Abstract
The relationships among the morphology, trophic ecology, and use of food resources by fish fauna in a pristine stream in Iguaçu National Park (INP), Brazil were evaluated. We expected that the trophic patterns would be explained by ecomorphological variations among fishes and the availability of food resources. Sampling was conducted quarterly from May 2015 to April 2016 using electrofishing. The stomach contents of 599 individuals belonging to nine species were analyzed using the volumetric method. Thirty-two morphological measures related to trophic ecology and body morphology were then converted into 10 ecomorphological indices. The fish assemblage consumed aquatic (Diptera, Ephemeroptera) and terrestrial (Hymenoptera) insects, other aquatic invertebrates, plants, and detritus/sediment. Therefore, the consumption frequencies of these food items were considered representative of their environmental availability. Furthermore, the fish fauna presented wide trophic niche breadths and little diet overlap. Principal component analysis (PCA) was used to classify the fishes’ ecomorphological variations into three ecomorphotypes. The benthonic ecomorphotype comprised species with more depressed bodies that eat detritus/algae and dipteran larvae (Ancistrus mullerae and Corydoras carlae). The nektobenthic ecomorphotype included species with elongated bodies and wider heads and mouths that eat aquatic insects and Aeglidae (Trichomycterus stawiarski and Rhamdia spp.). The nektonic ecomorphotype contained species with more compressed bodies and terminal mouths, which are generalist consumers of allochthonous and autochthonous resources. Morphology was significantly correlated with diet, which suggests that ecomorphological variations together with the availability of food in the environment are the main mechanisms underlying trophic segregation and coexistence among species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Feeding ecology and ecomorphology have long been assessed together to understand the trophic structuring of freshwater fish assemblages (Adite and Winemiller 1997; Pouilly et al. 2003; Teixeira and Bennemann 2007; Oliveira et al. 2010; Neves et al. 2015; Portella et al. 2016). Studies of feeding ecology have traditionally been concerned with what different species eat and the nature of the ecological relationships (i.e. competition, resource partitioning) among species that develop as a result of the use of food resources (Gerking 1994). Ecomorphological studies, in its turn, attempt to quantify morphological variations and their potential effects on performance, including food intake, in the species’ environment (Gatz Jr 1979; Delariva and Agostinho 2001; Kassam et al. 2004; Cochran-Biederman and Winemiller 2010; Portella et al. 2016). Therefore, both types of studies are complementary, as the former can identify the diet compositions of different species and the latter can then correlate these with their morphological variations (Oliveira et al. 2010; Neves et al. 2015). Therefore, such information makes it possible to predict a species’ diet from its morphology (Douglas and Matthews 1992; Franssen et al. 2015).
Variations in the feeding patterns of fish are strongly influenced by food resource availability (Prejs and Prejs 1987; Gerking 1994; Esteves and Aranha 1999), which plays a central role in both the distribution of species among trophic guilds (Pouilly et al. 2006; Wolff et al. 2013) and species coexistence (Barili et al. 2011). Food availability has been used to explain many of the differences, overlaps, and partitioning of diets and food resources among fish species (Dias et al. 2017). These trophic relationships may be mediated by the relative contribution of allochthonous and autochthonous materials that streams and their surroundings supply to fish assemblages (Wootton 1998; Russo et al. 2002; Ferreira et al. 2011). However, human activities have strongly impacted aquatic environments throughout the world (Collen et al. 2014), causing decreases in both the quantity and variety of food available. Therefore, studies contributing to our limited knowledge of the feeding ecology of fish, including information needed to develop and test theoretical predictions, have been limited to those conducted in totally pristine streams, without direct or indirect anthropogenic influences (Casatti 2002). As a consequence of this, it might be difficult to infer reliable trophic patterns in the fish fauna of streams in general based on the natural availability of food or even species morphology, if these inferences are just based on observations in impacted streams.
Although it is now difficult to find pristine streams, Iguaçu National Park (INP) in Brazil stands out for the extensive stretches of the Atlantic Forest in contains. These preserved forests have helped to preserve some of the watersheds of the lower Iguaçu River (e.g., Floriano River and Manoel Gomes Stream) in INP to an extent that is rare in other regions in south and southeastern Brazil (ICMBio 2009). In pristine streams like those in INP it is possible to make inferences about the relationships between the production in terrestrial communities and variations in the food resources they provide to fishes, since stream communities depend heavily on allochthonous resources coming from the surrounding vegetation (Dala-Corte et al. 2016; Lobón-Cerviá et al. 2016; Carvalho et al. 2017). Additionally, the heterogeneity and structural integrity of the habitat, together with the leaf matter input to pristine streams, contribute to the diversification of aquatic invertebrates in them (Ríos and Bailey 2006; Brown 2007) and the subsequent supply of autochthonous food resources to fishes (Russo et al. 2002; Pinto and Uieda 2007).
Various food resources can be exploited in different ways by fish (Gerking 1994), which may promote individual specializations in the use of food items (Araújo et al. 2010; Araújo et al. 2011). In this sense, populations of species regarded as generalists could be composed of specialist individuals (Araújo et al. 2010), which increases the inter-individual variations within a species and broadens the trophic niche of its populations (Abelha et al. 2001; Bolnick et al. 2003). Food availability may also increase the diet spectrum of the species (Novakowski et al. 2008; Mazzoni et al. 2012), as well as the trophic amplitude of guilds (Wolff et al. 2013). In addition, the variety of food available may promote specializations to different specific types of food items or categories, contributing to reduced trophic overlap among fishes (Dias et al. 2017).
Differences in food consumption may also be associated with morphological differences among species (Gatz Jr 1979; Watson and Balon 1984; Oliveira et al. 2010; Sampaio et al. 2013). In other words, the morphology of a particular species must be correlated to some extent with its diet, and this correlation can thus be used to predict the species’ trophic niche (Oliveira et al. 2010; Neves et al. 2015). When such correlations are positive, we may assume that species with similar morphologies use similar categories of food, and thus must share them to coexist (Ross 1986). However, since the relationship between morphology and diet must be interpreted as the result of tradeoffs between morphological adjustments and developmental constraints, it is necessary to control for phylogenetic influences on morphology before morphology can be viewed as being driven by diet variations (Douglas and Matthews 1992; Casatti and Castro 2006). Morphology-diet correlations can then be interpreted as the results of selection imposed on a species to improve its ability to obtain certain categories of food in the environment (Wainwright and Reilly 1994). However, this relationship has not always been consistently demonstrated by previous studies (e.g., Pouilly et al. 2003), and has been attributed to other influences, such as behavior, physiology, and the availability of resources. The last of these could be of substantial importance if, in the absence of its preferred foods, a species was forced to consume other food resources that are not fully predictable based on its morphology.
In the literature, it has been assumed that fishes, through their feeding activities, are the best samplers of the resources available in their environment, or at least those that are used (Mérona et al. 2003; Luz-Agostinho et al. 2006; Zupo et al. 2017). This type of approach is more reliable when based on the analysis of many species with a great diversity of morphotypes because any single species would have a relatively small influence on the estimates of available resources obtained. Therefore, the composition of fish diets in a pristine stream may represent the potential availability of food items there because it focuses on the resource that the fishes living there can effectively access. Thus, one can predict that patterns of food consumption and trophic amplitude among individuals and species, as well as the overlap in the use of food by these, are best explained by the availability of food resources in the environment. In addition, ecomorphological variations could explain more detailed differences in feeding tactics among organisms, and together with the food availability could also explain patterns of trophic segregation. To test these expectations, our objectives in this study were to: (i) describe fish diets in a pristine stream’s fish fauna; (ii) analyze the intra- and interspecific variations in fish diets in terms of their trophic amplitude and overlap; (iii) assess the ecomorphological variations in this fauna; and (iv) correlate the morphotypes observed with trophic patterns among the species in this fish fauna.
Methods
Study area
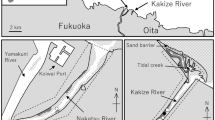
The Iguaçu River is isolated from the Paraná Basin by the Iguaçu Falls, which has resulted in speciation and high fish endemism (Baumgartner et al. 2012). According to Freshwater Ecoregions of the World (Feow 2018), the basin above the falls constitutes the Iguassu ecoregion (Abell et al. 2008). The principal channel, tributaries, and small headwater streams are mostly of moderate slope and flows through sandstone bedrock. In this study, sampling was conducted in Manoel Gomes Stream, a 3rd-order stream (Allan 1995) that drains into Floriano River, a tributary of the right bank of the Iguaçu River (Fig. 1). This stream has its drainage basin fully inside Iguaçu National Park (INP), Brazil. The INP is a Federal Conservation Unit comprising the largest forest remnant in the State of Paraná (PR), with an area of 260,000 ha and located in a contact region between the Semideciduous Seasonal Forest and the Mixed Ombrophilous Forest, two sensu lato Atlantic Forest physiognomies (Joly et al. 1999). The 50-m section sampled presents high structural complexity and is partially to heavily shaded by vegetation cover. The channel geomorphology includes riffles, with rocky bottom substrates and pools in which gravel, sand/silt, leaf litter, branches, and tree trunks are deposited. The channel is on average 4.0 m wide and 0.20–1.0 m deep. In addition, due to the high degree of shading, the water is relatively cold (19.19 ± 2.91 °C) and well-oxygenated (9.01 ± 0.73 mg/L-1), indicating good water quality (unpublished data).
Field sampling
The fish fauna was sampled quarterly from May 2015 to April 2016 using electrofishing in a 50-m stretch of a pristine stream. The electrofishing equipment consisted of a portable electrical generator (Honda, 220 V, output voltage from 400 to 600 V, 50–60 Hz, 3.4–4.1 A, 100 W), connected to two electrodes by a flexible multifilament cable 60 m in length. The specimens collected were anesthetized with eugenol and then fixed in 4% formalin. The fishes were identified according to Graça and Pavanelli (2007) and Baumgartner et al. (2012), and were gutted to remove the gastrointestinal tracts, which were then preserved in 70% ethanol. Voucher specimens were deposited in the Coleção Ictiológica do Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia) of the Universidade Estadual de Maringá, Maringá, PR, Brazil.
Laboratory analysis
Only species with a total abundance greater than five individuals were used in this study. In order to avoid issues during the identification and quantification of digested items, stomach contents were only analyzed if the stomach was more than 50% full. Food items were identified to the lowest possible taxonomic level using an optical microscope and stereomicroscope along with identification keys for macroinvertebrates (Mugnai et al. 2010) and algae (Bicudo and Bicudo 1970), and their abundances were then quantified using the volumetric method (Hyslop 1980; Marrero 1994). This method expresses each food item as a proportion of the total volume of all other food categories found in the sample. For species with an undefined stomach (i.e. members of the Loricariidae), the anterior third of the intestine was analyzed instead.
For the ecomorphological study of the captured fishes, 26 linear morphological measurements and six areal measurements of the fins and eyes were made by drawing outlines of these structures (Gatz Jr 1979; Oliveira et al. 2010; Pagotto et al. 2011). These measures were then assigned to the habitat, swimming behavior, and food intake of each of the nine species analyzed. The morphological variables were measured with a digital caliper on the left side of 30 specimens (or at least five specimens of species whose total number was less than 30). To calculate the six areas related to the fins, these were first drawn in plastic material on the fin and then scanned. The images were imported to the software Autodesk (2014) to calculate the internal areas of the fin images. The morphometric measurements were used to produce ten ecomorphological indices (Table 1). In addition, we coded the mouth orientation as a morphological trait as follows: 1, superior position; 2, terminal position; 3, oblique ventral position; or 4, ventral position (Baumgartner et al. 2012).
Data analyses
To characterize fish diets, the food contents in their stomachs were identified at the level of individual food items; however, some of these occurred at very low frequencies or abundances, and therefore they were grouped into broader categories (Table 2). The items Bacillariophyta, Chlorophyceae, and Cyanobacteria were grouped together as algae; the larvae and pupae of Psephenidae, Odonata, Coleoptera, and Diptera were grouped together as other aquatic insects; Gastropoda, Decapoda, Platyhelminthes, and Nematoda were grouped together as other aquatic invertebrates; adult Diptera, Orthoptera, and Hemiptera were grouped together as other terrestrial insects; and Oligochaeta, Diplopoda, and Araneae were grouped together as other terrestrial invertebrates. Detritus/Sediment was identified as a particular food item type, although it represented both inorganic and organic particles at different stages of decomposition.
The availability of food resources was considered herein as the amount of each food type consumed by all the analyzed species. This was assessed by standardizing the sampling effort (i.e. the volume of each food resource consumed) by means of the equitability index (EVAR) (Smith and Wilson 1996), wherein the proportion of food resources was distributed among species as follows:
where: S = the total volume of food resources (items or trophic categories) in the sample; and Xs = is the species’ abundance. Thus, the volume of food resources was also standardized for all species. Due to the presence of different fish ecomorphotypes in this study, the sampled fishes thus had the capacity to explore various habitat compartments, which may provide a more reliable evaluation of the overall availability of resources than a single ecomorphotype would (Dias et al. 2017). We then generated a histogram to represent the standardized food volume and the relative availability of the different items and categories of food resources.
The trophic guild to which each fish belonged was determined based on the volumetric data of the stomach contents by a procedure adapted from Mérona et al. (2001). Detritivorous fishes were those whose stomach contents were more than 50% debris/sediment and algae. Aquatic insectivorous fishes were those whose stomach contents were more than 50% aquatic insects. Carnivorous fishes were those whose stomach contents were more than 50% composed of several invertebrates and fishes. Detritivorous/Insectivorous fishes’ stomachs contained similar proportions of detritus and aquatic insects. Finally, omnivorous fishes were those for which none of the above statements were quite true, and whose stomachs contained items of multiple different origins (i.e. both animal and plant materials).
To test for possible significant differences in dietary composition among species, a permutational multivariate analysis of variance (PERMANOVA) of Bray-Curtis index values was used, with 999 random permutations (Anderson 2001). To determine whether and which food items or trophic categories contributed most to interspecific dissimilarities in diet, we performed a similarity percentage (SIMPER) analysis of the afore mentioned Bray-Curtis index values (Clarke 1993).
A principal component analysis (PCA) combined with the Hellinger transformation of square-root-transformed diet data (Legendre and Gallagher 2001) was used to summarize the differences in dietary patterns among the fish fauna. Hellinger’s distance provides a common ground that allows for the subsequent combination of multivariate analyses, and in the case of biological variables it yields a metric that is similar in performance to the Bray-Curtis dissimilarity index (Borcard et al. 2011; Basterretxea et al. 2013).
The niche breadth (i.e. dispersion of the diet in space) was measured through a permutational analysis of multivariate dispersions (PERMDISP; Anderson 2006). Recently, this analysis has become a common approach to verify intraspecific variability (Quirino et al. 2015) and assess differences in intra- and interspecific niche breadth (Correa and Winemiller 2014; Silva et al. 2017). The distance from the centroid of a group defined a priori (in this case, the species) is calculated in the PERMDISP through a principal coordinate analysis (PCoA). Calculation of the centroid of each group was performed using Bray-Curtis dissimilarity index values, which allowed the average dissimilarity in n individual observations within the group to be compared. In this case, the distance from the centroid (D) corresponds to the niche breadth. To test the null hypothesis that the intraspecific variability and niche breadth did not differ among the groups, the F-statistic was calculated for the comparison of the average distance of each sample from the centroid of the group. Subsequently, the p value associated with this F-statistic was obtained through 9999 permutations of the residual least-squares (Anderson 2006). Post-hoc pairwise comparisons were made using Tukey’s ‘Honestly Significant Difference’ (HSD) method. The assumption was that differences in the distance from the centroid among species indicate that some species have more restricted or broader diets than others do (Correa and Winemiller 2014; Silva et al. 2017; Neves et al. 2018).
Diet overlap was calculated for each sample (i.e. among all species, in each month), and was presented as a mean of four observations between each pair of species. The overlap was calculated based on the similarity matrix of volumetric data for different food items. We used the niche overlap index of Pianka (1973), described by the equation:
where: Ojk = Pianka’s niche overlap measure between species j and k; Pij = the proportion of food item i in the diet of species j; Pik = the proportion of food item i in the diet of species k; and n = the total number of food items. The overlap values ranged from 0 (no overlap) to 1 (total overlap), and were grouped into the following categories: low (overlap = 0–0.39), intermediate (overlap = 0.4–0.6), and high (overlap = 0.61–1) (Grossman 1986; modified by Corrêa et al. 2011).
The ecomorphological patterns within the fish assemblage were evaluated through another PCA, applied to the correlation matrix calculated for the 10 ecomorphological indices plus the coded mouth orientation variable. The criterion we adopted for the retention of the axes (principal components) followed the Broken-Stick randomization method, in which only those axes with eigenvalues greater than those generated at random are interpreted (Jackson 1993).
To investigate the relationship between the morphological variations and trophic patterns exhibited in the fish fauna, Spearman’s rank correlation coefficients were calculated. In this procedure, the correlation between the PCA scores from the selected axes (Broken-Stick criterion) of the different PCAs (PCA-morphology and PCA-diet) was tested.
However, the relationships between differences in diet and morphology among species may also be derived from the phylogenetic distances among species, in which case spurious correlations would be generated (Winemiller 1991; Douglas and Matthews 1992). Therefore, a partial correlation test (partial Mantel test) between two matrices (morphological × trophic) was run, while controlling for the effect of a third matrix (taxonomic) to evaluate possible phylogenetic effects on the relationship between diet and morphology. From the volumetric percentages of different food resources consumed by each species, we calculated a matrix of trophic similarity (Manhattan index). Similarly, the mean values of the ecomorphological indices were used to calculate an ecomorphological distance matrix (Euclidean distance) among species. The taxonomic distance matrix was constructed according to Douglas and Matthews (1992), in which a value of 1 was attributed to pairs of congeneric species, 2 to pairs of species belonging to different genera but the same family, 3 to pairs of species belonging to different families of the same order, and 4 to pairs of species belonging to different orders.
The PCAs and PERMDISP analysis were performed in R (www.rproject.org) (R Core Team 2014) using the vegan package (Oksanen et al. 2007). The Pianka niche overlap index was calculated using EcoSim 7.0 software (Gotelli and Entsminger 2006). The PERMANOVA, SIMPER, and MANTEL analysis were performed in PAST 2.08 (Paleontological Statistic Software) (Hammer et al. 2001). Spearman’s rank correlations and figures were constructed in the STATISTIC 7.0 program. The level of statistical significance adopted for all analyses was p < 0.05.
Results
Diet
The fish fauna was represented by a total of 1761 individuals belonging to 12 species in our samples. However, three of these species, of which fewer than five individuals were found, were not considered further in this study (Hisonotus yasi Almirón, Azpelicueta & Casciotta, 2004; Trichomycterus davisi Haseman, 1911; Trichomycterus spp.). The stomach contents of 599 specimens of the nine remaining species were analyzed (Table 2). The availability of food resources, assessed based on the volume of food resources ingested by these nine species, comprised a wide variety of autochthonous and allochthonous resources (Fig. 2). The most important food resources, which constituted the greatest proportions of food ingested, were larvae of Diptera (30.4%), plant material (15.2%), EPT (Ephemeroptera, Plecoptera, and Trichoptera) (15.2% combined), and detritus/sediment (11.2%).
The fish fauna was composed primarily of smaller-sized species, with a predominance of species belonging to the omnivorous and aquatic insectivorous guilds (Table 2). The main resources consumed were detritus/sediment, aquatic insects, other aquatic invertebrates, terrestrial insects, and terrestrial plants. Ancistrus mullerae (Bifi, Pavanelli & Zawadzki, 2009) and Corydoras carlae (Nijssen & Isbrucker, 1983) fed preferentially on detritus/sediment, along with low proportions of algae in the former species and high amounts of dipteran larvae in the latter. Astyanax species had varied diets composed of diverse autochthonous and allochthonous resources. In turn, Rhamdia branneri (Haseman, 1911) consumed mainly Aeglidae, which differed from its congener Rhamdia voulezi (Haseman, 1911), which predominantly fed on other aquatic insects and plant. The diets of Bryconamericus ikaa (Casciotta, Almirón & Azpelicueta, 2004) and Trichomycterus stawiarski (Miranda Ribeiro, 1968) were composed of larvae of aquatic insects, such as those of Ephemeroptera, Diptera, Plecoptera, and other taxa.
Significant differences in diet composition were observed among species (PERMANOVA; pseudo-F = 29.65, p = 0.0001). The food items that contributed most to the dietary differences among fish species were: Hymenoptera for Astyanax bifasciatus (Garavelho & Sampaio, 2010); other aquatic invertebrates for Astyanax gymnodontus (Eigenmann, 1911); Coleoptera for Astyanax minor (Garavelho & Sanpaio, 2010); algae for A. mullerae; Ephemeroptera (larvae) for B. ikaa and T. stawiarski; Aeglidae for R. branneri; and other aquatic insects for R. voulezi (SIMPER, Table 3).
Segregation in food consumption by the fish fauna was summarized in a PCA, which explained 45% of the total variation in the data. Positive values of the first principal component (PC1) segregated species ingesting detritus/sediment and algae, such as A. mullerae and C. carlae, from others. Positive values of PC2 segregated species (Astyanax and Rhamdia) that mainly consumed plants and terrestrial insects, while at negative scores of PC2 B. ikaa and T. stawiarski, which had greater intakes of larvae of Ephemeroptera and Diptera, were segregated (Fig. 3, Appendix Table S1).
Ordenation of the fish fauna of Manoel Gomes stream, Iguaçu National Park, Iguassu ecoregion, Brazil, produced by the first two axes of the principal components analysis (PCA 1 and PCA 2) applied to the correlation of 17 food items consumed by species. Food resource codes: Det: Detritus/Sediment; Pla: Plant; Alg: Algae; Eph: Ephemeroptera (larvae); Dip: Diptera (larvae); Oia: Other aquatic insects; Hym: Hymenoptera (adult); Oit: Other terrestrial insects. Symbols:  (filled blue circle): Astyanx bifasciatus;
(filled blue circle): Astyanx bifasciatus;  (filled green square): Astyanax gymnodontus;
(filled green square): Astyanax gymnodontus;  (red triangle): Astyanax minor; ■ (filled black square): Ancistrus mullerae; ○ (black circle): Bryconamericus ikaa; ◊ (black diamond): Corydoras carlae;
(red triangle): Astyanax minor; ■ (filled black square): Ancistrus mullerae; ○ (black circle): Bryconamericus ikaa; ◊ (black diamond): Corydoras carlae;  (filled dark red triangle): Rhamdia branneri; + (black plus symbol): Rhamdia voulezi;
(filled dark red triangle): Rhamdia branneri; + (black plus symbol): Rhamdia voulezi;  (filled gray circle): Trichomycterus stawiarski
(filled gray circle): Trichomycterus stawiarski
The trophic niche breadth differed significantly among species (PERMIDISP, F8.590 = 102.28, p < 0.5) (Fig. 4, Table 4). The lowest values were found for the most specialized species, such as A. mullerae and C. carlae. The species considered to be omnivorous and generalists, such as characins, presented high values of niche breadth. R. branneri stood out for having the widest niche breadth. C. carlae was the species with the highest intraspecific variation in its niche breadth, while Rhamdia spp. presented the lowest intraspecific variation.
Variation in diet breadth using PERMDISP analysis for nine fish species of the Manoel Gomes stream, Iguaçu National Park, Iguassu ecoregion, Brazil. Diet breadth was assessed as species dispersion in dietary space (i.e., greater distance to spatial centroid indicates larger dispersion and therefore a broader trophic niche). Box lower and upper endpoints represent the 25th and 75th quartiles, respectively. The horizontal bar inside each box represent centroid and average represent the diet breadth, respectively. See Table 4 for p value of comparisons among species. Species codes see Table 3
In general, the diet overlap was significantly low (<0.4) between 77.5% of the pairs of species (Fig. 5, Kruskal-Wallis test, H = 87.44, p < 0.0001). Higher niche overlap values were observed for A. bisfasciatus × A. gymnodontus (both feeding on aquatic and terrestrial insects), A. mullerae × C. carlae (feeding on detritus/sediment), B. ikaa × T. stawiarski (feeding on aquatic insects), and R. branneri × R. voulezi (feeding on aquatic insects).
Diet overlap values (mean ± SE) for species pairs sampled on the Manoel Gomes stream, Iguaçu National Park, Iguassu ecoregion, Brazil. Diet overlap: low (0–0.39), intermediate (0.4–0.6) or high (0.61–1). Species codes: Abi: Astyanax bifasciatus, Agy: Astyanax gymnodontus, Ami: Astyanax minor, Amu: Ancistrus mullerae, Bik: Bryconamericus ikaa, Cor: Corydoras carlae, Rbr: Rhamdia branneri, Rvo: Rhamdia voulezi, Tst: Trichomycterus stawiarski
Ecomorphological traits
Morphological measurements were taken of 224 individuals belonging to nine species. The fish fauna showed much interspecific ecomorphological variation, but presented several main, distinct morphotypes, including species with compressed bodies and caudal peduncles, as well as those with depressed bodies and ventral mouths (Fig. 6). The first two PCA axes retained for interpretation (per the Broken-Stick model) explained 75.3% of the total variance in the data (Appendix Table S2). Positive values of the first PCA axis (accounting for 42.49% of the explained variance) distinguished species with relatively wider heads and mouths, such as T. stawiarski, Rhamdia branneri, and Rhamdia voulezi, and this axis also captured the position of the eye for A. mullerae. Species with greater eye areas, more compressed bodies, and longer heads (Astyanax and B. ikaa) were segregated by negative values of axis 1. Positive values of PCA axis 2 (32.81% of explained variance) segregated species with more depressed bodies, greater caudal fin areas, and higher mouths, such as A. mullerae and Astyanax. Negative scores of this axis separated out species with wider heads and mouths, such as the two species of Rhamdia and T. stawiarski, and more compressed bodies, such as Astyanax and B. ikaa (Fig. 6).
Ordenation of the fish fauna of Manoel Gomes stream, Iguaçu National Park, Iguassu ecoregion, Brazil, produced by the first two axes of the principal components analysis (PCA 1 and PCA 2) applied to the correlation of 10 ecomorphological indices and the mouth orientation. Symbols:  (filled blue circle): Astyanx bifasciatus;
(filled blue circle): Astyanx bifasciatus;  (filled green square): Astyanax gymnodontus;
(filled green square): Astyanax gymnodontus;  (red triangle): Astyanax minor; ■ (filled black square): Ancistrus mullerae; ○ (black circle): Bryconamericus ikaa; ◊ (black diamond): Corydoras carlae;
(red triangle): Astyanax minor; ■ (filled black square): Ancistrus mullerae; ○ (black circle): Bryconamericus ikaa; ◊ (black diamond): Corydoras carlae;  (filled dark red triangle): Rhamdia branneri; + (black plus symbol): Rhamdia voulezi;
(filled dark red triangle): Rhamdia branneri; + (black plus symbol): Rhamdia voulezi;  (filled gray circle): Trichomycterus stawiarski
(filled gray circle): Trichomycterus stawiarski
Diet-ecomophology correlations
Significant correlations were found between PC1-morphology and PC1-diet scores (ρ = 0.56, p < 0.05) and between PC2-morphology and PC1-diet scores (ρ = 0.58, p < 0.05). Benthic species with larger heads and mouths, such as A. mullerae, tended to be those that consumed detritus/sediment and algae. Otherwise, nektonic species with compressed bodies (Astyanax and Bryconamericus) tended to be those that consumed insects (aquatic and terrestrial) and plant materials. Weak but significant negative correlations were found between PC1-morphology and PC2-diet (ρ = −0.17, p < 0.05) and between PC2-morphology and PC2-diet (ρ = −0.16, p < 0.05), meaning that greater body depression was associated with less consumption of drifting resources carried into the stream from the surroundings (plants and terrestrial insects).
The Mantel test demonstrated a significant correlation between the trophic distance matrix and the morphological matrix (r = 0.59, p = 0.0012). However, there was also a significant correlation between the morphological and taxonomic matrices (r = 0.62, p = 0.0006), which may indicate spurious relationships between trophic characteristics and morphology due to the phylogeny of the species. Therefore, a partial Mantel test was done to remove the effect of phylogeny from the assessment of the relationship between diet and morphology, and its results indicated that there was still a significant correlation between diet and morphology, regardless of taxonomy (r = −0.50, p = 0.0046).
Discussion
The integrity of the surrounding vegetation and the structural heterogeneity of the pristine stream evaluated in this study were clearly demonstrated by the variety of allochthonous and autochthonous resources we detected in the diets of its fish fauna. The high frequencies with which plants (leaves, seeds, and fruit) and terrestrial insects (adult Coleoptera and Hymenoptera) were consumed, as well as the substantial contributions of Diptera and EPT to fish diets, attest to the availability of these resources in the study site, which must be associated with the undisturbed condition of the environment there. These patterns have been reported in other studies, in which the influence of the adjacent forest on the availability of resources to stream fish fauna was confirmed (Sabino and Castro 1990; Pusey and Arthington 2003; Casatti 2010; Leite et al. 2015; Dala-Corte et al. 2016). The surrounding forest is also important to the maintenance of the trophic relationships among fishes within the stream fauna (Casatti et al. 2012; Silva et al. 2014; Lobón-Cerviá et al. 2016).
In environments with high resource availability, greater niche expansion is expected to occur in the diets of species that exhibit greater trophic plasticity (Schoener 1982; Abelha et al. 2001). The trophic breadths of the species analyzed herein were wide and varied, especially because they were fish that usually display generalist feeding behaviors (Esteves and Aranha 1999; Abelha et al. 2001; Oliveira et al. 2010), demonstrating that individuals had access to different food items in the studied system. In other words, the results highlighted individual differences in food resource usage, with probable specializations on different food resources by individuals and, consequently, the expansion of the niche of their species’ populations.
The wide diet breadth associated with high resource availability should be interpreted in more detail, considering that the variety and abundance of resources that a species can access should still be mediated by its morphological potentialities (Prado et al. 2016). In this sense, characins, which were identified to have the more compressed bodies and laterally positioned eyes indicative of nektonic species, would be allowed by this morphology to opportunistically use drifting resources, such as plants, terrestrial and aquatic insects, and the larvae of other fish species. This wide range of features thus demonstrates the potential to exploit all compartments of the environment. These species exhibit greater morphological diversification than others do, and have been considered morphological generalists (Mise et al. 2013a; Neves et al. 2015, 2018; Lopes et al. 2016). These species are able to ingest food items of any origin (Delariva et al. 2013; Silva et al. 2017; Neves et al. 2018) and that are normally not used, like fish larvae, and are only limited in what they can consume by the size of their mouth opening.
Bryconamericus ikaa presented similar morphological features to species of Astyanax except for the subterminal position of its mouth, which favored the intake of aquatic insects (Ephemeroptera and Diptera). This characteristic also provided this feeding habit to T. stawiarski. The shared aquatic insectivorous habit presented by these two species reflects their ability to explore lentic environments, foraging among the spaces in the rock bottom wherein insect larvae attain favorable developmental conditions (Casatti and Castro 1998; Russo et al. 2002; Barreto and Aranha 2006; Esteves et al. 2008; Rondineli et al. 2009; Ruaro et al. 2016; Uieda et al. 2016). In addition, B. ikaa and T. stawiarski were species that consumed a wide diversity of aquatic insects, and both showed wide niche breadths. Similar behaviors are favored in environments with high food resource availability (Silva et al. 2017; Neves et al. 2018), allowing some species to make opportunistic uses of shared resources. The Rhamdia species examined also showed wide niche breadths, which can be attributed to their consumption of various aquatic invertebrates (Aeglidae), aquatic insects, and plants. These species’ high trophic performance is related to their morphological characteristics, such as the large dimensions of their mouth and their relatively large head, which favor the capture of relatively larger food items in various parts of the environment (Mise et al. 2013b).
Narrow trophic breadths were observed for species with specialist morphological features, such as A. mullerae and C. carlae. The dorsoventrally flattened body and ventral mouth of A. mullerae provides superior attachment to the substrate and acquisition of food by scraping the rocks, giving this species a preferentially detritivorous diet. Corydoras carlae, which exhibited close positioning to A. mullerae in the morphospace despite its lower trophic amplitude, presented greater intraspecific variation. This may be related to the subterminal mouth and elongated snout of this species, which allows it to feed by suctioning in lentic muddy areas and acquire detritus/sediment in conjunction with aquatic insects, especially dipteran larvae.
Fish display different feeding strategies according to their biology (Gerking 1994). However, dietary tactics may also vary depending on ecological interactions and the availability of resources, which together may be responsible for species coexistence (MacArthur and MacArthur 1961; Vitt et al. 1997). The different fish body ecomorphotypes found herein presented different trophic requirements, as was verified by the wide variety of food resources consumed (allochthonous and autochthonous). In turn, these ecomorphotypes contributed to their being little trophic overlap between many of the pair of species analyzed. Nonetheless, genera (e.g., Astyanax, Rhamdia) and species with similar ecological and morphological characteristics, such as A. mullerae, C. carlae, B. ikaa, and T. stawiarski, presented greater overlap. The coexistence of such species may not necessarily be accompanied by competition, however, due to the high availability of resources in the habitat and differential consumption of some specific items by different species. Other aspects that should be considered are the differences in the habits and exploitation of microhabitats among species, which are considered important mechanisms for the use of shared resources and coexistence (Schoener 1974; Connell 1983; Mazzoni et al. 2012; Leitão et al. 2015; Barros et al. 2016).
Differential resource use, even if it is only partial, is an essential component of the trophic interactions within a community (Schoener 1974; Chase and Leibold 2003). In this regard, the ecomorphological variations exhibited in the fish fauna should play an important role in the coexistence of its species. In this study, three main ecomorphotypes were identified, including nektonic, nektobenthic, and benthonic (sensu Casatti and Castro 2006) ones. These ecomorphotypes are groups of species’ morphologies routinely identified in freshwater fish fauna (Oliveira et al. 2010; Pagotto et al. 2011; Neves et al. 2015). The presence of distinct ecomorphotypes together in the same habitat allows for the exploitation of dissimilar food resources by fish species throughout all compartments of the stream’s environment. Nevertheless, the diet differed both among species and ecomorphotypes, suggesting that inter-ecomorphotypic variation, together with food availability, mediates the relationships between consumption and trophic segregation in fish species.
Significant morphology-diet correlations were demonstrated in this study, which supports the hypothesis that morphology acts as one of the mechanisms responsible for dietary variations. Although little is known about the functional performance of different fish morphologies (Kassam et al. 2004), studies of morphological correlations with diet (Hugueny and Pouilly 1999; Cochran-Biederman and Winemiller 2010; Neves et al. 2015) have demonstrated indirect mechanisms capable of explaining the potential food intake by fishes. In our study, morphological traits allowing fish to feed on the benthos strongly constrained their use of drifting food items, whereas a laterally compressed body and terminal mouth broadened the food spectrum available to them. Therefore, the evolution of ecomorphotypes seems to largely reflect the action of selection on where food is acquired and across how broadly different categories (Wootton 1998; Oliveira et al. 2010). A probable functional interpretation based on the morphology-diet correlation suggests that the nektonic ecomorphotype is not very different from the ancestral condition of most fish, which can suck food into the mouth that is either drifting or located on the substrate (Gerking 1994). Accessing food resources on the bottom apparently selected for morphological specializations, such as a subterminal and ventral mouth, to improve the capture efficiency of benthic invertebrates and detritus/sediment by the nektobenthic and benthonic ecomorphotypes, respectively.
In summary, our results indicated that the varied composition, wide niche breadth, and low diet overlap in the fish assemblage of the pristine stream studied were all directly associated with the local availability of food resources. The ecomorphological variations observed also determined dietary differences among species, as well as among ecomorphotypes. Species with a nektonic morphology were associated with the consumption of allochthonous plants and insects, while those with a nektobenthic and benthonic morphology were associated with the consumption of aquatic insects and with detritus/sediment and algae, respectively. Therefore, food supply together with the morphological potentialities of different species acted as mechanisms of trophic segregation in this system, allowing the coexistence of species. Finally, our study emphasizes the importance of aquatic ecosystem integrity to the trophic and ecomorphological structuring of fish fauna, and provides information that can contribute to environmental comparisons and evaluation of disturbed streams.
References
Abelha MCF, Agostinho AA, Goulart E (2001) Plasticidade trófica em peixes de água doce. Acta Sci 23:425–434. https://doi.org/10.4025/actascibiolsci.v23i0.2696
Abell R, Thieme ML, Revenga C, Bryer M, Kottelat M, Bogutskaya N, Coad B, Mandrak N, Balderas SC, Bussing W, Stiassny MLJ, Skelton P, Allen GR, Unmack P, Naseka A, Ng R, Sindorf N, Robertson J, Armijo E, Higgins JV, Heibel TJ, Wikramanayake E, Olson D, López HL, Reis RE, Lundberg JG, Pérez MHS, Petry P (2008) Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58:403–414. https://doi.org/10.1641/B580507
Adite A, Winemiller KO (1997) Trophic ecology and ecomorphology of fish assemblages in coastal lakes of Benin, West Africa. Ecoscience 4:6–23. https://doi.org/10.1080/11956860.1997.11682371
Allan JD (1995) Stream ecology: structure and function of running waters. Chapman and Hall, London
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Araújo MS, Martins EG, Cruz LD, Fernandes FR, Linhares AX, Dos Reis SF, Guimarães PR Jr (2010) Nested diets: a novel pattern of individual-level resource use. Oikos 119:81–88. https://doi.org/10.1111/j.1600-0706.2009.17624.x
Araújo MS, Bolnick DI, Craig AL (2011) The ecological causes of individual specialization. Ecol Lett 14:948–958. https://doi.org/10.1111/j.1461-0248.2011.01662.x
Autodesk (2014) AutoCAD: graphic computation (Version 2014) [Software]. Autodesk Inc., San Rafael
Balon EK, Crawford SS, Lelek A (1986) Fish communities of the upper Danube River (Germany, Austria) prior to the new Rhein-Main-Donau connection. Environ Biol Fish 15:243–271
Barili E, Agostinho AA, Gomes LC, Latini JD (2011) The coexistence of fish species in streams: relationships between assemblage attributes and trophic and environmental variables. Environ Biol Fish 92:41. https://doi.org/10.1007/s10641-011-9814-2
Barreto AP, Aranha JMR (2006) Alimentação de quatro espécies de Characiformes de um riacho da Floresta Atlântica, Guaraqueçaba, Paraná, Brasil. Rev Bras Zool 23:779–788. https://doi.org/10.1590/S0101-81752006000300023
Barros G, Zuanon J, Deus C (2016) Effects of species co-occurrence on the trophic-niche breadth of characids in Amazon forest streams. J Fish Biol 90:326–340. https://doi.org/10.1111/jfb.13183
Basterretxea G, Catalán IA, Jordi A, Álvarez I, Palmer M, Sabatés A (2013) Dynamic regulation of larval fish self-recruitment in a marine protected area. Fish Oceanogr 22:477–495. https://doi.org/10.1111/fog.12035
Baumgartner GCS, Pavanelli CS, Baumgartner D, Bifi AG, Debona T, Frana VA (2012) Peixes do baixo Rio Iguaçu. Eduem, Maringá
Bicudo CEM, Bicudo RMT (1970) Algas de águas continentais brasileiras chave ilustrada para identificação de gêneros. Fundação Brasileira para o Desenvolvimento do Ensino de Ciências, São Paulo
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Amer Nat 161:1–28. doi: https://doi.org/10.1086/343878 PMID: 12650459
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, New York
Brown BL (2007) Habitat heterogeneity and disturbance influence patterns of community temporal variability in a small temperate stream. Hydrobiologia 586:93–106. https://doi.org/10.1007/s10750-006-0531-3
Carvalho DR, Castro DMP, Callisto M, Moreira MZ, Pompeu PS (2017) The trophic structure of fish communities from streams in the Brazilian Cerrado under different land uses: an approach using stable isotopes. Hydrobiologia 795:199–217. https://doi.org/10.1007/s10750-017-3130-6
Casatti L (2002) Alimentação dos peixes em um riacho do Parque Estadual Morro do Diabo, bacia do Alto Paraná, sudeste do Brasil. Biota Neotrop 2:1–14. https://doi.org/10.1590/S1676-06032002000200012
Casatti L (2010) Alterações no código florestal brasileiro: impactos potenciais sobre a ictiofauna. Biota Neotrop 10:31–34. https://doi.org/10.1590/S1676-06032010000400002
Casatti L, Castro RMC (1998) A fish community of the São Francisco River headwaters riffles, southeastern Brazil. Ichthyol Explor Freshw 9:229–242
Casatti L, Castro RMC (2006) Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the rio São Francisco, southeastern Brazil. Neotrop Ichthyol 4:203–214. https://doi.org/10.1590/S1679-62252006000200006
Casatti L, Teresa FB, Gonçalves-Souza T, Bessa E, Manzotti AR, Gonçalves CS, Zeni JO (2012) From forests to cattail: how does the riparian zone influence stream fish? Neotrop Ichthyol 10:205–214. https://doi.org/10.1590/S1679-62252012000100020
Chase JM, Leibold MA (2003) Ecological niches: linking classical and contemporary approaches. University of Chicago Press, London
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Cochran-Biederman JL, Winemiller KO (2010) Relationships among habitat, ecomorphology and diets of cichlids in the Bladen River, Belize. Environ Biol Fish 88:143–152. https://doi.org/10.1007/s10641-010-9624-y
Collen B, Whitton F, Dyer EE, Baillie JEM, Cumberlidge N, Darwall WRT, Pollock C, Richman NI, Soulsby AM, Böhm M (2014) Global patterns of freshwater species diversity, threat and endemism. Glob Ecol Biogeogr 23:40–51. https://doi.org/10.1111/geb.12096
Connell JH (1983) On the prevalence and relative importance of interspecifc competition: evidence from feld experiments. Am Nat 122:661–696. https://doi.org/10.1086/284165
Correa SB, Winemiller KO (2014) Niche partitioning among frugivorous fishes in response to fluctuating resources in the Amazonian floodplain forest. Ecology 95:210–224. https://doi.org/10.1890/13-0393.1
Corrêa CE, Albrecht MP, Hahn NS (2011) Patterns of niche breadth and feeding overlap of the fish fauna in the seasonal Brazilian Pantanal, Cuiabá River basin. Neotrop Ichthyol 9:637–646. https://doi.org/10.1590/S1679-62252011000300017
Dala-Corte RB, Becker FG, Melo AS (2016) Riparian integrity affects diet and intestinal length of a generalist fish species. Mar Freshw Res 68:1272–1281. https://doi.org/10.1071/MF16167
Delariva RL, Agostinho AA (2001) Relationship between morphology and diets of six neotropical loricariids. J Fish Biol 58:832–847. https://doi.org/10.1111/j.1095-8649.2001.tb00534.x
Delariva RL, Hahn NS, Kashiwaqui EAL (2013) Diet and trophic structure of the fish fauna in a subtropical ecosystem: impoundment effects. Neotrop Ichthyol 11:891–904. https://doi.org/10.1590/S1679-62252013000400017
Dias RM, Ortega JCG, Gomes LC, Agostinho AA (2017) Trophic relationships in fish assemblages of Neotropical floodplain lakes: selectivity and feeding overlap mediated by food availability. Iheringia Sér Zool 107:e2017035. https://doi.org/10.1590/1678-4766e2017035
Douglas ME, Matthews WJ (1992) Does morphology predict ecology? Hypothesis testing within a freshwater stream fish assemblage. Oikos 65:213–224. https://doi.org/10.2307/3545012
Esteves KE, Aranha JMR (1999) Ecologia trófica de peixes de riachos. In: Caramaschi EP, Mazzoni R, Peres-Neto PR (eds) Ecologia de peixes de riachos. Série Oecologia Brasiliensis, Rio de Janeiro, pp 157–182
Esteves KE, Lobo AVP, Faria MDR (2008) Trophic structure of a fish community along environmental gradients of a subtropical river (Paraitinga River, Upper Tietê River Basin, Brazil). Hydrobiologia 598:373–387. https://doi.org/10.1007/s10750-007-9172-4
Feow (2018) Freshwater Ecoregions of the World- 346: Iguassu. Available at http://www.feow.org/ecoregions/details/346. Accessed 2 Aug 2018
Ferreira A, Paula FR, Ferraz SFB, Gerhard P, Kashiwaqui EAL, Cyrino JEP, Martinelli LA (2011) Riparian coverage affects diets of characids in Neotropical streams. Ecol Freshw Fish 21:12–22. https://doi.org/10.1111/j.1600-0633.2011.00518.x
Franssen NR, Goodchild CG, Shepard DB (2015) Morphology predicting ecology: incorporating new methodological and analytical approaches. Environ Biol Fish 98:713–724. https://doi.org/10.1007/s10641-014-0306-z
Gatz AJ Jr (1979) Ecological morphology of freshwater stream fishes. Tulane Stud Zool Bot 21:91–124
Gerking SD (1994) Feeding ecology of fish. Academic Press, San Diego
Gotelli NJ, Entsminger GL (2006) EcoSim: null models software for ecology. Version 7. Acquired intelligence and Kesey- bear. Jericho, Vermont, USA. Http:// garyentsminger.com/ecosim.htmi. Accessed 30 Oct 2013
Graça WJ, Pavanelli CS (2007) Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Eduem, Maringá
Grossman GD (1986) Food resources partitioning in a rocky intertidal fish assemblage. J Biol 1:317–355. https://doi.org/10.1111/j.1096-3642.1986.tb00642.x
Hammer DA, Harper T, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hora SL (1922) Structural modifications in the fish of mountain torrents. Rec Indian Museum 24:31–61
Hugueny B, Pouilly M (1999) Morphological correlates of diet in an assemblage of west African freshwater fishes. J Fish Biol 54:1310–1325. https://doi.org/10.1111/j.1095-8649.1999.tb02057.x
Hyslop EJ (1980) Stomach contents analysis: a review of methods and their application. J Fish Biol 17:411–429. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x
Instituto Chico Mendes De Conservação Da Biodiversidade, ICMBio. Parque Nacional do Iguaçu (2009) http://www.icmbio.gov.br/parnaiguacu/guia-do-visitante.html. Accessed 28 Oct 2017
Jackson DA (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214. https://doi.org/10.2307/1939574
Joly CA, Aidar MPM, Klink CA, McGrath DG, Moreira AG, Moutinho P, Nepstad DC, Oliveira AA, Pott A, Rodal MJN, Sampaio EVSB (1999) Evolution of the Brazilian phytogeography classification systems: implications for biodiversity conservation. Cienc Cult 51:331–348
Kassam D, Adams DC, Yamaoka K (2004) Functional significance of variation in trophic morphology within feeding microhabitat-differentiated cichlid species in Lake Malawi. Anim Biol 54:77–90. https://doi.org/10.1163/157075604323010060
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. https://doi.org/10.1007/s004420100716
Leitão RP, Sánchez-Botero JV, Kasper D, Trivério-Cardoso V, Araújo CM, Zuanon J, Caramaschi EP (2015) Microhabitat segregation and fine ecomorphological dissimilarity between two closely phylogenetically related grazer fishes in an Atlantic Forest stream, Brazil. Environ Biol Fish 98:2009–2019. https://doi.org/10.1007/s10641-015-0423-3
Leite GF, Silva FTC, Gonçalves JFJ, Salles P (2015) Effects of conservation status of the riparian vegetation on fish assemblage structure in neotropical headwater streams. Hydrobiologia 762:223–238. https://doi.org/10.1007/s10750-015-2351-9
Lobón-Cerviá J, Mazzoni R, Rezende CF (2016) Effects of riparian forest removal on the trophic dynamics of a Neotropical stream fish assemblage. J Fish Biol 89:50–64. https://doi.org/10.1111/jfb.12973
Lopes VG, Nessimian JL, Da-Silva ER, Gomes JHC, Dias ACI, Souza LC, Branco CW (2016) Habitat heterogeneity on feeding habit of two sympatric and congeneric characidae fishes in two tropical reservoirs. Iheringia Sér Zool 106:e2016012. https://doi.org/10.1590/1678-4766e2016012
Luz-Agostinho KD, Bini LM, Fugi R, Agostinho AA, Júlio HF Jr (2006) Food spectrum and trophic structure of the ichthyofauna of Corumbá reservoir, Paraná river basin, Brazil. Neotrop Ichthyol 4:61–68. https://doi.org/10.1590/S1679-62252006000100005
MacArthur RH, MacArthur JW (1961) On bird species diversity. Ecology 42:594–598. https://doi.org/10.2307/1932254
Marrero C (1994) Métodos para Cuantificar Contenidos Estomacales en Peces. Talleres Gráficos de Liberil, Caracas
Mazzoni R, Marques PS, Rezende CF, Iglesias-Rios R (2012) Niche enlargement as a consequence of coexistence: a case study. Braz J Biol 72:267–274. https://doi.org/10.1590/S1519-69842012000200006
Mérona D, Santos GM, Almeida RG (2001) Short term effects of Tucuruí dam (Amazônia, Brazil) on the trophic organization of fish communities. Environ Biol Fish 60:375–392. https://doi.org/10.1023/A:1011033025706
Mérona B, Vigouroux R, Horeau V (2003) Changes in food resources and their utilization by fish assemblages in a large tropical reservoir in South America (petit-Saut dam, French Guiana). Acta Oecol 24:147–156. https://doi.org/10.1016/S1146-609X(03)00065-1
Mise FT, Fugi R, Pagotto JPA, Goulart E (2013a) The coexistence of endemic species of Astyanax (Teleotei: Characidae) is propitiated by ecomorphological and trophic variations. Biota Neotrop 13:21–28. https://doi.org/10.1590/S1676-06032013000300001
Mise FT, Tencatt LFC, Souza F (2013b) Ecomorphological differences between Rhamdia (Bleeker, 1858) populations from the Iguaçu River basin. Biota Neotrop 13:99–104. https://doi.org/10.1590/S1676-06032013000400010
Mugnai R, Nessimian JL, Baptista DF (2010) Manual de identificação de macroinvertebrados aquáticos do Estado do Rio de Janeiro. Technical Boocks, Rio de Janeiro
Neves MP, Delariva RL, Wolff LL (2015) Diet and ecomorphological relationships of an endemic, species-poor fish assemblage in a stream in the Iguaçu National Park. Neotrop Ichthyol 13:245–254. https://doi.org/10.1590/1982-0224-20140124
Neves MP, Da Silva JC, Baumgartner D, Baumgartner G, Delariva RL (2018) Is resource partitioning the key? The role of intra-interspecific variation in coexistence among five small endemic fish species (Characidae) in subtropical rivers. J Fish Biol 93:238–249. https://doi.org/10.1111/jfb.13662
Novakowski GS, Hahn NS, Fugi R (2008) Diet seasonality and food overlap of the fish assemblage in a pantanal pond. Neotrop Ichthyol 6:567–576. https://doi.org/10.1590/S1679-62252008000400004
Oksanen J, Kindt R, Legendre P, O'Hara B, Stevens MHH, Oksanen M, Suggests M (2007) The vegan package. Community Ecology Package. R package version 2.4–2. Retrieved from www.CRAN.R-project.org/package=vegan
Oliveira EF, Goulart E, Breda L, Minte-Vera CV, Paiva LRS, Vismara MR (2010) Ecomorphological patterns of the fish assemblage in a tropical floodplain: effects of trophic, spatial and phylogenetic structures. Neotrop Ichthyol 8:569–586. https://doi.org/10.1590/S1679-62252010000300002
Pagotto JPA, Goulart E, Oliveira EF, Yamamura CB (2011) Trophic ecomorphology of Siluriformes (Pisces, Osteichthyes) from a tropical stream. Braz J Biol 71:469–479. https://doi.org/10.1590/S1519-69842011000300017
Pankhurst NW (1989) The relationship of ocular morphology to feeding modes and activity periods in shallow marine teleosts from New Zealand. Environ Biol Fishes 26(3):201–211
Pianka ER (1973) The structure of lizard communities. Annu Rev Ecol Syst 4:53–74. https://doi.org/10.1146/annurev.es.04.110173.000413
Pinto TLF, Uieda VS (2007) Aquatic insects selected as food for fishes of a tropical stream: are there spatial and seasonal differences in their selectivity? Acta Limnol Bras 19:67–78
Portella T, Lobón-Cerviá J, Manna LR, Bergallo HG, Mazzoni R (2016) Eco-morphological attributes and feeding habits in coexisting characins. J Fish Biol 90:129–146. https://doi.org/10.1111/jfb.13162
Pouilly M, Lino F, Bretenoux JG, Rosales C (2003) Dietary-morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. J Fish Biol 62:1137–1158. https://doi.org/10.1046/j.1095-8649.2003.00108.x
Pouilly M, Barrera S, Rosales C (2006) Changes of taxonomic and trophic structure of sh assemblages along an environmental gradient in the upper Beni watershed (Bolivia). J Fish Biol 68:137–156. https://doi.org/10.1111/j.0022-1112.2006.00883.x
Prado AV, Goulart E, Pagotto J (2016) Ecomorphology and use of food resources: inter-and intraspecific relationships of fish fauna associated with macrophyte stands. Neotrop Ichthyol 14:e150140. https://doi.org/10.1590/1982-0224-20150140
Prejs A, Prejs K (1987) Feeding of tropical freshwater fishes: seasonality in resource availability and resource use. Oecologia 71:397–404. https://doi.org/10.1007/BF00378713
Pusey BJ, Arthington AH (2003) Importance of the riparian zone to the conservation and management of freshwater fish: a review. Mar Freshw Res 54:1–16. https://doi.org/10.1071/MF02041
Quirino BA, Carniatto N, Gaiotto JV, Fugi R (2015) Seasonal variation in the use of food resources by small fishes inhabiting the littoral zone in a Neotropical floodplain lake. Aquat Ecol 49:431–440. https://doi.org/10.1007/s10452-015-9535-2
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL http://www.R-project.org/. Accessed 10 Aug 2015
Ríos SL, Bailey RC (2006) Relationship between riparian vege- tation and stream benthic communities at three spatial scales. Hydrobiologia 553:153–160. https://doi.org/10.1007/s10750-005-0868-z
Rondineli GR, Carmassi AL, Braga FMS (2009) Population biology of Trichomycterus sp. (Siluriformes, Trichomycteridae) in Passa Cinco stream, Corumbataí River sub-basin, São Paulo state, southeastern Brazil. Braz J Biol 69:925–934. https://doi.org/10.1590/S1519-69842009000400022
Ross ST (1986) Resource partitioning in fsh assemblages: a review of feld studies. Copeia 1986:352–388. https://doi.org/10.2307/1444996
Ruaro R, Gubiani EA, Cunico AM, Moretto YP, Pitágoras A (2016) Comparison of fish and macroinvertebrates as bioindicators of Neotropical streams. Environ Monit Assess 188:235–255. https://doi.org/10.1007/s10661-015-5046-9
Russo MR, Ferreira A, Dias RM (2002) Disponibilidade de invertebrados aquáticos para peixes bentófagos de dois riachos da bacia do rio Iguaçu, Estado do Paraná, Brasil. Acta Sci Anim Sci 24:411–417. https://doi.org/10.4025/actascibiolsci.v24i0.2313
Sabino J, Castro RMC (1990) Alimentação, período de atividade e distribuição espacial dos peixes de um riacho da floresta Atlântica (Sudeste do Brasil). Rev Bras Biol 50:23–36
Sampaio ALA, Pagotto JPA, Goulart E (2013) Relationships between morphology, diet and spatial distribution: testing the effects of intra and interspecifc morphological variations on the patterns of resource use in two Neotropical cichlids. Neotrop Ichthyol 11:351–360. https://doi.org/10.1590/S1679-62252013005000001
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39. https://doi.org/10.1126/science.185.4145.27
Schoener TW (1982) The controversy over interspecifc competition: despite spirited criticism, competition continues to occupy a major domain in ecological thought. Am Nat 70:586–595. http://www.jstor.org/stable/27851730. Accessed 15 Sept 2015
Silva JC, Gubiani EA, Delariva RL (2014) Use of food resources by small fish species in Neotropical rivers: responses to spatial and temporal variations. Zoologia (Curitiba) 3:435–444. https://doi.org/10.1590/S1984-46702014000500004
Silva JC, Gubiani ÉA, Neves MP, Delariva RL (2017) Coexisting small fish species in lotic neotropical environments: evidence of trophic niche differentiation. Aquat Ecol 51:275–288. https://doi.org/10.1007/s10452-017-9616-5
Smith B, Wilson JB (1996) A consumer’s guide to evenness indices. Oikos 76:70–82
Teixeira I, Bennemann ST (2007) Ecomorfologia refletindo a dieta dos peixes em um reservatório no sul do Brasil. Biota Neotrop 7:67–76
Uieda VS, Alves MIB, Silva EI (2016) Invertebrados bentônicos: relação entre estrutura da fauna e características do mesohabitat/benthic invertebrates: relationship between the fauna structure and mesohabitat features. Rev Ambient Água 11:676–688
Vitt LJ, Zani PA, Lima ACM (1997) Heliotherms in tropical rain forest: the ecology of Kentropyx calcarata (Teiidae) and Mabuya nigropunctata (Scincidae) in the Curua-Una of Brazil. J Trop Ecol 13:22. https://doi.org/10.1017/S0266467400010415
Wainwright PC, Reilly SM (1994) Ecological morphology: integrative organismal biology. University of Chicago Press, Chicago
Watson DJ, Balon EK (1984) Ecomorphological analysis of fish taxocenes in rainforest streams of northern Borneo. J Fish Biol 25:371–384. https://doi.org/10.1111/j.1095-8649.1984.tb04885.x
Willis SC, Winemiller KO, Lopez-Fernadez H (2005) Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142:284–295. https://doi.org/10.1007/s00442-004-1723-z
Winemiller KO (1991) Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol Monogr 61:343–365. https://doi.org/10.2307/2937046
Wolff LL, Carniatto N, Hahn NS (2013) Longitudinal use of feeding resources and distribution of fish trophic guilds in a coastal Atlantic stream, southern Brazil. Neotrop Ichthyol 11:375–386. https://doi.org/10.1590/S1679-62252013005000005
Wootton RJ (1998) The ecology of teleost fishes. Kluwer Academic Publisher, Dordrecht
Zupo V, Alexander TJ, Edgar GJ (2017) Relating trophic resources to community structure: a predictive index of food availability. R Soc Open Sci 4:160515. https://doi.org/10.1098/rsos.160515
Acknowledgements
We thank the Universidade Estadual do Oeste do Paraná (UNIOESTE) for the support needed to perform the analyses, Fundação Araucária for granting a scientific-initiation scholarship to the first author, and members of the LIEB (Laboratory of Ichthyology, Ecology and Biomonitoring) at UNIOESTE for helping with field collections and the processing of parts of the studied materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Fish were collected with authorization from the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (license numbers 30,182 and 25,039–1). All sampling and handling procedures were approved by the Ethics Committee on Animal Use of the Universidade Estadual do Oeste do Paraná (CEUA) in accordance with their protocols for the ethical and methodological use of fish (project approved on February 11, 2014).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 49 kb)
Rights and permissions
About this article
Cite this article
Baldasso, M.C., Wolff, L.L., Neves, M.P. et al. Ecomorphological variations and food supply drive trophic relationships in the fish fauna of a pristine neotropical stream. Environ Biol Fish 102, 783–800 (2019). https://doi.org/10.1007/s10641-019-00871-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-019-00871-w