a tissue homogenizer (Ultra-Turrax T 25, IKA Labortechnik, Germany) to prepare the enzyme source. The AChE activity in the homogenate was measured at 25°C as described by Ellman et al. (1961) with Acetylthiocholine iodide (final concentration 0.50 mM in the reaction mixture) using a computer controlled recording spectrophotometer (GBC Cintra 10e) with thermostated cuvette holders. Substrate blanks without tissues and tissue blanks without substrate were used to correct the measured activities. All reagents were from Sigma-Aldrich (St. Louis, MO, USA). Brain AChE activity was expressed in nmoles min−1 mg−1 of wet brain weight.
Gill histology
Gills of randomly selected three fish from each sampling site in the field study and three fish from each of the control and pesticide exposed aquaria in the laboratory study were used for histological evaluation. The gill tissues were fixed in neutral buffered formalin, and processed using standard histological procedures. The sections were cut at 5–7 μm thickness and stained with Haemotoxin and Eosin (Bucke 1989). Abnormalities of the gill tissues in the fish were identified under the light microscope. For quantitative evaluation of gill abnormalities, all secondary lamellae in randomly selected eight consecutive primary lamellae of each gill were examined and the number of secondary lamellae which, exhibit a specific abnormality and the total number of secondary lamellae observed from each gill were recorded. The data are presented as percentages of occurrence of specific abnormality.
Statistical analysis
The data on CF and brain AChE were tested for normal distribution using Anderson Darling test for normality. Influence of body size of R. caverii on brain AChE activity was tested by calculating Pearson product moment correlation coefficients using the data from the fish collected from the reference site. The data on CF and brain AChE activities of the fish collected during the field study were analysed using two-way ANOVA with sampling stage and sampling site as factors. The data on CF and brain AChE activity of the fish exposed to different pesticides in the laboratory with and without pre-exposure to Paraquat along with the respective control fish were compared using one-way ANOVA. When the data were significantly different, Tukey’s multiple comparison test was used for pair wise comparison of means as appropriate. The data on gill abnormalities of the fish collected from the field study and laboratory study were analysed using non-parametric Kruskal–Wallis test. All the statistical analysis was carried out following Zar (1999). The results were determined to be statistically significant if P ≤ 0.05.
Results
Pesticide analysis
Fenthion was the only detected pesticide in the water samples. The water samples collected from the sampling site C at the sampling stage 3 (2 days after application of Phenthoate and Fenthion) contained 2 μg l−1 of Fenthion. The pesticides in the other sites were below the detection limits (<2 μg l−1). No pesticides were detected in the water collected from the sampling site A, which was considered as the reference site.
CF and brain AChE activity of fish collected from the field
Condition factor of R. caverii sampled from the five sites varied from 0.68 to 0.85 g cm−3. Two-way ANOVA revealed that there were no site specific (F (4, 180) = 1.10, P > 0.05) or sampling stage specific (F (3, 180) = 1.09, P > 0.05) significant differences or interaction between different sampling sites and sampling stages (F (12, 180) = 1.57, P > 0.05) in relation to the condition factor.
Correlation analysis revealed that there was no significant correlation between the brain AChE activity of R. caverii and body weight (0.8–3.07 g) or body length (4.2–8.4 cm) of the fish sampled from the reference site (r = 0.257 for body weight, r = 0.092 for body length, df = 48, P > 0.05). Two-way ANOVA on brain AChE activity of fish collected during the field study revealed that there were highly significant differences with respect to different sampling sites (F (4, 180) = 15.95, P = 0.001) and sampling stages (F (3, 180) = 51.87, P = 0.001) and interaction between different sampling sites and stages (F (12, 180) = 6.03, P = 0.001).
Sampling site-related statistical comparisons of the brain AChE activity of the fish (Fig. 2, 3) revealed that there were no significant sampling site-related differences in the fish collected at sampling stage 1 (14 days before application of any pesticide) and stage 2 (7 days after application of Paraquat). However, at the sampling stage 3 (2 days after application of Fenthion and Phenthoate), the AChE activities of fish collected from the sites C (75% inhibition) and D (65% inhibition) were significantly lower than that of the fish collected from the sites A and B. At the fourth sampling stage (65 days after application of Fenthion and Phenthoate), the brain AChE activities of the fish collected from sampling sites C–E were also depressed by 50, 56 and 32% of the activity of the fish in the reference site, respectively.
Brain AChE activity of Rasbora caverii collected from different sampling sites at four sampling stages. Bars represent mean brain AChE activity (± SD). Bars with unlike letters are significantly different from each other (ANOVA, Tukey’s test). Site A: reference site (Akuragoda Wewa); Site B: the main canal running through the abandoned rice fields; Site C: feeder canal running through cultivated rice fields; Site D: the main canal running through cultivated rice fields; Site E: the area where the main canal joins with Kelani river
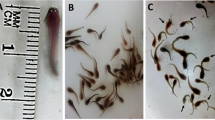
a Gill lamellae of Rasbora caverii collected from the reference site, PL Primary lamellae, SL Secondary lamellae. b Gill lamellae of R. caverii collected from a canal running through rice fields showing abnormal multiple divisions in the apical portion of the secondary lamellae (AD) and club shaped deformities (CD). c Close up view of a primary lamellae showing abnormal multiple divisions in the apical portion of the secondary lamellae (H and E stain)
Sampling stage-related statistical comparison showed that brain AChE activities of R. caverii collected from site A (reference site), and site B (area of the main canal running through the abandoned rice fields) were not significantly different, at four sampling stages (P > 0.05). However, the brain AChE activities of R. caverii collected from the sites C and D at sampling stages 3 and 4 were significantly lower than those of the fish collected from the same locations at sampling stages 1 and 2 where the samples were taken before application of any pesticide and 7 days after application of Paraquat (P < 0.05). The AChE activity of the fish collected from the site E at sampling stage 4 was significantly different from that of the fish collected from the same location at the first and second sampling stages (P < 0.05).
CF and brain AChE activity of fish exposed to pesticides in the laboratory
One-way ANOVA indicated that condition factor of the fish in different treatment groups and the controls were not significantly different from each other (F (9, 170) = 1.24, P > 0.05). The survival of control fish and the fish exposed to pesticides for 2 days was 100%. When the fish pre-exposed to Paraquat were subsequently exposed to the other pesticides, the survival of fish was reduced to 70–80%.
Exposure of fish to 2 μg l−1 Paraquat for 2 or 9 days had no significant effect on brain AChE activity (Table 1). However, the AChE activity of the fish exposed to 3 μg l−1 Fenthion or 5 μg l−1 Phenthoate for 2 days was significantly lower (54 and 77% inhibition, respectively) than that of the controls. When the fish were exposed only to a combination of 3 μg l−1 Fenthion and 5 μg l−1 Phenthoate for 2 days, the AChE activity was inhibited by 75%. However, pre-exposure to Paraquat for 7 days had no significant effect on the degree of inhibition induced by 5 μg l−1 Phenthoate exposure for 2 days (Table 1). Prior exposure to Paraquat for 7 days further increased the degree of AChE inhibition caused by Fenthion or a mixture of Fenthion and Phenthoate.
Histological changes in the gills
Most of the gill lamellae of R. caverii collected from the sampling sites C and D at the sampling stage 3, exhibited hyperplasia, hypertrophy and enlargement of secondary lamellae forming club shaped deformities. These abnormalities were also observed in the gill lamellae of fish collected from sampling sites A, B and E, but the percentage occurrence of these abnormalities was significantly lower than those in the fish collected from sampling sites C and D (P < 0.05, Table 2). Abnormalities in the gill lamellae of the fish that were exposed to pesticides in the laboratory were much lower than those were observed in the samples collected from the site C and D. There was no significant difference in the percentage occurrence of these abnormalities between the control fish and the pesticide exposed fish in the laboratory (P > 0.05, Table 3). In addition to the above-mentioned abnormalities, prominent multiple divisions (two or more parts) of secondary gill lamellae at the apical portion (Fig. 2) were observed in the gills of fish collected from sampling sites C and D. This specific abnormality was not observed in the fish that were exposed to pesticides under controlled laboratory conditions.
Discussion
Environmental fate of pesticides are affected by various factors such as pesticide application rates, half life of the pesticide in soil, half life of the pesticide in water, climate/weather conditions, soil characteristics, etc (Brown et al. 1995). In the present study, a mixture of Fenthion and Phenthoate was applied once (0.6, and 1.12 kg ha−1, respectively) to the rice fields during the cultivation season to control the insect pests. If these pesticides were directly applied to water, expected highest concentration of Fenthion and Phenthoate in 10 cm depth of water just after the application would have been 0.60 and 1.12 mg l−1, respectively. However, such high levels of Fenthion and Phenthoate in nearby water bodies cannot be expected even just after the application as they were sprayed to the rice plants. Surface run-off, spray drifts and leaching through the soil are possible indirect routes of pesticide contamination in the rice field associated waterbodies. It has been reported that Fenthion adsorb fairly strongly to soil particles and less likely to move or leach through soil (Khan 1977; Wauchope et al. 1992). Phenthoate did not persist in soil for more than few days upon aerial application as sprays to Citrus and date palm orchards and evaporation appeared to be the major process by which dissipation of the insecticide occur (Al-Omar et al. 2004). In the present study, Phenthoate was not detectable in the water samples collected from rice field associated waterbodies 2 days after the application of Fenthion and Phenthoate as sprays to the rice fields. Fenthion was the only detected pesticide in water samples. Fenthion was present at 2 μg l−1 in the feeder canal running through the cultivated paddy fields 2 days after pesticides application to the nearby rice fields. As there was no rain in the area on the day of Fenthion and Phenthoate application and during 2 days after the application, it is unlikely that these pesticides have entered the nearby water sources through surface run-off or leaching. The pesticides sprayed to the rice fields might have entered the water sources through aerial drift, as slight wind was present towards the west direction on the date of application of these pesticides to the rice fields (6 km h−1, Meteorological Department, Colombo, Sri Lanka). It is possible that most of the insecticides especially Phenthoate that have entered the water sources may have been diluted and/or degraded during 2 days. In a previous study, the average concentration of Phenthoate in river water just after application as sprays to orchards was reported as 15.45 mg l−1, which was reduced to 1.31 mg l−1 by 48 h indicating nearly 92% reduction of Phenthoate in water within 2 days (Al-Omar et al. 2004).
Whitehead et al. (2005) emphasized the importance of adopting cholinesterase activity measurements into region specific monitoring programmes to assess in—stream effects of pesticide contaminations in native species. Even though agrochemicals are widely used in Sri Lanka in agriculture, no monitoring programmes are conducted to assess the pesticide impact. The present study demonstrated significant inhibition (65–75%) of brain AChE activity in R. caverii residing the feeder canal and main canal which run through cultivated rice fields 2 days after the application of Fenthion and Phenthoate to the rice fields even though only detected pesticide in the water samples by chemical analysis was 2 μg l−1 of Fenthion. High inhibition of brain AChE may reflect the exposure of fish to the original concentrations of the insecticides in water. Insecticides induced inhibition restored partly (50–56%) by 65 days after the insecticides application. As OP insecticides irreversibly bind with the AChE forming a firm bond, normal activity of the enzyme can only be restored by de novo synthesis of unaffected enzyme, which continues at a regular pace in the fish body (Fulton and Key 2001). Hence, full restoration of Fenthion and Phenthoate induced AChE inhibition in R. caverii takes more than 65 days, and the neurotoxicity may persist until the activity restores. Previous studies with different fish species have suggested that brain AChE inhibition levels of >70% are associated with mortality in most of the fish species. Sublethal effects on stamina have been reported for some species of fish in association with brain AChE inhibition levels as low as 50% (Fulton and Key 2001).
In the present study, the concentrations of Paraquat, Fenthion and Phenthoate used to expose R. caverii in the laboratory were 2, 3 and 5, respectively, which represented the 0.5% of the estimated highest concentration in 10 cm depth of water if the pesticides were directly applied to waterbodies. Lethal toxicity data of these pesticides for R. caverii are not available. Range of lethal toxicity data of Paraquat, Fenthion and Phenthoate for several fish species listed by Pesticide action Network, North America (http://www.pesticideinfo.org, retrieved on 19 July 2006) based on data obtained by United States Environmental Protection Agency indicate that LC50 data for Paraquat range from 6,125 (for Rhomdia sapo, black cat fish) to 610,025 μg l−1 (for Gambusia affinis, Western mosquito fish). LC50 data of Fenthion range from 2.8 (for G. affinis) to 17,000 μg l−1 (for Angilla japonica, Japanese eel). LC50 data for Phenthoate range from 2 (for Seriola quinqueradiata, yellow tail) to 2,400 μg l−1 (for Carassius auratus, gold fish). These toxicity data indicate that the concentrations of Fenthion and Phenthoate used in the present study are acutely toxic to some fish species whereas tested concentration of Paraquat is not acutely toxic to any of the fish species listed.
The results of the laboratory study showed significant inhibition of brain AChE activity induced by Fenthion (54% inhibition at 3 μg l−1) and Phenthoate (77% inhibition at 5 μg l−1) in R. caverii and these two insecticides do not have a synergistic effect on brain AChE activity of R. caverii at the tested concentration levels. Inhibition of brain AChE activity in fingerlings (26–29%) and in the fry (38–40%) of tilapia (Oreochromis mossambicus) after 10 weeks exposure to Fenthion (Pathiratne, 1999) in the laboratory was very much lower than the inhibition levels recorded in the present study where R. caverii were exposed to very much lower concentrations of Fenthion (3 μg l−1) for 2 days. High percentage inhibition of brain AChE activity due to short term exposure to very low concentration of Fenthion indicates that R. caverii, which is an indigenous fish species is more sensitive to exposure to Fenthion than the exotic species, O. mossambicus.
Previous studies indicate significant inhibition of AChE activity in a few species of fish exposed to relatively higher concentrations of Paraquat (Nemesók et al. 1985; Di Marzio and Tortorelli 1994). In the present study, brain AChE of the fish were not affected significantly either at 7 days after application of Paraquat to the rice fields or laboratory exposure of fish to 2 μg l−1 Paraquat for 9 days. The concentration of Paraquat used in the study may have been too low to cause significant inhibitions in brain AChE activity of R. caverii.
In rice cultivation seasons, the fish residing in waterbodies close to the rice fields are pre-exposed to Paraquat before being exposed to insecticides because Paraquat is applied to agricultural fields during the field preparation period. In the present study, the percentage inhibition of brain AChE activity of R. caverii exposed to 3 μg l−1 Fenthion or a mixture of 3 μg l−1 Fenthion and 5 μg l−1 Phenthoate after pre-exposure to Paraquat in the laboratory was found to be significantly higher than that of the fish exposed to only 3 μg l−1 Fenthion or a mixture of 3 μg l−1 Fenthion and 5 μg l−1 Phenthoate. Percentage survival of fish pre-exposed to Paraquat was lower than the percentage survival of fish that were not pre-exposed to Paraquat. The increased toxic action of Fenthion or a mixture of Fenthion and Phenthoate due to pre-exposure to Paraquat observed in the present study may be due to the stress induced in the fish due to Paraquat pre-exposure. The results therefore indicate that long-term exposure to Paraquat could affect the survival of R. caverii populations in the rice field associated waterbodies.
Histopathological biomarkers in the gills may be valuable as indicators of the general health of the fish and mirror effects of parasitic infections and exposure to a variety of anthropogenic pollutants. Even though, AChE can be considered as a specific biomarker for OP and CM pesticides, gill lesions are only unspecific responses to chemical exposures and/or parasite infections. In the present study, hyperplasia, hypertrophy and club shaped deformities in the secondary gill lamellae were recorded in R. caverii collected from the field. These abnormalities in the gills following pesticide exposure have been reported earlier in different species of fish (Murty 1986; Fanta et al. 2003). Parasites also can provoke hyperplasia and hypertrophy of gill epithelial cells and club shaped deformities in the secondary lamellae (Post 1985). However in the present study, no parasites were found in association with the gills of sampled fish. Prominent abnormal divisions of the secondary gill lamellae to two or more ‘comb like’ divisions at the apical region were observed in R. caverii from the sampling sites running through the cultivated rice fields. To the best of our knowledge, such an abnormality in the gill lamellae of fish has not been reported in the literature by earlier workers. This specific abnormality in R. caverii collected only from canals running through the rice fields may have been due to long-term repeated exposure of fish populations to agrochemicals used in the field during different cultivation seasons. It is not known whether this condition is due to a teratogenic effect. Short-term exposure to the selected pesticides in the laboratory (viz. Paraquat, Fenthion, Phenthoate) did not induce abnormal division of the secondary gill lamellae or significant increase in hyperplasia, hypertrophy or club shaped deformities probably due to the relatively short duration of exposure to very low levels of the pesticides. As the status of the gill histology in R. caverii before application of the pesticides in the studied culture cycle are not known, it is difficult to relate the abnormal divisions seen in the fish to the exposure of the three pesticides in the present study. However, histological changes in the gills allowed differentiating sampling sites running through the rice fields from the sampling sites located in the distant water bodies. Histopathological changes observed in R. caverii could cause detrimental effects to the proper functioning of the gills of the fish thereby affecting the vital functions of the organ such as respiratory gas exchange, osmoregulation, acid base balance and ammonia excretion.
Condition factor is a morphological parameter indicative of the health status of the whole body of the fish related to environmental availability of food and energy resources. This factor may be affected if the availability of food is limited or if the food consumption of fish is impaired due to stress factors (Van der Oost et al. 2003). Our results did not show significant alterations in the CF of R. caverii regardless of the sampling site or sampling stages. De la Torre et al. (2005) also found similar results when they used CF in a native fish species (Cnesterodon decemmaculatus) in field evaluations. Results indicate that CF may not be a sensitive enough biomarker to measure environmental stress in natural environments.
Conclusion
The present study is a field application of integrated use of several biomarkers to evaluate the pesticide impact on populations of R. caveri, an indigenous fish commonly found in rice fields associated waterbodies in Sri Lanka. Exposure of R. caverii to low concentrations of Fenthion and Phenthoate could markedly inhibit its brain AChE activity. The fish collected from canals running through rice fields exhibited significant AChE inhibition in the brain tissues and prominent histopathological changes in its gills including prominent abnormal divisions at the tips of the secondary gill lamellae. However, condition factor of the fish was not affected by the exposure to pesticides used in this study. The response of brain AChE and histological changes in the gills of the fish allowed for the differentiation of sampling sites following insecticide applications to the rice fields. Pesticide responsive changes in R. caverii detected in the present study can be considered as biomarkers of the early warning signals of pesticide impact on non-target native fish populations inhabiting rice field associated water bodies. Since R. caveri is an indigenous fish widely distributed in freshwater environments, it may be considered as a surrogate species in ecotoxicological risk evaluations of agrochemicals in the region.
References
Al-Omar MA, Al-Suhaily RH, Kamel Y (2004) Distribution and dissipation of Phenthoate insecticide following aerial application. Bull Environ Contam Toxicol 73:825–831
Bucke D (1989) Histology. In: Austin B, Austin DA (eds) Methods for the microbiological examination of fish and shellfish. Ellis Horwood Limited, West Sussex
Brown CD, Carter AD, Hollis JM (1995) Soil and pesticide mobility. In: Roberts TR, Kearney PC (eds) Environmental behaviour of agrochemicals. Wiley, West Sussex
Coppage DL, Bradeich E (1976) River pollution by anti-cholinesterase agents. Water Res 10:19–24
De La Torre FR, Ferrari L, Salibián A (2005) Biomarkers of a native fish species (Cnesterodon decemmaculatus) application to the water toxicity assessment of a peri-urban polluted river of Argentina. Chemosphere 59:577–583
De Mel GWJLMVTM, Pathiratne A (2005) Toxicity assessment of insecticides commonly used in rice pest management to the fry of common carp, Cyprinus carpio, a food fish culturable in the rice fields. J Appl Ichthyol 21:146–150
Di Marzio WD, Tortorelli MC (1994) Effects of paraquat on survival and total cholinesterase activity in fry of Cnesterodon decemmaculates (Pisces, Poecillidae). Bull Environ Contam Toxicol 52:274–278
Ecobichon DJ (1992) Toxic effects of pesticides In: Amdur M, Doull J, Klaassen C (eds) Casarett and Doull’s toxicology. The basic science of poisons. McGraw-Hill, New York, pp 565–622
Ellman GL, Courtney KD, Anders V Jr, Featherstone RM (1961) A new and rapid colourimetric determination of acetylcholiesterase activity. Biochem Pharmacol 7:85–95
Fanta E, Rios FS, Romão S, Vianna ACC, Freiberger S (2003) Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorous in water and food. Ecotoxicol Environ Saf 54(2):119–130
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorous insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Gruber SJ, Munn MD (1998) Organophosphate and carbamate insecticides in agricultural waters and cholinesterase inhibition in common carp (Cyprinus carpio). Arch Environ Contam Toxicol 35:391–396
Khan MAQ (1977) Pesticides in aquatic environments. Plenum Press, NY
Kozlovskaya VI, Mayer FL, Menzikova OV, Chuyko GM (1993) Cholinesterase of aquatic animals. Rev Environ Contam Toxicol 132:117–142
McCarthy JF, Shugart LR (1990) Biomarkers of environmental contamination. Lewis Publishers, FL
Murty AS (1986) Toxicity of pesticides to fish, vol 2. CRC Press Inc., Boca Raton, FL
Nemesók J, Orban L, Asztalos B, Buǵas ZS, Nemeth A, Boross L (1985) Investigations on paraquat toxicity in fishes. Water Int 10:79–81
Pathiratne A (1999) Toxicity of Fenthion in Lebaycid to tilapia, Oreochromis mossambicus (Peters): effects on survival, growth and brain acetylcholinesterase activity. J Natl Sci Found Sri Lanka 27(2):79–91
Pethiagoda R (1991) Freshwater fishes of Sri Lanka. Wildlife Heritage Trust of Sri Lanka, Sri Lanka
Post G (1985) Text book of fish health. T. F. H. Publications Inc., Neptune City, NJ
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Wauchope RD, Buttler TM, Hornsby AG, Augustijn-Beckers PWM, Burt JP (1992) SCS/ARS/CES Pesticide Properties data base for environmental decision-making. Rev Environ Contam Toxicol 123:1–155
Whitehead A, Anderson SL, Ramirez A, Wilson BW (2005) Cholinesterase in aquatic biomonitoring: assay optimization and species specific characterization for a California native fish. Ecotoxicology 14:597–606
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wijeyaratne, W.M.D.N., Pathiratne, A. Acetylcholinesterase inhibition and gill lesions in Rasbora caverii, an indigenous fish inhabiting rice field associated waterbodies in Sri Lanka. Ecotoxicology 15, 609–619 (2006). https://doi.org/10.1007/s10646-006-0101-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-006-0101-5