Abstract
Seeds of Zea mays L. were exposed to aflatoxine B1 (AFB1), aflatoxine G1 (AFG1) and selenium (Se) alone and in combination and allowed to germinate. Phytohormone levels of GA-like substances (GAs), trans-Zeatin (t-Z) and Indole-3-acetic acid (IAA) were determined by High Performance Liquid Chromatography (HPLC) when the roots of the germinating seeds reach 1.5–3.0 cm in length. The levels of endogenous hormones decreased in seeds treated with AFB1 and AFG1 compared to control; however an increase was noted in seeds exposed to AFG1 and Se together. AFB1 and Se treatment caused reduced hormone levels in most of the treatments. When plants were exposed to Se alone, the highest levels of GAs, t-Z and IAA were observed in the application of 800 ppm Se. The highest levels of GAs, t-Z and IAA were observed when seeds were treated with 0.2 ppm AFG1 + 8 ppm Se, 0.2 ppm AFG1 + 8 ppm Se and 0.2 ppm AFG1 + 0.08 ppm Se, respectively, whereas the lowest levels of the hormones were observed in 0.2 ppm AFB1 + 8 ppm Se, 0.2 ppm AFB1 + 0.08 ppm Se and 0.1 ppm AFB1, respectively. In conclusion, the levels of phytohormones were reduced by the treatment of AFB1 and AFG1 alone. However Se removed the negative effect of AFB1 on phytohormones, but not AFB1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aflatoxines are secondary metabolites produced by toxigenic strains of Aspergillus flavus Link ex. Fries and Aspergillus parasiticus. These fungi grow rapidly on a variety of natural substrates and the consumption of fungi-contaminated food can cause serious health hazards in humans and animals. Aflatoxine B1 (AFB1) is a highly toxic and carcinogenic metabolite produced by Aspergillus species on agricultural crops (Leontopoulos et al. 2003). The mutagenic effect of aflatoxine G1 (AFG1) on Neurospora crassa and on the larvae of the Egyptian cotton leaf worm was reported (Ong 1971; Abdou et al. 1984). Different concentration of AFG1 inhibited chlorophyll, carotenoid, protein and lipid content and reduced growth and germination of Zea mays L. and Vicia faba seeds (El-Naghy et al. 1999). It is well documented that mycotoxines produced by specific filamentous fungi also cause significant reductions in crop yield and economic losses (Gourama and Bullerman 1995; Gqaleni et al. 1996; Bathnagar and Garcia 2001).
The involvement of Aspergillus sp. as plant pathogens has not been seriously taken into consideration, but there is accumulating evidence that the aflatoxines are phytotoxic compounds. The phytotoxic effects of the aflatoxines have been investigated with respect to seed germination and inhibition of root and hypocotyl elongation (Reiss 1978; Dashek and Llewellyn 1983). Aflatoxines were reported to be carcinogenic fungal metabolites of A. flavus and were prevalent contaminant in cotton grown in southwestern USA (Busley and Wogan 1979; Groopman et al. 1981).
Maize is one of the most important cereals grown in the world. It is commonly consumed directly as food, indirectly as additive in most of food products and used as animal fodder (Lutz 1994). Maize may be contaminated with storage fungi, some of which produce mycotoxine that could be harmful for animal and human health.
Aflatoxine content may vary with the season and storage time (Lillehoj and Zuber 1988). In Vietnam it was observed that toxin contamination was higher during the rainy season and increased with increasing storage time. Ahmad (1993) reported that aflatoxine contamination in storage was dependent on the storage system. It is well documented that AFB1 produced by A. flavus and A. parasiticus, is a potent hepatocarsinogen in many animal species (Hsieh et al. 1977), and has been implicated as an aetiological agent in the development of hepatocellular carcinomena in several African and Asian communities (Wogan 1992).
Selenium is a mineral which has important effect on some metabolic process in plants and animals (Chow and Gairola 1984; Gerald et al. 1998). Se is a key component of various enzymes, of which glutathione peroxidase is the best characterized. Indeed, internationally recommended daily intake of Se is set on the basis of the level necessary to saturate the DNA repair enzyme. However, it is also a component of thioredoxin reductase, and several other enzymes, which are not currently identified (Ferguson and Philpott 2004). A number of lines of evidence suggest that Se is an important micronutrient in protection against cancer (Schrauzer et al. 1977; El-Bayoumy 2001). A number of studies have shown that cancer patients have slightly lower Se level than healthy controls (Combs 1997).
Se deficiency would be expected to induce genetic damage as a result of causing excessive oxidative stress. Se deficiency has been implicated in playing a role in the development of many diseases, including cancer, cardiovascular and immune disorders (Rayman 2000). On the other hand, excessive intake of Se may result in oxidative damage leading to genomic instability (Spallholz 1994).
Se affects local extinctions of fish and birds in cooling reservoirs of coal-fired power plants (Lemly 1985) and in wetland and river ecosystems receiving drainage from seleniferous agricultural lands (Presser and Ohlendorf 1987; Skorupa 1998; Hamilton 1999). Although Se is nutritionally essential, the window is narrow between essential concentrations in food and those that cause adverse effects (Hodson and Hilton 1983). Shi et al. (1994) suggested that Se could effectively inhibit AFB1-induced DNA damage and it might be partially responsible for anticarcinogenic effect against AFB1. Chen et al. (1982a) found comparable dietary levels of Se (2 mg/g as selenite) that reduce the formation of covalent DNA adducts of Aflatoxine in the chick (Chen et al. 1982b).
Plant growth regulators that exert a variety of biological activities are important for growth and development of plants (Sembdener and Parthier 1993). Some investigations directed toward the elimination of preharvest aflatoxine contamination through the enhancement of natural defensive systems were presented in the cotton plant. In a recent study, natural elicitors were combined with methyl jasmonate (MJ), a natural plant hormone, to evaluate its effects on phytoalexin production and aflatoxine B1 (Zeringue 2002). However, the effects of the AFG1 and AFB1 on GAs, t-Z and IAA remained unexplored until now.
Aflatoxines are joined in food chain in different ways and make important detrimental damages in the metabolism of organisms (Hela et al. 2000). Se is also an important component of food chain. However in different doses, the positive and negative effects of Se on organisms have been reported (Wilbur 1983). In the present study, the effects of separate and combined application of AFB1, AFG1 and Se on the plant hormones were investigated. We hope that the present study will help new investigation in determining the interactions between phytohormones, aflatoxines and Se.
The seeds have been shown to be naturally contaminated with various levels of aflatoxins in the field (Anderson et al. 1975) and during storage (Bilgrami and Sinha 1992) The contaminants (aflatoxines) are serious problem for seed germination that causes important economic loss. The contaminants are also carried to other organisms (human and animals) via food chain resulted in metabolic disorder. It is a common knowledge that phytohormones play a remarkably influential role for seed germination. The research is an introductory work to state the interaction between phytohormones, Se and aflatoxines.
Material and methods
Germination conditions of seed
Aflatoxin G1 (AFG1), Aflatoxin B1 (AFB1) and sodium selenite were obtained from Sigma Chemical Company, USA. Zea mays L. seeds were obtained from Department of Field Crops, Faculty of Agriculture, Ataturk University (Turkey). Seeds in equal size of Zea mays L. were chosen and the surface of seeds was sterilized with 2.5% (w/v) sodium hypochlorite (NaOCl) for 3 min. Then the seeds were washed four times with tap water, and dried using sterile filter paper. The seeds were soaked in sterile distilled water for 1 h and then fifteen seeds were put in petri dishes on four layers of sterile Whatman (No. 1) filter paper for germination. Each experiment was performed in triplicate under same conditions. The concentrations of Selenium (Se), AFB1 and AFG1 were chosen according to the relevant literature (Chen et al. 1982a, b; McLean et al. 1995; El-Naghy et al. 1999). Following solutions were added into each plate. Solution-free (control), 0.08, 8.00 ve 800 ppm Se alone, 0.1, 0.2 ppm AFG1 and AFB1 alone, and in combination with 0.08, 8.00, 800 ppm of selenium (Se), explanation in detail: 0.1 ppm AFG1 + 0.08 ppm Se, 0.1 ppm AFG1 + 8 ppm Se, 0.1 ppm AFG1 + 800 ppm Se, 0.2 ppm AFG1 + 0.08 ppm Se, 0.2 ppm AFG1 + 8 ppm Se, 0.2 ppm AFG1 + 800 ppm Se, 0.1 ppm AFB1 + 0.08 ppm Se, 0.1 ppm AFB1 + 8 ppm Se, 0.1 ppm AFB1 + 800 ppm Se, 0.2 ppm AFB1 + 0.08 ppm Se, 0.2 ppm AFB1 + 8 ppm Se, 0.2 ppm AFB + 800 ppm Se. The Petri dishes were incubated in the dark at 25°C for germination. When the roots reached 1.5–3.0 cm in length the plantlets were harvested and put in deep-freeze (−80°C).
Extraction, purification and determination of phytohormones
The analysis of trans-Zeatin (t-Z) and Indole-3-acetic acid (IAA) was performed according to Kuraishi et al (1991) and Battal et al (2004). One gram of frozen sample was powdered in liquid nitrogen. Then cold methanol was added and stored at 4°C for 24 h in dark. The samples were homogenized in an Ultra Tissue Lysis (Ultrasonic Processor, Jenway Ltd. Essex, UK) and filtered through a filter paper (Whatman No. 1). Then the filtrates were collected. The residue was reprocessed in the same way as mentioned above and combined with the former one in order to minimize the loss of phytohormones. The filtrates were filtered through PTFE filters (0.45 μm). Methanol was removed at 35°C under reduced pressure. The extracts were redissolved in K2PO4 buffer (pH 8.5) and centrifuged at 10,000 × g for 1 h at 4°C. Then, the supernatants were put in flask (25 cm3), each containing 1 g polyvinylpolypyrolidone (PVPP, Sigma Chemical Co. UK), well mixed and filtered through Whatman filter paper (No. 1). The filtrates were introduced to Sep-Pak C18 cartridges (Waters, Hichrom Ltd. UK) (Machackova et al. 1993). The hormones were adsorbed by cartridges and the remnants were removed. The hormones were eluated from cartridge with 80% methanol and collected in vials. The hormone extracts were injected into High Performance Liquid Chromatography (HPLC) to detect t-Z and IAA.
The analysis of Gibberellic acid-equivalents (GAs) was performed according to Fujioka et al. (1986), Cakmak et al. (1989) and Wang et al. (2002) with some modifications. The frozen tissue samples (1 g) were ground to powder in liquid nitrogen and homogenized in 3 ml of methanol. The homogenate was mixed with 80% methanol, kept for overnight at 4°C and then filtered. The residue was reextracted with 80% methanol for 4 h, refiltered and combined with the former supernatant. Methanol was removed under reduced pressure at 35°C. The aqueous residue adjusted to pH 2.5 (2 M HCl). This solution was then partitioned with equal volumes of ethyl acetate and the combined organic phases were partitioned with 5% (m/v) sodium bicarbonate (3 × 1/5 volume) and separated GAs were injected into HPLC.
The isocratic system was used for HPLC analysis. The extracts in the vials were injected into HPLC equipped with Waters 6000A pump (Waters, Hicrom Ltd. Uk); Ultraviolet detector (Unicam Analytical Systems, Cambridge, UK) and μBondapak C18 column (Waters, Hicrom Ltd. Uk) using acetonitrile (12.00%; pH 4.98) as the mobile phase. The flow rate, pressure and wavelength were selected to be 2 ml min−1, 2,000 psi and 265 nm, respectively. Under these conditions, the retention time of GA3, t-Z, and IAA were determined as 2.85, 3.88 and 7.17 min for standards, respectively. GA3 was used as the standard for determining GAs.
Results were expressed as means of different experiments ± standard error of triplicates. Analysis of variance was conducted using one way ANOVA test using SPSS for Microsoft Windows and means were compared by Duncan test at the 0.05 level of confidence.
Results
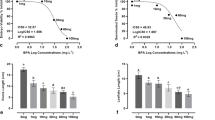
The highest and lowest phytohormones level were observed in 800 and 0.08 ppm Se applications respectively, when the Se treatment considered alone (Figs. 1a, 3a, 5a).
The level of Gibberellic acid-equivalents (GAs) declined in germinating seed treated with 0.1 ppm AFG1 compared to control. However the change was not statistically significant (P ≥ 0.05). The statistically significant increase (P ≤ 0.05) was determined in GAs level depending on the various concentrations of Se applied with 0.1 ppm AFG1. The highest level of GAs was determined in the application of 0.1 ppm AFG1 + 0.08 ppm Se (Fig. 1a).
The level of GAs decreased with the application of 0.2 ppm AFG1 alone. A significant increase (P ≤ 0.05) in GAs level was determined in the treatments of 0.2 ppm AFG1 + 800 and 8 ppm Se. The highest level of GAs was observed in the application of 0.2 ppm AFG1 + 8 ppm Se whereas GAs level was found lower than the control in the case of 0.2 ppm AFG1 + 0.08 ppm Se application (Fig. 1b).
When AFB1 was applied both alone and with Se, a decrease in GAs levels were observed in all the experiments. The lowest levels of GAs for 0.1 ppm AFB1 application were determined in 0.1 ppm AFB1 + 800 ppm Se and 0.1 ppm AFB1 + 0.08 ppm Se (Fig. 2a) they were found statistically significant (P ≤ 0.05), whereas the lowest level of GAs for 0.2 ppm AFB1was determined in the applications of 0.2 ppm AFB1 + 8 ppm Se and 0.2 ppm AFB1 + 0.08 ppm Se (Fig. 2b). All of the changes observed in 0.2 ppm AFB1 application were found significant (P ≤ 0.05).
t-Z levels decreased in plants exposed to 0.1 ppm AFG1 while it was significantly increased (P ≤ 0.05) in case of Se application with AFG1. The highest level of t-Z was found in the treatment of 0.1 ppm AFG1 + 800 ppm Se (Fig. 3a). A slight decline occurred in t-Z levels of plant treated with 0.2 ppm AFG1. However significant rise in t-Z levels (P ≤ 0.05) was seen in all the treatment of 0.2 ppm AFG1 with Se. A sharp increment in t-Z was observed in the application of 0.2 ppm AFG1 + 8.00 ppm Se (Fig. 3b).
The application of AFB1 alone and with Se significantly decreased t-Z levels (P ≤ 0.05) as shown in Fig. 4a and b.
Also 0.1 and 0.2 ppm AFG1 decreased IAA levels. On the other hand, IAA levels significantly (P ≤ 0.05) increased with the application of AFG1 plus Se. The highest IAA levels were observed in the treatment of 0.1 ppm AFG1 + 8 ppm Se and 0.2 ppm AFG1 + 0.08 ppm Se (Fig. 5a, b).
IAA levels decreased at the certain levels in the entire specimen exposed to AFB1 alone and accompanied with Se. The lowest level of IAA was found in the applications of 0.2 ppm AFB1 and 0.2 ppm AFB1 + 800 ppm Se (Fig. 6a, b). All changes were found statistically significant (P ≤ 0.05) except for 0.1 ppm AFB1 + 0.08 ppm Se.
Discussion
It is well known that all the forms of aflatoxines have toxic effect on living organisms (Shi et al. 1994). The findings in the present study showed that the separate application of AFB1 and AFG1 reduced whereas Se increased plant hormone levels compared to the controls. These results on aflatoxines are in agreement with the literature claiming that the substances inhibit the growth and developmental process in plants (Ahmad 1993; Reiss 1978; Dashek and Llewellyn 1983). AFG1 suppressed the synthesis of phytohormones. However, Se appears to remove the negative effect of AFG1 on phytohormones, when it was applied with AFG1. The levels of most of the phytohormones increased with the application of Se + AFG1, but not Se + AFB1 and AFG1 and AFB1 alone. The results indicate that Se could eliminate the fluctuating levels of phytohormones affected by AFG1 and protect plants by increasing the synthesis of phytohormones. In the present study, Se applications were observed to increase phytohormones level compared to controls. The findings are in a good agreement with the Shi et al. (1994) who reported that selenium prevents the damages of aflatoxines on DNA.
Application of AFB1 both alone and with Se reduced hormone levels compared to the controls and this negative effect is attributed to high toxicity of AFB1 (Leontopoulos et al. 2003). Se does not reverse the effect of AFB1 as it does with AFG1. It can be speculated that the detrimental results of AFB1 on the level of phytohormones is higher than that of AFG1 in terms of physiological processes in the plant and that none of the application of Se might eliminate the influence of AFB1 on fluctuation of pyhtohormone levels. On the contrary, Se may have completely caused a decrease in phytohormone level when it was applied with AFB1.
The present study suggest that Se have binary effect on plant metabolism behaving as either suppressing phytohormone synthesis or preventing the effects of aflatoxines depending on the concentration and the way of application. Biswas et al. (1997) also reported the negative effect of selenium on chromosomes. The preventing effect of Se on phytohormone fluctuation caused by AFG1 is probably not involved in all of the variety of aflatoxines. The finding can attract attentions on the other aflatoxins. However the interaction of the aflatoxines and phytohomones should be taken into consideration. It may affect the certain physiological and biochemical process in different forms depending on the various applications.
Furthermore, GAs may play an important role against destructive effect of AFG1 as do t-Z and IAA (Farmer and Ryan 1992; Gundlach et al. 1992; Andresen et al. 1992; Bell and Mullet 1991; Creelman et al. 1992). GAs might be synthesized in the highest levels to eliminate the negative effect of AFG1. On the other hand, the phytohormones, however, may have been inhibited by AFB1.
Consequently, it was observed that the separate application of AFG1 and AFB1 may have inhibiting effect on plant hormone levels in seeds and Se has little effect on AFB1’s inhibition on phytohormone levels when they were applied together. Probably Se promoted AFB1’s injury. However, the separet application of Se promote phytohormones level. These findings also suggest forward the result that Se could have different effects against the various kinds of aflatoxines.
References
Abdou RF, Megella SE, Azab SG (1984) Mutogenic effect of aflatoxin B1 and G1 on the Egyptian cotton leaf-worm Spodoptera littoralis (Boisd). Mycopathologia 88:23–26
Ahmad SK (1993) Mycoflora changes and aflatoxin production in stored blackgram seeds. J Stored Prod Res 29:33–36
Anderson HW, Nehring EW, Wichser WR (1975) Aflatoxin contamination of corn in the field. J Agric Food Chem 23:775–782
Andresen I, Becker W, Schluter K, Burges J, Parthier B (1992) The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum Vulgare). Plant Mol Biol 19:193–204
Bathnagar D, Garcia S (2001) Aspergillus. In: Labbe RG, Garcia S (eds) Guide to foodborne pathogens. John Wiley and Sons, New York, pp 35–49
Battal P, Aslan A, Turker M, Uzun Y (2004) The effect of gaseous air pollutant sulfur dioxide on phytohormone levels in some lichens. Fresinus Environ Bull 13(5):436–440
Bell E, Mullet JE (1991) Lipoxygenase gene expression is modulated in plants by water deficit, wounding, and methyl jasmonate. Mol Gen Genet 230:456–462
Bilgrami KS, Sinha KK (1992) Aflatoxins their biological effects and ecological significance. In: Bhatnager D, Lillehoj EB, Arora DK (eds) Handbook of applied mycology, vol 5. Marcel Dekker, New York, pp 59–86
Biswas S, Talukder G, Sharma A (1997) Selenium, salts and chromosome damage. Mutat Res 390:201–205
Busley WF, Wogan GN (1979) Food-borne mycotoxins and alimeniary mycotoxicosis. In: Reiman HP, Bryan FL (eds) Food-borne infections and intoxications, 2nd edn. Academic Press, New York, pp 519–610
Cakmak I, Marschner H, Bangert F (1989) Effect of zinc nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other phytohormones in bean (Phaseolus vulgaris L.). J Exp Bot 40:404–412
Chen J, Goetchius MP, Campbell TC, Combs GFJR (1982a) Effects of dietary selenium and vitamin E on hepatic mixed-function oxidase activities and in vivo covalent binding of aflatoxin B1 in rats. J Nutr 112:324–349
Chen J, Goetchius MP, Combs GFJR, Campbell TC (1982b) Effects of dietary selenium and vitamin E on covalent binding of aflatoxin to chick liver cell macromolecules. J Nutr 112:350–355
Chow CK, Gairola GC (1984) Influence of dietary vitamin E and selenium on metabolic activation of chemicals to mutagens. J Agric Food Chem 32:443–447
Combs GFJR (1997) Selenium and cancer. In: Garewal H (ed) Antioxidants and disease prevention. CRC Press, New York, pp 97–113
Creelman RA, Tierney ML, Mullet JE (1992) Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA 89:4938–4941
Dashek WV, Llewellyn GC (1983) Mode of action of the hepatocarcinogens, aflatoxins in plant systems: a review. Mycopatologia 81:83–94
El-Bayoumy K (2001) The protective role of selenium on genetic damage and on cancer. Mutat Res 475:123–139
El-Naghy MA, Fadl-Allah EM, Samhan M (1999) Effects of Aflotoxin G1 on germination, growth and metabolic activities of some crop plants. Cytobiosis 97:87–93
Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of woundinducible proteinase inhibitors. Plant Cell 4:129–134
Ferguson LR, Philpott M (2004) Dietary cancer and prevention using antimutagens. Toxicology 198:147–159
Fujioka S, Sakurai A, Yamaguchi I, Murofiishi N, Takahashi N, Kaihari S, Takimota A, Cleland CF (1986) Flowering and Endogenous levels of plant hormones in Lemna species. Plant Cell Physiol 27:151–160
Gerald F, Combs GFJR, William PG (1998) Chemopreventive agents: selenium. Pharmacol Ther 79:179–192
Gourama H, Bullerman LB (1995) Antimycotic and antiaflatoxigenic effect of lactic acid bacteria a review. J Food Protect 57:1275–1280
Gqaleni N, Smith JE, Lacey J (1996) Co-production of aflatoxins and cyclopiazonic acid in isolates of Aspergillus flavus. Food Addit Contam 13:677–685
Groopman JP, Croy RG, Wogan GN (1981) In vitro reactions of aflatoxin B1 aducted DNA. Proc Natl Acad Sci USA 78:5445–5449
Gundlach H, Muller MJ, Kutchan TM, Zenk MH (1992) Jasmonic acid is a signal transducer in elicitor-induced plant cell structures. Proc Natl Acad Sci USA 89:2389–2393
Hamilton SJ (1999) Hypothesis of historical effects from selenium on endangered fish in the Colorado River Basin. Hum Ecol Risk Assess 5:1153–1180
Hela K, Cardwell K, Setamou FM, Phoehlig HM (2000) The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, west Africa. J Stored Prod Res 36:365–382
Hsieh DPH, Wong ZA, Wong JJ, Michas C, Ruebner BH (1977) Comparative metabolism of aflatoxin. In: Rodricks JV, Hesseltine CW, Mehlman MA (eds) Mycotoxins in human and animal health. Pathotox Publishers, Park Forest South, pp 37–50
Hodson PV, Hilton JW (1983) The nutritional requirements and toxicity to fish of dietary and waterborne selenium. Ecol Bull (Stockholm) 35:335–340
Kuraishi S, Tasaki K, Sakurai N, Sadatoku K (1991) Changes in levels of cytokinins in etiolated squash seedlings after illumination. Plant Cell Physiol 2:585–591
Lemly AD (1985) Toxicology of selenium in a freshwater reservoir: implications for environmental hazard evaluations and safety. Ecotoxicol Environ Saf 10:314–338
Leontopoulos D, Siafaka A, Markaki P (2003) Black olives as substrate for Aspergillus parasiticus growth and aflatoxin B1 production. Food Microbiol 20:119–126
Lillehoj EB, Zuber MS (1988) Distribution of toxin-producing fungi in mature maize kernels from diverse environments. Trop Sci 28:19–24
Lutz C (1994) The functioning of the maize market in Benin: spatial and temporal arbitrage on the market of a staple food crop. PhD Thesis, University of Amsterdam
Machackova L, Krekule J, Seidlova F, Strnad M (1993) Cytokinins in photoperiodic introduction of flowering Chenopodium species. Physiol Plant 87:160–166
McLean M, Watt MP, Berjak P, Dutton MF 1995. Aflatoxine B1-its effects on an in vitro plant system. Food Addit Contam 12(3):435–443
Ong T (1971). Mutogenic activities of aflatoxins B1 and G1 in Neurospora crassa. Mol Gen Genet 111:159–160
Presser TS, Ohlendorf HM (1987) Biogeochemical cycling of selenium in the San Joaquin Valley. Environ Manage 11:805–821
Rayman MP (2000) The importance of selenium to human health (review). Lancet 356:233–241
Reis J (1978) Effects of mycotoxins on higher plants, algae, fungi and bacteria. In: Wyllie D, Morehouse LG (eds) Mycotoxic fungi, mycotoxins, mycotoxicoses: an encylopaedic handbook mycotoxicoses of man and plants: mycotoxin control and regulatory Practices. Marcel Dekker Inc, New York, pp 119–143
Schrauzer GN, White DA, Schneider CJ (1977) Cancer mortality correlation studies-III: statistical associations with dietary selenium intakes. Bioinorg Chem 7:23–31
Sembdener G, Parthier B (1993) The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol 44:569–589
Shi CY, Chua SC, Lee HP, Ong CN (1994) Inhibition of aflatoxin B1-DNA binding and adduct formation by selenium in rats. Cancer Lett 82:203–208
Skorupa JP (1998) Selenium poisoning of fish and wildlife in nature: lessons from twelve real-world examples. In: Frankenberger W, Engberg RA (eds) Environmental chemistry of selenium. Marcel Dekker, New York, pp 315–354
Spallholz JE (1994) On the nature of selenium toxicity and carcinostatic activity. Free Rad Biol Med 17:45–64
Wang Q, Little CHA, Sheng CO, Pharis RP (2002) Effect of exogenous gibberellin A4/7 on tracheid production, longitudinal growth and the levels of indole-3-acetic acid and gibberellins A4, A7 and A9 in terminal shoot of Pinus sylvestris seedlings. Physiol Plant 86:202–208
Wilbur COG (1983) Selenium – A potential environmental poison and a necessary food constituent. Springfield, IL, pp 134
Wogan GN (1992) Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res 52:2114–2118
Zeringue HJ (2002) Effects of methyl jasmonate on phytoalexin production and aflatoxin control in the developing cotton boll. Biochem System Ecol 30:497–503
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ağar, G., Türker, M., Battal, P. et al. Phytohormone levels in germinating seeds of Zea mays L. exposed to selenium and aflatoxines. Ecotoxicology 15, 443–450 (2006). https://doi.org/10.1007/s10646-006-0079-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-006-0079-z