Abstract
This study investigated the physiological and biochemical impacts of organophosphate flame retardants (OPFRs) on wheat (Triticum aestivum L.) germination and growth performance in the presence and absence of copper. The study evaluated seed germination, growth, OPFRs concentrations, chlorophyll fluorescence index (Fv/Fm and Fv/F0), and antioxidant enzyme activity. It also calculated the root accumulation of OPFRs and their root-stem translocation. At the germination stage, at a concentration of 20 μg·L−1 OPFR exposure, wheat germination vigor, root, and shoot lengths were significantly decreased compared to the control. However, the addition of a high concentration of copper (60 mg·L−1) decreased by 80%, 82%, and 87% in the seed germination vitality index and root and shoot elongation, respectively, compared to 20 μg·L−1 of OPFR treatment. At the seedling stage, a concentration of 50 μg·L−1 of OPFRs significantly decreased by 42% and 5.4% in wheat growth weight and the photochemical efficiency of photosystem II (Fv/Fm) compared to the control. However, the addition of a low concentration of copper (15 mg·L−1) slightly enhanced the growth weight compared to the other two co-exposure treatments, but the results were not significant (p > 0.05). After 7 days of exposure, the activity of superoxide dismutase (SOD) and malondialdehyde (MDA) (indicates lipid peroxidation) content in wheat roots significantly increased compared to the control and was higher than in leaves. MDA contents in wheat roots and shoots were decreased by 18% and 6.5% when OPFRs were combined with low Cu treatment compared with single OPFRs treatment, but SOD activity was slightly improved. These results suggest that the co-exposure of copper and OPFRs enhances reactive oxygen species (ROS) production and oxidative stress tolerance. Seven OPFRs were detected in wheat roots and stems, with root concentration factors (RCFs) and translocation factors (TFs) ranging from 67 to 337 and 0.05 to 0.33, respectively, for the seven OPFRs in a single OPFR treatment. The addition of copper significantly increased OPFR accumulation in the root and aerial parts. In general, the addition of a low concentration of copper promoted wheat seedling elongation and biomass and did not significantly inhibit the germination process. OPFRs could mitigate the toxicity of low-concentration copper on wheat but had a weak detoxification effect on high-concentration copper. These results indicated that the combined toxicity of OPFRs and Cu had antagonistic effects on the early development and growth of wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus flame retardants (OPFRs) are commonly used in daily and commercial products as substitutes for traditional flame retardants (FRs) (Du et al. 2019). The global consumption of OPFRs has increased from 100,000 tons in 1992 to 680,000 tons in 2015 (Hou et al., 2016). In China, annual OPFR emissions rose from approximately 670 tons per year in 2014 to 1000 tons per year in 2018 (He et al. 2021). Due to the lack of chemical binding to materials, OPFRs are prone to release into the environment through volatilization and abrasion (Du et al. 2019). This has led to increased concern about their environmental behavior, fate, and biological toxicity. Previous studies have detected the presence of OPFRs in various environmental media, including house dust (He et al. 2015), atmosphere (Wang et al. 2020), water (Picó et al. 2021), soil (Ji et al. 2019), and sediment (Hu et al. 2017). OPFRs can be transferred from contaminated soil or water to plants and enter organisms and human bodies through food chains (Wan et al. 2016), such as aquatic organisms (McGoldrick et al. 2014; Garcia-Garin et al. 2020), natural vegetation (Picó et al. 2021), human breast milk (Chen et al. 2021), and urine (Wang et al. 2021a). Toxicity studies have indicated that OPFRs can cause negative effects on development, neurotoxicity, and oxidative stress in animals, including humans (Yan et al. 2021).
Several studies have explored the impact of growing conditions on the accumulation of OPFRs in plants. In the natural environment, the concentration of OPFRs in wheat stems collected near a plastic waste disposal site was slightly higher than that in roots (range: 11–51 ng·g−1 DW) (Wan et al. 2016), which was in contrast to the distribution of OPFRs in wheat grown in hydroponic conditions. As cereal crops, such as wheat, maize (Zea mays L.), and other grains, form a staple part of the Chinese diet, they are considered important indicators of environmental pollution and are thought to be sensitive to it. The early stages of seed germination and growth are crucial and vulnerable periods in the plant’s lifecycle (He et al. 2021). Zhang et al. suggested that a low concentration of OPFRs could stimulate the growth of rice (Oryza sativa L.) seedlings within a specific range of concentrations (100.0–500.0 μg⋅mL−1) (Zhang et al. 2022a). Hu et al. (2021) found that OPFRs at 500 ng⋅g−1 had small or negligible phytotoxicity to zucchini (Cucurbita pepo L.), soybean (Glycine max (Linn.) Merr.), lettuce (Lactuca sativa var. ramosa Hort.), and tomato (Solanum lycopersicum L.) but slightly inhibited the growth of maize biomass. Until recently, there was limited knowledge about the toxic effects and dose-response relationship of OPFRs on the early germination of plants.
Many pollutants are co-existent in the real environment, including in the agricultural soil. The simultaneous presence of heavy metals and organic pollutants in soils may lead to synergistic or antagonistic effects on plants. For instance, Wang et al. (2015b) found that polybrominated diphenyl ethers (PBDEs) could decrease maize biomass when Cu was also present, while the presence of PBDEs alone could not. Because copper can increase the permeability of root cell membranes and disrupt root defense mechanisms. At present, there are very few studies on the effects of the simultaneous presence of OPFRs and heavy metals on plant physiology. Only Hu et al. (2021) found that Cu significantly increased accumulation of OPFRs in wheat plants; this could be attributed to the strong interactions between Cu2+ and OPFRs via cation-π complexation (Deng et al. 2018). In addition, most studies focused on the seedling growth of plants in response to the co-exposure of OPFRs and heavy metals, while the present paper mainly focuses on the early germination stage, which is a sensitive and vital key for plant life cycle.
Wheat is a major grain crop in northern and central China. High concentrations of metals in wheat have been shown to inhibit enzymes involved in photosynthetic reactions (Lin and Jin, 2018), leading to severe oxidative damage (Madejón et al. 2009). Wang et al. (2015b) found that the presence of copper and OPFRs can cause root cell membrane damage in maize seedling but also stimulate their anti-oxidative stress response. Hu et al. (2021) observed that the addition of copper significantly inhibited the growth of wheat seedlings and increased their root uptake of OPFRs but had no noticeable effect on root-stem translocation. The chlorophyll fluorescence parameter (Fv/Fm) is a measure of the maximum quantum efficiency of PSII photochemistry in dark-adapted leaves and is related to leaf chlorophyll concentration (Pilon et al. 2006; Ambrosini et al. 2018). High concentrations of the triphenyl phosphate (TPhP) group have been shown to significantly decrease the Fv/Fm value of marine diatoms. To date, most studies on the effect of pollution on plants have focused on specific plant types, high concentrations of exposure, and single pollutants. It is therefore crucial to examine the physiological and biochemical effects of single and co-pollution with varying concentrations of copper and OPFRs on early germination and growth of wheat.
Several recent studies have investigated the root uptake, accumulation, and translocation of OPFRs in plants grown in hydroponics conditions. These studies have been related to various factors such as specific plant species, plant parts, lipids, proteins, and genes (Wan et al. 2016; Liu et al. 2019; Bonato et al. 2022). Additionally, the accumulation of OPFRs in plants is influenced by their hydrophobicity (log Kow) and functional groups. In this study, three alkyl-OPFRs (TPrP, TiBP, and TnBP), two aromatic-OPFRs (TPP and TCP), and two chlorinated-OPFRs (TCiPP and TDCPP) were selected for examination. The study aimed to investigate the phytotoxic effects of different concentrations of OPFRs in the absence and presence of copper on wheat seed germination and growth, including germination index, length index, biomass, tissue distribution, and antioxidant enzyme levels. Furthermore, the relationships between wheat root concentration factors (RCFs) and translocation factors (TFs) for all OPFRs and the octanol-water partition coefficient (log Kow) of OPFRs were analyzed under the co-existence of Cu in wheat.
Materials and methods
Chemicals and reagents
Wheat seeds (Triticum aestivum L.) were purchased from the Jiangsu Academy of Agricultural Science (Nanjing, China). Tripropyl phosphate (TPrP), tri-iso-butyl phosphate (TiBP), tributyl phosphate (TnBP), tris (1-chloro-2-propyl) phosphate (TCiPP), tris (1,3-dichloro-2-propyl) phosphate (TDCPP), triphenyl phosphate (TPP), and tricresyl phosphate (TCP) were obtained from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The properties of seven OPFRs are listed in Table SI (Supporting Information (SI)). The HPLC-grade solvents including hexane, ethyl acetate, dichloromethane (DCM), methanol, acetonitrile, and acetone were purchased from Sigma-Aldrich (St. Louis, MO, USA). The purity of all reagents was higher than 95%. CuSO4·5H2O was obtained from Jinke Chemical Corporation (Shanghai, China) with a purity of 98%. Enzyme kits such as superoxide dismutase (SOD) and malondialdehyde (MDA) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Experiment design
Wheat (Triticum aestivum L.) seed germination
We select full and uniform-size seeds, according to the reported methods (Yanyu et al. 2022), Seeds were sterilized for 30 min in 3% (Volume ratio) H2O2 aqueous solution, rinsed with deionized water three times, and soaked for 6 h in aqueous solution. Then, wheat seeds were planted in a petri dish (diameter of 90 mm) with moist filter paper soaked in 10 mL of exposure solution.. After, wheat seeds were incubated at 25 °C for 24 h avoiding lights. Seven OPFR standard materials were dissolved with methanol and then diluted in double-distilled water with the concentration of 10, 20, and 50 μg·L−1 of OPFRs, respectively as a single OPFR exposure solution. The concentration of methanol in aqueous solution was far less than 0.1% (v/v). The single copper treatment group was set at three concentrations of 15, 30, and 60 mg⋅L−1 of copper. Three combination OPFRs and copper groups was set as three levels of 15, 30, and 60 mg⋅L−1 of Cu2+ mixing into the initial concentration of OPFRs (20 μg⋅L−1). The solution pH value was adjusted to about 6.5. We added the distilled water every 2 days to eliminate changes in concentration caused by wheat growth and water evaporation. The control group was set as the same volume of distilled water containing 0.1% methanol. All treatments were carried out in three replicates, and each replicate contained 10 wheat seeds.
This study observed the seed germination process and measured the number of germinated seeds, the root and shoot lengths, and the biomass at different times post-exposure (1, 3, 5, 7, and 10 days). According to the literatures (Ranal et al. 2009; Yanyu et al. 2022), germination percentage (or germinability) is the germination percentage at the end of germination. Final seed germination was recorded at 5 days. Germination vigor (potential or energy) is the proportion of germination number of tested seeds within 3 days. Germination vigor and germination percentage were calculated within 3 and 5 days, respectively. The inhibition rate of the root and shoot elongation at different time points (3, 5, 7, and 10 days) was calculated.
Wheat seedling growth
Selective seeds were germinated and grown up 5 leaves with a culture solution of 1/2 Hoagland nutrient and then incubated for 5 days in an artificial climate box. The single OPFR exposure groups and combination OPFRs and Cu groups were designed as the same as the seed germination, but the control group was set in the 150 mL of culture solution.
The seedlings were grown in an incubator at 25 °C with a relative humidity of 65%, a light cycle of 14h/10 h day/night, and light intensities of 250 μmol.m−2s−1 for 7 days. The seedlings randomly changed in their positions and compensated for evaporation loss with double distilled water every day. The seedlings were harvested after 7-day exposures. The root lengths and shoot lengths and biomass were measured. The potential maximum photosynthetic capacity (Fv/Fm) and potential activity (Fv/Fo) of PSII in leaves were detected by a chlorophyll fluorometer (Yaxin-1161G, Beijing, China). Before the assay, wheat leaves were protected from the light with thick tin foil for more than 20 min and measured with the mode of OJIP fluorescence kinetics curve. And then seedlings were divided into two groups. One group was used to detect OPFR and Cu concentration in roots and shoots, and the other group was used to extract the crude enzymes and measure the antioxidant enzyme activities with an assay kit (Nanjing jiancheng Bioengineering Institute, China).
Chemical analysis
The detection method of OPFRs in plant tissues had slightly modified according to the previous reports (Santín et al. 2016). In particular, 0.2 g of freeze-dried tissue samples was grinded, sieved, and then weighed and extracted by ultrasonic at 35 °C for 20 min with a 10-mL mixture of ethyl acetate and acetone (volume ratio 3:2). After, the extract were combined, concentrated to near dryness under a nitrogen stream, and then redissolved in 1 mL of hexane. The obtained extracts were introduced into a solid phase extraction (SPE) column (florisil, 0.5g, 5mL) that was preconditioned with 10 mL of ethyl acetate and 10 mL of n-hexane in order. Then all OPFRs were eluted with 5 mL of ethyl acetate. The eluant was collected, concentrated, and then re-dissolved in 200 μL of acetonitrile. The obtained acetonitrile solution was filtered using a nylon filter (0.22 μm) for analysis.
A total of 0.2 g of powder samples was digested with 1 mL of hydrogen peroxide and 5 mL of nitric acid in a 25-mL Teflon crucible at 120 °C for 2 h, then removed the acid, and diluted in 10 mL of re-distilled water, filtered using a PES filter (0.22 μm) for analysis. The instrumental conditions are shown in the supporting information (SI).
Formula calculations
In the early seed germination stage, the germination performance including germination vigor (potential), germination percentage, germination index, and vitality index were described. Their formulas were as follows.
Root concentration factors (RCFs) and translocation factors (TFs) express the root uptake and root-stem translocation ability of OPFRs in wheat seedling (Liu et al. 2019). Their formulas were as follows.
Here, Croot, eq, and Cshoot, eq are each OPFR concentration in the roots and shoots, respectively, after 7-day exposure (μg·g−1), and Csolution is the initial concentration of OPFRs in exposure solution (μg·L−1).
Statistical analysis
One-way ANOVA analysis and least significant difference (LSD) were performed using SPSS18.0 software for substantial differences between mean, and data were expressed as mean ± standard deviation. The trend graphs in different treatment groups were plotted by Origin 2019B, and linear regression was fitted to the relationship between RCFs and TFs and the hydrophobicities of OPFRs.
Results and discussion
Wheat germination
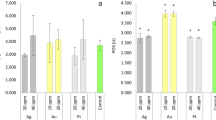
In the early seed germination stage, the performance of germination vigor (GV), germination percentage (GP), germination index (GI), and vitality index (VI) in different treatment groups are presented in Fig. 1A, B, C, and D. At the concentrations of 10, 20, and 50 μg·L−1 of OPFRs, the Gv was reduced by 8.54%, 14.64%, and 22.01%, respectively. The GV, GP, GI, and VI were significantly decreased with increasing concentrations of OPFRs compared to the control group (p < 0.05). Meanwhile, in the presence of 15, 30, and 60 mg·L−1 of Cu, the GV decreased by 18.44%, 26.90%, and 42.06%, respectively. After 24 h of single OPFR exposure, 3 to 5 seminal roots were observed along with plasmolyzed seeds, while in the case of Cu, seed germination was delayed and only plasmolyzed seeds (or short radicle) were observed (Fig. 1H). Our results showed that copper had a more severe suppression effect on wheat germination than OPFRs, especially with a 42% decrease in the VI value at a high concentration of copper (60 mg·L−1) (Fig. 1D). Bae et al. (2016) also found that 50 and 100 mg⋅kg−1 Cu reduced on legumes grass germination by 40% and 70%, respectively. Interestingly, in the presence of 30 mg⋅L−1 Cu, the GV, GP, and GI significantly decreased but did not increase significantly (p > 0.05) in the low concentration of Cu (15 mg⋅L−1), compared to 20 μg⋅L−1 of OPFRs. Some studies suggested that the presence of copper reduced the toxicity of sulfadiazine to wheat seedlings to some extent, as seen in an increase in germination percentage (Xu et al. 2017).
Response of wheat to OPFRs and Cu2+ ions during germination stage, A, B, C, D, E, F, and, G Germination vigor, germination percentage, germination index, vitality index, root length, shoot length, fresh weight, respectively. H Wheat seed germination after 24 h of exposure. Different letters indicate significant differences at p < 0.05. Error bars indicate standard deviation (n=3)
The root and shoot growth of wheat was significantly impacted by the increasing concentration of OPFRs and the addition of Cu, as shown in Fig. 1E and F (p < 0.05). After a 5-day exposure, the inhibition rates on root elongation reached 14.6% at a high concentration of OPFRs (50 μg⋅L−1) and 97.4% at a high concentration of Cu (60 mg⋅L−1) (Table S2). The addition of a high concentration of copper (60 mg·L−1) decreased by 82% and 87% in wheat root and shoot elongation compared to 20 μg·L−1 of OPFRs treatment. The presence of OPFRs reduced the capacity of the plant to uptake water and nutrients, causing a delay in root and stem elongation, and biomass growth (Luo et al. 2021). The fresh weight of wheat decreased slightly under single OPFR stress but increased significantly with the addition of a low concentration of copper compared to 20 μg⋅L−1 of OPFRs (p < 0.05) and decreased at the addition of a high concentration of Cu (Fig. 1G). The root biomass of the maize seedling decreased more than young and mature leaves in heavy metals (Zn, Ni, Cd, and Cu) treatment (Abdelgawad et al. 2019). The EC50 values of OPFRs and Cu for the wheat fresh weight were calculated, and the results showed that the EC50 value of Cu in the presence of OPFRs was slightly higher than that of single Cu exposure (Table S4). This indicated that a moderate concentration of OPFRs can alleviate the inhibitory effect of Cu on wheat growth. However, adding 20 μg⋅L−1 OPFRs did not moderate the inhibitory effect of a high concentration of Cu (60 mg⋅L−1) on wheat germination during the early seed germination stage.
Growth response
During the seedling growth stage, as shown in Fig. 2A, B, C, and D, OPFRs continued to inhibit the growth of wheat seedlings compared to the control, and the root and shoot lengths, as well as fresh weights, were negatively impacted by increasing OPFR concentration (p < 0.05). For instance, a concentration of 50 μg·L−1 of OPFRs significantly decreased by 42% in wheat growth weight compared to the control. This outcome was different from previous studies that reported low concentrations of OPFRs could stimulate the growth of rice seedlings within a specific concentration range (Zhang et al. 2022a). The reason for this discrepancy may be due to the specific plant species, and the concentration of OPFRs not reaching the threshold needed to stimulate plant hormones at lower levels (Wani et al. 2012).
The results of the seedling growth stage showed a different response compared to the germination stage. The addition of a low concentration of Cu had a positive impact on the physiological indices of the wheat seedlings, particularly in root and stem growth, when compared to the 20 μg·L−1 of OPFRs. However, a medium concentration of Cu had a slight negative impact on root and stem length and fresh weight, but not significantly. Additionally, the high concentration of copper was observed to result in a thicker wheat root and the increment of root fresh weight, as shown in Fig. 2H. These findings indicate that at a certain level, Cu could alleviate the inhibitory effect of OPFRs on the growth of wheat seedlings. Research has shown that copper can reduce the toxicity of sulfadiazine (SDZ) in wheat seedlings (Xu et al. 2017) and that OPFRs can significantly reduce the growth of four plants (zucchini, soybean, lettuce, and tomato) under the addition of Cu but significantly increased the biomass of maize seedlings (Hu et al. 2021). Our results are likely more similar to maize as both wheat and maize seedlings are grasses.
The effects of OPFRs on Fv/Fm and Fv/Fo in wheat leaves in the absence and presence of Cu are illustrated in Fig. 2E and F. The Fv/Fm and Fv/Fo values of wheat leaves decreased with the increase in the concentration of OPFRs, compared to the control group. For instance, a concentration of 50 μg·L−1 of OPFRs decreased by 5.4% in Fv/Fm. The Fv/Fm values of bacteria (Microcystis aeruginosa) increased significantly when exposed to 5–10 mg⋅L−1 of TDCPP and 50 mg⋅L−1 of TCEP (Zhang et al. 2022b). However, the Fv/Fm value of microalgae (chaetoceros meulleri) was severely reduced by 3.2 mg⋅L−1 of triphenyl phosphate (TPhP) (Wang et al. 2021b). The species of the organism and the initial concentrations of OPFRs can influence the photosynthesis of plants and their stress resistance abilities. The trend of change in Fv/Fo was more significant than that of Fv/Fm, indicating that Fv/Fo values were more intuitive indicators of the impacts of OPFRs on the photosynthetic capacity of plants. Interestingly, the Fv/Fm and Fv/Fo values of wheat leaves decreased when 30 mg⋅L−1 of Cu was added but further increased when the low concentration of 15 mg⋅L−1 of Cu was present, which was consistent with the seedling growth weight.
The level of malondialdehyde (MDA) in plant cells reflects the level of membrane peroxidation. High MDA levels indicate severe membrane damage. After a 7-day exposure to OPFRs (at concentrations of 10, 20, 50 μg⋅L−1), the MDA content in wheat roots increased compared to the control, with a higher increase in the roots than in the leaves (as shown in Fig. 3A). Liu et al. (2020) also found that the levels of lipid peroxidation (as indicated by MDA content) were significantly increased in response to a treatment of 4 mg⋅L−1 TDCPP.
Superoxide dismutase (SOD) is the first and most important line of defense for plant against reactive oxygen species (ROS). The wheat root exposed to high levels of OPFRs showed a significant increase in SOD activity compared to the control (as shown in Fig. 3B). Furthermore, the addition of copper increased the SOD activity and MDA content in the wheat roots and shoots compared to single OPFRs exposure, but no significant difference. Only co-exposure of OPFRs and high level of copper (60 mg⋅L−1) showed a significant increase in the SOD activity in wheat roots and shoots compared to single OPFR exposure and the other two combined treatments (as shown in Fig. 3B). These results indicate that co-exposure of Cu and OPFRs increase SOD activity in the wheat plant, enhance superoxide anion free radical (·O2−) production and oxidative stress tolerance (Wang et al. 2015a; Zhang et al. 2021b). Meanwhile, the SOD activity in wheat first increases, after slightly decreasing with the increase of Cu concentration compared to other Cu treatments, suggesting that high levels of Cu can damage of plant tolerance mechanism.
Uptake and translocation of OPFRs in wheat seedling
Previous research has shown that OPFRs are absorbed by the root epidermis and then introduced into the root system via exosomes (Gong et al. 2020). Our results indicate that all OPFRs were detected in both wheat roots and stems, with higher concentrations in roots (ranging from 0.85 to 12.71 μg·g−1) than in stems (ranging from 0.28 to 1.12 μg·g-−1) (Table 1). The root concentration factors (RCFs) for OPFRs appeared to decrease with increasing initial concentrations (10, 20, and 50 μg·L−1) in the hydroponic solution, with the exception of TPrP. The RCFs for OPFRs ranged from 67 to 337 (Fig. 4), with the average RCFs for seven OPFRs sorted from high to low as TCIPP > TPrP > TiBP > TDCPP > TnBP > TPP > TCP, which is consistent with previous studies (Wan et al. 2017; Liu et al. 2019; Hu et al. 2021; Bonato et al. 2022). The addition of Cu at concentrations of 15, 30, and 60 mg·L−1 resulted in a 14%, 9%, and 23% increase in the concentration of TCIPP in the roots and a corresponding increase in the RCFs. Similar trends were observed for other OPFRs, with the exception of TnBP and TiBP (Table 1). The presence of Cu significantly enhanced the absorption and accumulation of OPFRs in wheat roots. This is attributed to the impact of metals on the lipid synthesis and hydrophobicity of roots through neutralizing the negative charge of root cell wall surface (Deng et al. 2018) as well as the coordination of π-system organic compounds via cation-π interaction (Chen et al. 2020), which concurred with the findings of Hu et al. (2021).
Seven OPFRs were found in wheat stems, and the average translocation factors (TFs) were between 0.05 and 0.33 for all OPFRs in a single OPFR treatment. The lowest TF values were found for TPP and TCP, ranging from 0.05 to 0.06, while TiBP, TnBP, and TDCPP had TF values ranging from 0.11 to 0.15, and TPrP and TCiPP had TF values between 0.25 and 0.33. Compared to the RCF value, adding Cu significantly increased the TF values of all seven OPFRs compared to the single OPFRs treatment. For instance, adding Cu (at concentrations of 15, 30, and 60 mg·L−1) increased the concentrations of TCiPP and TPP in the leaves by 1.71, 1.34, 1.87 times, and 2.25, 1.94, and 2.5 times, respectively. Zhang et al. (2021a) found that adding high concentrations of copper (100–400 μmol·L−1) enhanced the accumulation of perfluoroalkanoic acids (PFAAs) in wheat roots and their translocation to the aboveground part, which may be due to the damage of wheat root cell membranes and the increase of electrolyte leakage.
Effects of the hydrophobicities of OPFRs on RCFs and TFs
Additionally, the RCF values of OPFRs were observed to have a strong significant relationship with their hydrophobicity (p < 0.05), as shown in Fig. 5. The correlation between the RCF values and log Kow values of OPFRs was strongest in the presence of a high concentration of Cu (60 mg⋅L−1). The TF value of the seven OPFRs was ranked as TPP > TCP > TDCPP > TCIPP > TiBP > TPrP, which corresponded well with their hydrophobic properties. Specifically, OPFRs with electron ring substituents (TPP and TCP) showed stronger translocation from roots to stems, which is similar to the findings of Hu et al. (2021). Our study confirmed that the ability of non-ionized organic pollutants to enter the vascular bundle and transfer from roots to stems is dependent on their hydrophobicity, within a range of log Kow values from 1 to 5.
Conclusions
During the germination stage, wheat seeds exposed to OPFRs had a significant negative impact on the wheat germination percentage, germination index, root vigor index, and root and shoot lengths. However, the inhibition of Cu on wheat germination and growth was mitigated in the presence of OPFRs (as shown in Fig. 6). The combined toxicity of OPFRs and Cu showed antagonistic effects on the early germination and seedling growth of wheat. Different phenotypic indicators (lengths index) were even more sensitive to the combined pollutants in the early life of wheat. During the wheat seedling stage, the length, weight, and the photochemical efficiency of the leaf were inhibited in all treatment groups compared to the control, leading to elevated levels of SOD and MDA (as shown in Fig. 6). The addition of Cu enhanced the root uptake and radial translocation of OPFRs, increasing potential ecological risks.
Availability of data and materials
All data generated or analyzed in this study are included in this manuscript and in the published articles.
References
Abdelgawad H, Zinta G, Badreldin AH, Selim S, Abuelsoud W (2019) Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ Pollut 258:113705. https://doi.org/10.1016/j.envpol.2019.113705
Ambrosini VG, Rosa DJ, Bastos de Melo GW, Zalamena J, Cella C, Simão DG, Souza da Silva L, Pessoa dos Santos H, Toselli M, Tiecher TL, Brunetto G (2018) High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol Biochem 128:89–98. https://doi.org/10.1016/j.plaphy.2018.05.011
Bae J, Benoit DL, Watson AK (2016) Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ Pollut 213:112–118
Bao Y, Pan C, Li D, Guo A, Dai F (2022) Stress response to oxytetracycline and microplastic-polyethylene in wheat (Triticum aestivum L.) during seed germination and seedling growth stages. Sci Total Environ 806. https://doi.org/10.1016/J.SCITOTENV.2021.150553
Bonato T, Beggio G, Pivato A, Piazza R (2022) Maize plant (Zea mays) uptake of organophosphorus and novel brominated flame retardants from hydroponic cultures. Chemosphere 287. https://doi.org/10.1016/j.chemosphere.2021.132456
Chen J, Xia X, Chu SQ, Wang H, Gan JJ (2020) Cation-π interactions with coexisting heavy metals enhanced the uptake and accumulation of polycyclic aromatic hydrocarbons in spinach. Environ Sci Technol 54(12):7261–7270. https://doi.org/10.1021/acs.est.0c00363
Chen X, Zhao X, Shi Z (2021) Organophosphorus flame retardants in breast milk from Beijing, China: occurrence, nursing infant’s exposure and risk assessment. Sci Total Environ 771. https://doi.org/10.1016/j.scitotenv.2021.145404
Deng S, Ke T, Wu Y, Zhang C, Hu Z, Yin H, Guo L, Chen L, Zhang D (2018) Heavy Metal Exposure alters the uptake behavior of 16 priority polycyclic aromatic hydrocarbons (PAHs) by pak choi (Brassica chinensis L.). Environ Sci Technol 52:13457–13468. https://doi.org/10.1021/acs.est.8b01405
Du J, Li H, Xu S, Zhou Q, Jin M, Tang J (2019) A review of organophosphorus flame retardants (OPFRs): occurrence, bioaccumulation, toxicity, and organism exposure. Environ Sci Pollut Res 26:22126–22136. https://doi.org/10.1007/s11356-019-05669-y
Garcia-Garin O, Sala B, Aguilar A, Vighi M, Víkingsson GA, Chosson V, Eljarrat E, Borrell A (2020) Organophosphate contaminants in North Atlantic fin whales. Sci Total Environ 721. https://doi.org/10.1016/j.scitotenv.2020.137768
Gong X, Wang Y, Pu J, Zhang J, Sun H, Wang L (2020) The environment behavior of organophosphate esters (OPEs) and di-esters in wheat (Triticum aestivum L.): uptake mechanism, in vivo hydrolysis and subcellular distribution. Environ Int 135. https://doi.org/10.1016/j.envint.2019.105405
He C-T, Zheng J, Qiao L, Chen S-J, Yang J-Z, Yuan J-G, Yang Z-Y, Mai B-X (2015) Occurrence of organophosphorus flame retardants in indoor dust in multiple microenvironments of southern China and implications for human exposure. Chemosphere 133:47–52. https://doi.org/10.1016/j.chemosphere.2015.03.043
He J, Wang Z, Zhao L, Ma H, Huang J, Li H, Mao X, Huang T, Gao H, Ma J (2021) Gridded emission inventory of organophosphorus flame retardants in China and inventory validation. Environ Pollut 290. https://doi.org/10.1016/j.envpol.2021.118071
Hou R, Xu Y, Wang Z (2016) Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 153:78–90. https://doi.org/10.1016/j.chemosphere.2016.03.003
Hu B, Jiang L, Zheng Q, Luo C, Zhang D, Wang S, Xie Y, Zhang G (2021) Uptake and translocation of organophosphate esters by plants: impacts of chemical structure, plant cultivar and copper. Environ Int 155. https://doi.org/10.1016/j.envint.2021.106591
Hu Y-X, Sun Y-X, Li X, Xu W-H, Zhang Y, Luo X-J, Dai S-H, Xu X-R, Mai B-X (2017) Organophosphorus flame retardants in mangrove sediments from the Pearl River Estuary, South China. Chemosphere 181:433–439. https://doi.org/10.1016/j.chemosphere.2017.04.117
Ji Y, Wang Y, Yao Y, Ren C, Lan Z, Fang X, Zhang K, Sun W, Alder AC, Sun H (2019) Occurrence of organophosphate flame retardants in farmland soils from Northern China: primary source analysis and risk assessment. Environ Pollut 247:832–838. https://doi.org/10.1016/j.envpol.2019.01.036
Lin MZ, Jin MF (2018) Soil Cu contamination destroys the photosynthetic systems and hampers the growth of green vegetables. Photosynthetica 56:1336–1345. https://doi.org/10.1007/s11099-018-0831-7
Liu Q, Tang X, Jian X, Yang Y, Ma W, Wang Y, Zhang X (2020) Toxic effect and mechanism of tris (1,3-dichloro-2-propyl)phosphate (TDCPP) on the marine alga Phaeodactylum tricornutum. Chemosphere 252. https://doi.org/10.1016/j.chemosphere.2020.126467
Liu Q, Wang X, Yang R, Yang L, Sun B, Zhu L (2019) Uptake kinetics, accumulation, and long-distance transport of organophosphate esters in plants: impacts of chemical and plant properties. Environ Sci Technol 53:4940–4947. https://doi.org/10.1021/acs.est.8b07189
Luo Q, Li Y, Wu Z, Wang X, Wang C, Shan Y, Sun L (2021) Phytotoxicity of tris-(1-chloro-2-propyl) phosphate in soil and its uptake and accumulation by pakchoi (Brassica chinensis L. cv. SuZhou). Chemosphere 277. https://doi.org/10.1016/j.chemosphere.2021.130347
Madejón P, Ramírez-Benítez JE, Corrales I, Barceló J, Poschenrieder C (2009) Copper-induced oxidative damage and enhanced antioxidant defenses in the root apex of maize cultivars differing in Cu tolerance. Environ Exp Bot 67:415–420. https://doi.org/10.1016/j.envexpbot.2009.08.006
McGoldrick DJ, Letcher RJ, Barresi E, Keir MJ, Small J, Clark MG, Sverko E, Backus SM (2014) Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ Pollut 193:254–261. https://doi.org/10.1016/j.envpol.2014.06.024
Picó Y, Campo J, Alfarhan AH, El-Sheikh MA, Barceló D (2021) A reconnaissance study of pharmaceuticals, pesticides, perfluoroalkyl substances and organophosphorus flame retardants in the aquatic environment, wild plants and vegetables of two Saudi Arabia urban areas: environmental and human health risk assessment. Sci Total Environ 776. https://doi.org/10.1016/j.scitotenv.2021.145843
Pilon M, Abdel-Ghany SE, Cohu CM, Gogolin KA, Ye H (2006) Copper cofactor delivery in plant cells. Curr Opin Plant Biol 9:256–263. https://doi.org/10.1016/j.pbi.2006.03.007
Ranal MA, Santana DG, Ferreira WR, Mendes-Rodrigues C (2009) Calculating germination measurements and organizing spreadsheets. Brazilian. Journal of Botany 32(4). https://doi.org/10.1590/S0100-84042009000400022
Santín G, Eljarrat E, Barceló D (2016) Simultaneous determination of 16 organophosphorus flame retardants and plasticizers in fish by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1441:34–43. https://doi.org/10.1016/j.chroma.2016.02.058
Wan W, Huang H, Lv J, Han R, Zhang S (2017) Uptake, translocation, and biotransformation of organophosphorus esters in wheat (Triticum aestivum L.). Environ Sci Technol 51:13649–13658. https://doi.org/10.1021/acs.est.7b01758
Wan W, Zhang S, Huang H, Wu T (2016) Occurrence and distribution of organophosphorus esters in soils and wheat plants in a plastic waste treatment area in China. Environ Pollut 214:349–353. https://doi.org/10.1016/j.envpol.2016.04.038
Wang H, Tang X, Sha J, Chen H, Sun T, Wang Y (2015a) The reproductive toxicity on the rotifer Brachionus plicatilis induced by BDE-47 and studies on the effective mechanism based on antioxidant defense system changes. Chemosphere 135:129–137. https://doi.org/10.1016/j.chemosphere.2015.03.090
Wang L-M, Luo D, Li X, Hu L-Q, Chen J-X, Tu Z-Z, Sun B, Chen H-G, Liu L, Yu M, Li Y-P, Pan A, Messerlian C, Mei S-R, Wang Y-X (2021a) Temporal variability of organophosphate flame retardant metabolites in spot, first morning, and 24-h urine samples among healthy adults. Environ Res 196. https://doi.org/10.1016/j.envres.2020.110373
Wang S-c, Gao Z-Y, Liu F-F, Chen S-Q, Liu G-Z (2021b) Effects of polystyrene and triphenyl phosphate on growth, photosynthesis and oxidative stress of Chaetoceros meülleri. Sci Total Environ 797. https://doi.org/10.1016/j.scitotenv.2021.149180
Wang S, Wang Y, Luo C, Jiang L, Song M, Zhang D, Wang Y, Zhang G (2015b) Could uptake and acropetal translocation of PBDEs by corn be enhanced following cu exposure? Evidence from a root damage experiment. Environ Sci Technol 50:856–863. https://doi.org/10.1021/acs.est.5b04030
Wang Y, Li Z, Tan F, Xu Y, Zhao H, Chen J (2020) Occurrence and air-soil exchange of organophosphate flame retardants in the air and soil of Dalian, China. Environ Pollut 265. https://doi.org/10.1016/j.envpol.2020.114850
Wani PA, Khan MS, Zaidi A (2012) Toxic effects of heavy metals on germination and physiological processes of plants. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0730-0_3
Xu Y, Yu W, Ma Q, Zhou H, Jiang C (2017) Toxicity of sulfadiazine and copper and their interaction to wheat ( Triticum aestivum L.) seedlings. Ecotoxicol Environ Saf 142:250–256. https://doi.org/10.1016/j.ecoenv.2017.04.007
Yan Z, Jin X, Liu D, Hong Y, Liao W, Feng C, Bai Y (2021) The potential connections of adverse outcome pathways with the hazard identifications of typical organophosphate esters based on toxicity mechanisms. Chemosphere 266. https://doi.org/10.1016/j.chemosphere.2020.128989
Zhang L, Wang Q, Chen H, Yao Y, Sun H (2021a) Uptake and translocation of perfluoroalkyl acids with different carbon chain lengths (C2–C8) in wheat (Triticum acstivnm L.) under the effect of copper exposure. Environ Pollut 274. https://doi.org/10.1016/j.envpol.2021.116550
Zhang Q, Yao Y, Wang Y, Zhang Q, Cheng Z, Li Y, Yang X, Wang L, Sun H (2021b) Plant accumulation and transformation of brominated and organophosphate flame retardants: a review. Environ Pollut 288. https://doi.org/10.1016/j.envpol.2021.117742
Zhang W, Wang H, Bai Q, Li X (2022a) Lipocalin-mediated organophosphate esters (OPEs) active uptake and accumulation in rice (Oryza sativa L.). Environ Exp Bot 200. https://doi.org/10.1016/j.envexpbot.2022.104910
Zhang X, Ai S, Wei J, Yang X, Huang Y, Hu J, Wang Q, Wang H (2022b) Biphasic effects of typical chlorinated organophosphorus flame retardants on Microcystis aeruginosa. Ecotoxicol Environ Saf 241. https://doi.org/10.1016/j.ecoenv.2022.113813
Acknowledgements
We thank the reviewers and the editor; their comments greatly improved the initial draft of this manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos .22076134, 21876125, and 41807142) and the Graduate Research & Practice Innovation Program of Jiangsu Province (SJCX21_1398).
Author information
Authors and Affiliations
Contributions
DXD proposed the idea of research and experimental design and wrote the original draft. SJX and YYS contributed to figures and tables. JXW, GYS, XDW, and HLW provided a critical review and substantially revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Our results presented in this study have not been published before, nor have they been published elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Tables S1–S4 (DOCX 88 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, D., Wang, J., Xu, S. et al. The physiological effect of organophosphate flame retardants (OPFRs) on wheat (Triticum aestivum L.) seed germination and seedling growth under the presence of copper. Environ Sci Pollut Res 30, 70109–70120 (2023). https://doi.org/10.1007/s11356-023-27312-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27312-7