Abstract
A priority issue in ecology and biogeography is understanding the patterns in species diversity and the causal factors of their distribution, which allows the generation of information for conservation strategies. The longitudinal distribution of fishes and their relationships with environmental variables were studied in the Guayalejo-Río Tamesí system (northeastern Mexico) from February 2000 to July 2001. A total of 5918 fish were caught in 27 collections along an altitudinal gradient in the main river course, from high mountain (1500 masl) to coastal plain near Tampico. Forty-three native and five exotic species, belonging to 35 genera in 23 families, were identified. Cluster analyses identified four major fish habitats in the river system. A distinctive euryhaline marine fish habitat (1) occurs near the mouth with native and two exotic species. Two other habitats consist essentially of freshwater fish species that are distributed along the longitudinal gradient. One of these habitats (habitat 4) shows greater diversity, as per the Shannon index value, and also includes amphidromous fish, in addition to two exotic freshwater fish; the other (habitat 2) includes freshwater, euryhaline and three exotic species. The changes in the frequency of occurrence and the abundance of Gambusia vittata, Astyanax mexicanus, and Xiphophorus variatus contribute to explaining differences between these habitats. Another habitat (3) is represented by two sampling sites located near the mouth and consist of freshwater and euryhaline fish and three exotic cyprinids with broad salinity tolerance. The low abundance and richness of exotic species suggest little impact on native fish fauna in this river. The fish assemblage of the Guayalejo-Tamesí river system species changes along a longitudinal gradient with the addition, replacement and presence of indicator species. Upstream fish fauna is mostly composed of freshwater species, some of them generalists that inhabit the entire longitudinal gradient, others that are restricted to certain sites, and the remainder of species is an assemblage composed of a mixture of euryhaline freshwater and marine species near the mouth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mechanisms that explain changes in fish assemblage along a river have been well studied in both temperate and tropical rivers (Winemiller and Leslie 1992; Trujillo-Jiménez et al. 2002; Ibañez et al. 2009: Bhatt et al. 2012; Mercado-Silva et al. 2012; Mejía-Mojica et al. 2014; Carvajal-Quintero et al. 2015; Chea et al. 2016; Askeyev et al. 2017). Two hypotheses have been proposed to explain such changes: one that alludes to the concept of “biozonation”, and another that places emphasis on the addition of species. Both share the idea that environmental heterogeneity along the river is the main factor that drives changes in fish assemblage (Petry and Schulz 2006). As a result, the river continuum concept (sensu Vannote et al. 1980) was incorporated to explain the longitudinal changes in fish assemblages on temperate pristine rivers and streams, but have been strongly questioned (Statzner and Higler 1985; Miranda and Raborn 2000). However, this concept inspired theoretical and practical research in streams and rivers around the world (Statzner and Higler 1985; Ibáñez et al. 2011). Previous work on freshwater fish assemblages reported diverse relationships between species diversity and the longitudinal gradient, such as inverse relationships (Jaramillo-Villa et al. 2010), unimodal (optimal in mid-elevations) or nonlinear (without correlation) patterns (Rahel and Hubert 1991; Grenouillet et al. 2004; Li et al. 2009; Askeyev et al. 2017), and even an increase in species diversity in high altitude zones (Carvajal-Quintero et al. 2015). Nonetheless, a case of ecological convergence has recently been found between species richness (assemblage) in four continents with comparable environmental conditions (i.e., assemblage position in the stream’s longitudinal continuum, Ibáñez et al. 2011). It should be obvious that species richness in a river or stream depends on historical events (Ricklefs and Schluter 1993), but also on the constraints that environmental factors exert on them. For example, structural features of the rivers such as the width and depth of the channel, the diversity of the substrate, the order of the currents, etc., as well as biotic relationships such as predation, competition and disease, have been associated with different fish assemblage patterns (Matthews 1998; Tejerina-Garro et al. 2005). In the rivers of northeastern Mexico, such studies are rare, and it is important to know the changes in fish assemblage along the river due to human activity.
The Río Pánuco is located between the Sierra Madre Oriental and the Gulf of Mexico in the Tamaulipean ecoregion of northeastern Mexico (Contreras-Balderas 1969; CONABIO 2000). The main tributary of this river is the Rio Guayalejo-Tamesí system (hereinafter referred to as the river system), which drains an area of 15,257 km2 (INEGI 1983). Darnell (1962) studied the fish community structure of this system; his inspection provided the first extensive inventory (60 species; 23 freshwater and 37 peripheral, of which 12 were exclusive or endemic). However, since this first inspection, no comprehensive studies are available on the fish along the river or their relationship with environmental variables. The basic knowledge of species composition contrasts with the deterioration of habitats, especially in the middle and lower parts of the basin (García-De León et al. 2005). Changes in water flow and the introduction of exotic species pose major threats to native fish assemblages (Hughes et al. 2005; Hermoso et al. 2011; Mendoza et al. 2014). Our study provides updated baseline information that may be useful for future conservation of fish fauna, land use planning and holistic watershed management. We are interested in knowing how the fish assemblage is determined by environmental factors along the channels’ longitudinal gradient and report the new species introduced in the system after Darnell’s (1962) inventory.
Material and methods
Study area
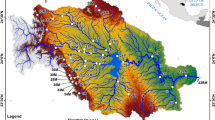
The Río Guayalejo-Río Tamesí system is located in east-central Mexico, in the northern part of the Tampico embayment. In the south, this embayment is drained by the lower section, the Río Pánuco, where these two branches join (22°13′N, 97°51′W) before entering the Gulf of Mexico (INEGI 1983). The northernmost tributary, the Río Guayalejo, originates in the Sierra Madre Oriental at 3400 m. The Sabinas, Frío, Boquilla, and Mante rivers join to form the Río Tamesí, which assumes a meandering course that borders the states of Tamaulipas and Veracruz (Fig. 1). Tributary rivers start as springs, large ponds or wetlands in shallow valleys in the foot-slopes of the Sierra Madre Oriental.
Sites along the Río Guayalejo-Río Tamesí system. See Table 1 for codes
Fish collection
Fish were collected in the river system from February 2000 through July 2001. The study area was divided into three elevation zones: mountain and high elevations (200–1500 m), plains and low elevations (50–200 m), and river mouth (0–50 m). The sampling sites are described in Table 1. Ejido Aldama, located ~60 km north of the main course of the river system, was included because it does not belong to any of the major basins of Tamaulipas. This study was conducted at the Technological Institute of Ciudad Victoria in Tamaulipas, where despite the lack of any animal care protocol, the animals were all collected under standard care procedures.
To collect fish, a combination of fishing gears was used, following standard fish-collecting procedures (Sooley et al. 1998). In the mountain zone, fish were collected with minnow seines, and supported with electrofishing equipment for an average collecting time of 30 min for each sample. In the plains zone, we used electrofishing gear, a minnow seine, and experimental gillnets 180 m long and consisting of eight panels, each 23 m long with 1, 1.5, 2, 2.5, 3, 3.5, 4, and 5-in. meshes. At these sites, the experimental gillnets remained in place for 12 h. In the mouth zone, where the channels are deeper, we used a trammel net (180 m long with a 3-in. internal mesh) and the experimental gillnets described above, both of which were in place for 12 h. The elevation and coordinates of each site were measured with a GPS unit.
The fish were anesthetized and subsequently fixed in 10% formalin neutralized with sodium borate and then preserved in 50% isopropanol solution. The identification of each species was based on Álvarez del Villar (1969), Castro-Aguirre et al. (1999), Page and Burr (1991), McEachran and Fechhelm (1998), and Miller et al. (2005), as well as specific literature for some taxa.
Alkalinity, pH, dissolved carbon dioxide, hardness (amount of dissolved calcium and magnesium in the water, mg/l, Wetzel 2001), transparency, total dissolved solids, and temperature were measured at each site; conductivity (μS/cm) and dissolved oxygen (mg/l) were measured with a conductivity meter and an oxygen meter, respectively. Salinity (ppt) was measured with a refractometer. Water samples collected in each site were fixed immediately in order to determine the quantity of nutrients (ppm) as nitrate, nitrite, and phosphate, all of which were measured with a spectrophotometer (SMART model, LaMotte, Chestertown, MD). To characterize environmental heterogeneity at each site, we considered 6 quantitative and 17 qualitative variables (Moyle and Nichols 1973; Table S1).
Statistical analysis
To describe fish habitats, the presence-absence of each species was recorded (Table S2) and used to quantify the similarity in species composition between sampling sites, using an agglomerative hierarchical two-way cluster analysis. For this analysis, we determined the Euclidian distances between sampling sites and a complete linkage-clustering algorithm, using PRIMER6 software (Clarke 1993; Clarke and Warwick 2001, 2005). The significance of the clusters was tested by similarity profile analysis (SIMPROF, PRIMER6). To detect significant differences in the dissimilarity values between fish habitats, we applied a one-way analysis of similarity of the significance of the habitats defined a priori (ANOSIM). The average similarity and percentage of contribution of each species to the identity of each habitat were determined using the similarity percentage routine (SIMPER) in PRIMER 6 (Clarke and Warwick 2001).
A canonical correspondence analysis (CCA) was performed to define the relationships between each type of habitat and environmental variables. The environmental matrix contained the average of the physicochemical parameters and qualitative variables of the environment that had the highest value in explaining the variables at the 95% confidence level, in an initial CCA (Table 2a). These variables were the maximum depth of the channel, the percentage of water riffles, hardness, the average depth, the percentage of backwater, channel width, and the percentage of floating macrophytes. Diversity (Shannon index), dominance, and evenness of species in each habitat were calculated using PAST 2.07 diversity software (Hammer et al. 2001).
Results
We collected 5918 fish belonging to 23 families, 35 genera, and 48 species, taken at 27 collection sites in the study area (Tables 1 and S2). Of 48 species, 43 were native and five were exotic (Cyprinus carpio, Ctenopharyngodon idella, Hypophthalmichthys molitrix, Micropterus salmoides, and Oreochromis aureus). Exotic species were only present in some places of lower elevation (Micropterus salmoides and Oreochromis aureus) and in virtually all the sites of the mouth region (five species). Exotic species were more numerous in the mouth area, but none was important in terms of abundance with respect to native species (Fig. 2). Globally, four species accounted for 75.9% of abundance (Astyanax mexicanus, Gambusia vittata, Poecilia mexicana, and Herichthys labridens, Table S2). Most river species were restricted in distribution along the longitudinal gradient; 43 species were found at fewer than six sites. Five species were found at many locations along the river: Astyanax mexicanus (24 sites), Poecilia mexicana (19 sites), Herichthys labridens (16 sites), Gambusia vittata (11 sites), and Poecilia formosa (11 sites) (Table S2).
Fish habitats
The dendrogram of fish species similarity defined four fish habitats (groups of sampling sites) with a separation of 3.6% distance (Fig. 3). These groupings were statistically significant (ANOSIM: R = 0.519, P < 0.05); all pairwise comparisons were also statistically significant (Table S3).
Habitat 1 (marine habitat) was mainly represented by marine species and was the most distinct. It included one sampling location (Fig. 3; Tables 1 and 3) of high salinity (Table S4). Habitat 2 (mountain and plain habitat) included eight sites in the mountain zone, six sites in the plains zone, and one site in the mouth zone, and contained mainly freshwater and peripheral species (Fig. 3; Tables 1 and 3). Habitat 3 (close to the mouth) included a few freshwater species and a number of peripheral species at two sites close to the mouth (Fig. 3; Tables 1 and 3), and habitat 4 (plain and mountain habitat) was similar to habitat 2, containing five sites in the plains, three at the mouth, and one in the mountain zone, and was essentially characterized by freshwater fish (Fig. 3; Tables 1 and 3).
Since habitat 1 was characterized by high salinity and dominated by marine species, and some of them (euryhaline species) were distributed upstream, it was not included in the CCA. The CCA had three components that accounted for 54.2% of the total variation, considering seven assessed environmental variables (Table 2b). Variation in hardness and percentage of riffles were positively correlated, and channel (width and depth) with percentage of backwater were negative on the first axis. This axis shows no separation between the three habitats (Fig. 4).
On the second axis, the percentage of floating macrophytes was positively correlated, while maximum depth and average depth were negatively correlated (Fig. 4). This axis shows a better separation between the sites of habitat 4 (correlated with a greater presence of macrophytes) and those of habitat 2, which show greater correlation with maximum depth. Sites in habitat 2 show a higher average maximum depth (2.9 m) in relation to habitat 4 (average 1.27 m). Habitat 3 had sites with broader channels and high percentages of backwaters.
The highest diversity (average Shannon index; H′ = 2.19) and the lowest dominance (average D = 0.17) and evenness (average E = 0.47) were found at sites near the mouth of the river (Table 4); habitat 1 at Puente Moralillo, Table 1). In contrast, the highest dominance (average D = 0. 58) and evenness (average E = 0.58), and the lowest diversity (average H′ = 0.82), were recorded in habitat 2 at higher elevations (average 349 m), where Gambusia vittata and Astyanax mexicanus were the most abundant species (Table 4).
Discussion
Darnell’s (1962) ground-breaking study of fishes from Río Guayalejo and its tributaries was carried out by field collections and complemented with previous records from Jordan and Dickerson (1908). However, Darnell’s study did not assess the component of brackish and marine fishes entering coastal lagoons and near river mouths, or their seasonal variation. Furthermore, Darnell lacked taxonomic revisions for some freshwater fish taxa, mainly in the families Cyprinidae and Poeciliidae. All these reasons account for the differences observed between our study and that of Darnell. Indeed, one notorious change is the occurrence of exotic species in the system (Table S5). The higher abundance and richness of exotic species occurred in sections of the river near the mouth with warmer waters and greater maximum depths and channel width (Table S2). It is important to mention that Darnell (1962) did not record the presence of exotic fish species in the study area due to the high levels of introduction of exotic fishes in Mexico during the 1970s and 1980s (Contreras-Balderas and Escalante 1984; Contreras-Balderas et al. 2004, 2008). We believe that exotic species may have been introduced in the river system in the 1970s, which means that these species have already been interacting with native species for at least 30 years – long enough to disperse throughout the system. However, invasion of the whole river system did not seem to have occurred, at least during sampling. At higher elevations, no exotic species was observed. It is possible that species of tropical origin, such as tilapia, show low yields in northern areas of Mexico with low temperature during winter (Kapetsky 1997), which limits the breeding activity of this African cichlid fish.
Fish habitats and their relationship with environmental factors
Cluster analyses identified four major fish habitats in the river basin. A very different euryhaline marine fish habitat (habitat 1) occurs downstream near the mouth of the river with interchange of flows between the sea and river. Three species (Elops saurus, Bairdiella chrysoura and Brevoortia gunteri) distinguish this type of habitat located in Puente Moralillo, all of which are of euryhaline marine origin and penetrate to coastal lagoons or rivers with sandy bottoms to feed and reproduce (Table S6). Elops saurus and Bairdiella chrysoura feed on small fish and crustaceans, and Brevoortia gunteri feeds on plankton. This habitat also contains two exotic cyprinids in low abundance (Cyprinus carpio and Hypophthalmichthys molitrix). These are related to benthos and are tolerant, over brief periods, to backwaters and low concentrations of oxygen. The former is omnivore and the latter feeds on phytoplankton and zooplankton (Freyhof and Kottelat 2008; Zhao 2011).
Two other habitats (2 and 4) consist essentially of freshwater fish that are distributed along the longitudinal gradient of the channel, but are distinguished by the greater or lesser percentage of floating macrophytes, and the depth of the channel (Fig. 4). Both habitats include generalist species (Astyanax mexicanus, Poecilia mexicana and Herichthys sp.) of neotropical origin as well as species typical to all Atlantic basins, with a high dispersion capacity (Miller and Smith 1986; Miller et al. 2005). This last fish species inhabits practically all the environments along the longitudinal gradient of the river; other species such as Gambusia vitatta show more restricted distribution upstream, in an area characterized by high levels of hardness and presence of riffles or Xiphophorus variatus confined to shallower environments with floating macrophytes (Fig. S1). However, downstream, habitat 4 includes amphidromous species, in which adults are strictly freshwater (Agonostomus monticola and Gobiomorus dormitor, McDowall 1997; Ribeiro and Villalobos 2010), and exotic species with low abundance (Micropterus salmoides and Oreochromis aureus), while habitat 2 has a clear influence of euryhaline marine fish (Dorosoma petenense and Megalops atlanticus) (Table S6). The frequency of occurrence and abundance of some species (Gambusia vittata, Astyanax mexicanus and Xiphophorus variatus) also contributed to the differences between the two habitats (Table 5). Habitat 3 is also composed of generalist freshwater species (Astyanax mexicanus), but shows a large proportion of euryhaline marine species (Dorosoma petenenese, Ariopsis felis, Centropomus undecimalis, and Gobiomorus dormitor) and three exotic cyprinids in sites close to the mouth of the river (Cyprinus carpio, Ctenopharyngodon idella, and Hypophthalmichthys molitrix) with tolerance to the salinities of the estuarine environment (Cudmore and Mandrak 2004; Freyhof and Kottelat 2008; Zhao 2011); however, the habitat also exhibits low species richness and abundance.
Various environmental, geographic and topographic features are often described as determinants of patterns of species richness along longitudinal gradients (Ricklefs and Schluter 1993; Matthews 1998; Tejerina-Garro et al. 2005). How these factors impact the richness of species has been debated (Benke et al. 2011). Our study, the fish species diversity decreases with high elevations, a pattern commonly reported by other authors (Edwards and Contreras-Balderas 1991; Jaramillo-Villa et al. 2010). In order to understand this, we evaluated abiotic factors and found that factors on an intra-basin scale such as salinity, depth, percentage of riffles and backwaters, hardness, channel width and percentage of floating macrophytes could prevent colonization and limit upstream and downstream dispersion, which could act as ecological barriers or filters (Contreras-Balderas 1969; Ruiz-Campos et al. 1985; Contreras-Balderas et al. 2002). For example, Astyanax mexicanus and Herichthys cyanoguttatus are generalist species with dietary habits related to the availability and diversity of food in the particular habitat (Miller 1966; Mitchell et al. 1977). These characteristics allowed them to have an extensive distribution and high abundance, in particular for Astyanax mexicanus, what could be interpreted as species with high tolerance to different environmental conditions, while other species with less environmental tolerance, such as Gambusia affinis and G. regani, are restricted to lenthic biotopes or weedy backwater conditions with virtually no current (Darnell 1962; Miller et al. 2005, Table S6). In addition, many freshwater fish species are bentho-pelagic but have different food requirements; for example, the most representative species of habitat 2, Astyanax mexicanus, feeds on insects, crustaceans and worms; others, such as Gambusia vitatta and Herichthys labridens, are omnivores, and Poecilia mexicana feeds mainly on detritus; this feeding partitioning exhibited by all these species enables them to avoid competition and allows stability in the fish community along the river (Miller and Smith 1986; Tejerina-Garro et al. 2005; Hoeinghaus et al. 2007; Winemiller et al. 2008). The same situation occurs in species of marine origin that enter the river for feeding (Table S6).
In summary, the structure of the fish community of the Guayalejo-Tamesí river system is represented by one upstream fauna composed of mostly freshwater species – some of them generalists that inhabit the entire longitudinal gradient, and others that inhabit more restricted areas – and a downstream assemblage composed of a mixture of the more abundant upstream elements and species of marine origin, both of which are tolerant to brackish water near the mouth. The low abundance of exotic species and the richness of upstream and downstream freshwater species may indicate few changes in this type of rivers in the region (Ruiz-Campos et al. 1985; Edwards and Contreras-Balderas 1991).
The river continuum concept establishes that physical conditions of streams or rivers from upstream to downstream areas create strong constraints on assemblage structure linked to food availability (Vannote et al. 1980). However, the original concept was modified to make it flexible and more applicable (Statzner and Higler 1985). One modification that emerged with studies of modern ecology is that physical connectivity may account for differences in freshwater assemblages between sites (Miranda and Raborn 2000). Recently, Ibañez et al. (2009) provided evidence, from the comparative study of headwater streams in four continents, that the richness of fish assemblage and trophic structure converged along the continuous river to a substantial degree. The Guayalejo-Tamesí river system seems to be consistent with this principle, in particular with regard to species addition and replacement and the occurrence of indicator species along a longitudinal gradient. These findings have been reported by other studies (Grenouillet et al. 2004; Petry and Schulz 2006; Grosmann et al. 2010; Bhatt et al. 2012; Carvajal-Quintero et al. 2015; Chea et al. 2016; Silva et al. 2016; Askeyev et al. 2017).
In terms of conservation, the least disturbed area is the upper part of the river system (habitats 2 and 3); however, without proper regulation and enforcement, tourism and mining could seriously affect fish diversity (García-De León et al. 2005). In the plains and mouth (habitats 1, part of 2, and 3), fish diversity has been heavily affected by several dams, exotic species, pollution with agricultural fertilizers and pesticides, ranching, siltation, and pollution associated with petroleum operations (García-De León et al. 2005; Rodríguez Rodríguez et al. 2012).
The Mexican government continues to deliver programs aimed at introducing exotic fish (Ibáñez et al. 2011). According to our results, exotic species might have a lower impact; however, the effects of exotic species on freshwater ecosystems are widely known (Moyle and Nichols 1973; Contreras-Balderas and Escalante 1984; Mendoza et al. 2014), so it is advisable to continue to monitor the system, since other factors may emerge with long-term ecological data (Grosmann et al. 2010). Unfortunately, at both the federal and state level, the programs that exist in this region for conserving natural resources and maintaining the biodiversity of fish are practically nonexistent (Gobierno de Tamaulipas 2011). Extensive work on the part of the Mexican government is necessary to promote comprehensive strategies in using and managing aquatic resources. This should begin with establishing a baseline of information on the biological diversity and environmental services provided by balanced riverine ecosystems.
References
Álvarez del Villar J (1969) Claves para Peces Mexicanos. I.N.I.B.P. Serv Invest Pesq Est 1:1–156
Askeyev A, Askeyev O, Yanybaev N, Askeyev I, Monakhov S, Marić S, Hulsman K (2017) River fish assemblages along an elevation gradient in the eastern extremity of Europe. Environ Biol Fish 100:585–596
Benke M, Brandle M, Albrecht C, Wilke T (2011) Patterns of freshwater biodiversity in Europe: lessons from the spring snail genus Bythinella. J Biogeogr 38:2021–2032
Bhatt JP, Manish K, Pandit MK (2012) Elevational gradients in fish diversity in the Himalaya: water discharge is the key driver of distribution patterns. PLOSOne 7:e46237
Carvajal-Quintero JD, Escobar F, Alvarado F, Villa-Navarro FA, Jaramillo-Villa Ú, Maldonado-Ocampo JA (2015) Variation in freshwater fish assemblages along a regional elevation gradient in the northern Andes, Colombia. Ecol Evol 5(13):2608–2620
Castro-Aguirre J, Espinosa Pérez HS, Schmitter Soto JJ (1999) Ictiofauna estuarino-lagunar y vicaria de México. Editorial Limusa, México, D.F. 711 p
Chea R, Lek1 S, Ngor P, Grenouillet G (2016) Large-scale patterns of fish diversity and assemblage structure in the longest tropical river in Asia. Ecol Freshw Fish. https://doi.org/10.1111/eff.12301
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analyses and interpretation. PRIMER-E, Plymouth, UK
Clarke KR, Warwick RM (2005) PRIMER-6 computer program. Natural Environment Research Council, Plymouth, UK
CONABIO (2000) Estrategia Nacional sobre la Biodiversidad de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City
Contreras-Balderas S (1969) Perspectivas de la ictiofauna en las zonas áridas de México. In: Box TW, Rojas-Mendoza P (eds). Proceedings: International Symposium of Increasing Food Production in Arid Lands. 22–25 April 1968, Monterrey, México. Texas Technological Collage, Lubbock, pp 293–304
Contreras-Balderas S, Escalante CMA (1984) Distribution and known impacts of exotic fishes in Mexico. In: Courtenay WR, Stauffer JR (eds) Distribution, biology and management of exotic fishes. The John Hopkins University Press, Baltimore, pp 102–130
Contreras-Balderas S, Edwards RJ, Lozano-Villano ML, García-Ramírez ME (2002) Fish biodiversity changes in the lower Rio Grande/Rio Bravo, 1953-1996. Rev Fish Biol Fish 12:219–240
Contreras-Balderas S, Lozano-Vilano ML, García Ramírez ME, García-De León FJ, Gutiérrez-Tirado S (2004) Continental fishes and water of Tamaulipas state, Mexico. Pp. 283-298. In: Lozano-Vilano ML, Contreras-Balderas AJ (eds) Homenaje al doctor Andrés Reséndez Medina. Universidad Autónoma de Nuevo León, México
Contreras-Balderas S, Ruiz-Campos G, Schmitter-Soto JJ, Díaz-Pardo E, Contreras-McBeath T, Medina-Soto M, Zambrano-González L, Varela-Romero A, Mendoza-Alfaro R, Ramírez-Martínez C, Leija-Tristán A, Almada-Villela P, Hendrickson DA, Lyons J (2008) Freshwater fishes and water status in México: a country-wide appraisal. Aquat Ecosyst Health Manag 11(3):246–256
Cudmore B, NE Mandrak (2004) Biological synopsis of grass carp (Ctenopharyngodon idella). Can MS Rpt Fish Aquatic Science 2705: v + 44p
Darnell RM (1962) Fishes of the Río Tamesí and related coastal lagoons in east-central México. Pub Inst Mar Sci University of Texas 8:300–361
Edwards JR, Contreras-Balderas S (1991) Historical changes in the ichthyofauna of the lower Rio Grande (Río Bravo del Norte), Texas and Mexico. Southwest Nat 36(2):201–212
Freyhof J, Kottelat M (2008) Cyprinus carpio. The IUCN red list of threatened species 2008: e.T6181A12559362. https://doi.org/10.2305/IUCN.UK.2008.RLTS.T6181A12559362.en. Downloaded on 31 Aug 2017
García-De León FJ, Gutiérrez Tirado D, Hendrickson D, Espinosa Pérez H (2005) Fish of the continental water of Tamaulipas: diversity and conservation status. In: Carton JL, Ceballos G, Felger RS (eds) Biodiversity, ecosystem, and conservation in Northern México. Oxford University Press, pp 138–166
Gobierno de Tamaulipas (2011) Plan Estatal de Desarrollo Tamaulipas 2011–2016. (Government development plan for 2011–2016 Tamaulipas, Mexico.) http://www.cidge.gob.mx/wp-content/uploads/2013/05/TAMAULIPAS.pdf. Accessed 15 Jan 2016
Grenouillet G, Pont D, Hérissé C (2004) Within-basin fish assemblage structure: the relative influence of habitat versus stream spatial position on local species richness. Can J Fish Aquat Sci 61:93–102
Grosmann GD, Ratajczak RE, Farr MD, Wagner MC, Petty JT (2010) Why there are fewer fish upstream. In: Gido KB, Jackson DA (eds) Community ecology of stream fishes: concepts, approaches, and techniques. American fisheries society, Bethesda, pp 63–81
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. http://palaeo-electronica.org/2001_1/past/issue1_01.htm Accessed 14 Jan 2016
Hermoso V, Clavero M, Blanco-Garrido F, Prenda J (2011) Invasive species and habitat degradation in Iberian streams: an analysis of their role in freshwater fish diversity loss. Ecol Appl 21(1):175–188
Hoeinghaus DJ, Winemiller KO, Birnbaum JS (2007) Local and regional determinants of stream fish assemblage structure: inferences based on taxonomic vs.functional groups. J Biogeogr 34:324–338
Hughes RM, Rinne JN, Calamusso B (2005) Historical changes in large river fish assemblages of the Americas: a synthesis. Am Fish Soc Symp 45:603–612
Ibañez C, Belliard J, Hughes RM, Irz P, Kamdem-Toham A, Lamouroux N, Tedesco PA, Oberdorff T (2009) Convergence of temperate and tropical stream fish assemblages. Ecography 32:658–670
Ibáñez AL, Espinosa-Pérez H, García-Calderón JL (2011) Recent data on the distribution of the exotic species used in Mexican freshwater fisheries based on fish stocking. Revista Mexicana de Biodiversidad 82:904–914
INEGI (1983) Síntesis geográfica del estado de Tamaulipas. Dirección General de Geografía, Mexico City
Jaramillo-Villa U, Maldonado-Ocampo JA, Escobar F (2010) Altitudinal variation in fish assemblage diversity in streams of the Central Andes of Colombia. J Fish Biol 76:2401–2417
Jordan DS, Dickerson MC (1908) Notes on a collection of fishes from the Gulf of Mexico at Vera Cruz and Tampico. Proc. U. S. Nat Mus 34:11–22
Kapetsky JM (1997) Geography and constrains on inland fishery enhancement. In: Petr T (ed) Inland fishery enhancements fisheries technical paper 374. FAO, Roma, pp 37–63
Li J, He Q, Hua X, Zhou J, Xu H, Chen J, Fu C (2009) Climate and history explain the species richness peak at mid-elevation for Schizothorax fishes (Cypriniformes: Cyprinidae) distributed in the Tibetan Plateau and its adjacent regions. Glob Ecol Biogeogr 18:264–272
Matthews WJ (1998) Patterns in freshwater fish ecology, 2nd edn. Chapman and Hall
McEachran JD, Fechhelm JD (1998) Fishes of The Gulf of Mexico. University of Texas Press, Austin, Vol. II
McDowall RM (1997) The evolution of diadromy in fishes (revisited) and its place in phylogenetic analysis. Rev Fish Biol Fish 7:443–462
Mejía-Mojica H, Contreras-MacBeath T, Ruiz-Campos G (2014) Relationship of environmental and geographic factors and the distribution of exotic fishes in tributaries of the Rio Balsas Basin. Mex Environ Biol Fishes. https://doi.org/10.0007/s10641-014-0298-8
Mendoza R, Ramírez-Martínez C, Aguilera C, Meave del Castillo ME (2014) Principales vías de introducción de las especies exóticas. In: Mendoza R, Koleff P (eds) Especies acuáticas invasoras en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, pp 43–73
Mercado-Silva N, Lyons J, Díaz-Pardo E, Navarrete S, Gutiérrez-Hernández A (2012) Environmental factors associated with fish assemblage patterns in a high gradient river of the Gulf of Mexico slope. Revista Mexicana de Biodiversidad 83:117–128
Miller RR (1966) Geographical distribution of Central American freshwater fishes. Copeia 1966(4):773–802
Miller RR, Smith ML (1986) Origin and geography of the fishes of central Mexico. Pp. 487-518. In: Hocutt CH, Wiley EO (eds) The zoogeography of north American freshwater fishes. John Wiley & Sons, New York
Miller RR, Minckley WL, Norris SM (2005) Freshwater fishes of Mexico. The University of Chicago Press, Chicago
Miranda LE, Raborn SW (2000) From zonation to connectivity: fluvial ecology paradigms of the 20th century. Polskie Archiwum Hydrobiologii 47:5–19
Mitchell RW, Russell WH, Elliott WR (1977) Mexican eyeless characin fishes, genus Astyanax: environment, distribution, and evolution. Texas Tech Press, Lubbock
Moyle PB, Nichols RD (1973) Ecology of some native and introduced fishes of the Sierra Nevada foothills in Central California. Copeia:478–489
Page LM, Burr BM (1991) A field guide to freshwater fishes: north America north of Mexico. Houghton Mifflin Company, Boston
Petry AC, Schulz UH (2006) Longitudinal changes and indicator species of the fishfauna in the subtropical Sinos River, Brazil. J Fish Biol 69:272–290
Rahel FJ, Hubert WA (1991) Fish assemblages and habitat gradients in a rocky mountain-great plains stream: biotic zonation and additive patterns of community change. Trans Am Fish Soc 120:319–332
Ribeiro TC, Villalobos GU (2010) Distribution of Agonostomus monticola and Brycon behreae in the Río Grande de Térraba, Costa Rica and relations with water flow. Neotropical Ichthyology 8:841–849
Ricklefs RE, Schluter D (1993) Species diversity: regional and historical influences. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities: historical and geographic perspectives. The University of Chicago Press, Chicago, pp 350–363
Rodríguez Rodríguez H, García Guevara N, Cantero Medina D, Carreón Pérez A, Andrade Limas EC (2012) Pago por servicios ambientales en la cuenca del Río Guayalejo, Tamaulipas, México. Papeles de Geografía 55-56:167–178
Ruiz-Campos G, Torres-Morales M, Contreras-Balderas S (1985) Peces del Río Álamo, subcuenca del Bravo, México II. Estructura y dinámica de la comunidad íctica. Publicaciones Biológicas, Universidad Autónoma de Nuevo León, Mexico 2:51–75
Silva JC, Gubiania ÉA, Pianaa PA, Delariva RL (2016) Effects of a small natural barrier on the spatial distribution of the fish assemblage in the Verde River, upper Paraná River Basin, Brazil. Braz J Biol 76(4):851–863
Sooley DR, Luiker EA, Barnes MA (1998) Standard methods guide for freshwater fish and fish habitat surveys in Newfoundland and Labrador: rivers & streams. Fisheries and Oceans, St. John’s, NF
Statzner B, Higler B (1985) Questions and comments on the river continuum concept. Can J Fish Aquat Sci 42:1038–1043
Tejerina-Garro FL, Maldonado M, Ibañez C, Pont D, Roset N, Oberdorff T (2005) Effects of natural and anthropogenic environmental changes on riverine fish assemblages: a framework for ecological assessment of rivers. Braz Arch Biol Technol 48:91–108
Trujillo-Jiménez P, López-López E, Díaz-Pardo F, Camargo JA (2002) Patterns in the distribution of fish assemblages in Río Amacuzac, Mexico: influence of abiotic factors and biotic factors. Rev Fish Biol Fish 20:457–469
Vannote RL, MinshallGW CKW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Wetzel R (2001) Limnology: lake and river ecosystems. Third edition. Academic Press, San Diego
Winemiller KO, Leslie MA (1992) Fish assemblages across a complex, tropical freshwater/marine ecotone. Environ Biol Fish 34:29–50
Winemiller KO, Agostinho AA, Pellegrini Caramaschi E (2008) Fish ecology in tropical streams. In: Dudgeon D (ed) Tropical stream ecology pages. Elsevier/Academic Press, San Diego, pp 107–146
Zhao H (2011) Hypophthalmichthys molitrix. The IUCN red list of threatened species 2011: e.T166081A6168056. https://doi.org/10.2305/IUCN.UK.2011-2.RLTS.T166081A6168056.en. Downloaded on 31 Aug 2017
Acknowledgements
We thank Dean A. Hendrickson of the University of Texas in Austin; Héctor Espinosa- Pérez, Leticia Huidobro-Campos, Dalia Angélica Daza-Zepeda, and Xavier Valencia from the National Collection of Fishes at the Universidad Nacional Autónoma de México; and Salvador Contreras-Balderas (in memoriam), María de Lourdes Lozano Vilano and María Elena García-Ramírez from the Fish Collection of the Universidad Autónoma de Nuevo León, for their support in the identification and verification of species. Students at the Laboratory of Integrative Biology at the Instituto Tecnológico de Ciudad Victoria (ITCV) provided help with field collections. ITCV provided their facilities for this study. This study was funded by the CONACYT-SIREYES-19980606034 project awarded to FJGL. Finally, we are grateful to the anonymous reviewers, who helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Table S1
(XLSX 11.3 kb)
Table S2
(XLSX 19.5 kb)
Table S3
(XLSX 13.6 kb)
Table S4
(XLSX 8.82 kb)
Table S5
(XLSX 15.4 kb)
Table S6
(XLSX 14.3 kb)
Figure S1
Canonical correspondence analysis (CCA) showing the effect of the environmental variables on the abundance of fish in the assemblages. The lines and numbers in red indicate the environmental variables (5 = maximum depth, 12 = percentage of riffles, 4 = average depth, 11 = percentage of backwaters, 6 = channel width, and 10 = percentage of floating macrophytes) (TIFF 10313 kb)
Rights and permissions
About this article
Cite this article
García-De León, F.J., Hernández Sandoval, A.I., Contreras-Catala, F. et al. Distribution of fishes in the Río Guayalejo-Río Tamesí system and relationships with environmental factors in northeastern Mexico. Environ Biol Fish 101, 167–180 (2018). https://doi.org/10.1007/s10641-017-0689-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0689-8