Abstract
White muscle has been traditionally used in determining δ 13C and δ 15N signatures in fish but ethics and the current conservation status of many species mean the application of non-lethal sampling procedures is mandatory. In this study we test whether fins and scales can be used as non-lethal alternatives of muscle tissue for stable isotope analysis by comparing their δ 13C and δ 15N signatures, in two native (Barbus haasi, Squalius laietanus) and three introduced fish species (Rutilus rutilus, Lepomis gibbosus and Alburnus alburnus), collected in rivers from north-eastern Spain. Our results showed fins and scales were generally enriched in 13C and depleted in 15 N compared to muscle, and that both tissues were generally a moderate predictor of the isotopic composition of muscle for most species with the exception of Ebro chub (R 2 = 0.59–0.97). This study highlights the importance of determining the isotopic relationship between lethal and non-lethally sampled tissues before making straightforward equivalences, and also pinpoints the need for a more comprehensive understanding of how isotopic discrimination occurs in fins, scales and muscle tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stable isotope analysis of C and N (hereafter δ 13C and δ 15N, respectively) is a common method in studies of animal ecology (Vander Zanden et al. 1999; Post 2002). The predictive enrichment in δ 15N throughout trophic levels (i.e. 3–4 ‰) enables the determination of the animal trophic position, and the similarity in δ 13C between diet and consumer allows the determination of foraging habitat (Vander Zanden et al. 1997). White muscle tissue has been traditionally used in determining the δ 13C and δ 15N signatures in fish due to the low lipid content and its easy homogenization compared to other tissues such as gonads or liver (Pinnegar and Polunin 1999; Jardine et al. 2005; Sinnatamby et al. 2008). Preference for low lipid content tissues is based on 13C depletion in lipids as a result of discrimination against the heavier isotope during lipogenesis (DeNiro and Epstein 1977; Pinnegar and Polunin 1999). However, other tissues obtained by a non-lethal sampling may be equally suitable for stable isotope analysis. Poor knowledge regarding the isotopic similarity between lethally and non-lethally sampled tissues limits the adoption of these non-lethal alternatives for future studies of stable isotopes (Kelly et al. 2006; Fincel et al. 2012; Tronquart et al. 2012). A reduction of lethally sampled tissues currently broadens the scope of many studies, particularly for threatened/endangered species, while reducing the number of animals used in research is also needed according to animal welfare standards (Maceda-Veiga 2013).
Examining how non-lethally sampled tissues compare in isotopic signatures with the muscle tissue is the first step for the progressive replacement of the white muscle in stable isotope analysis (Jardine et al. 2005; Blanco et al. 2009; Tronquart et al. 2012). Non-lethally sampled tissues, such as fins and scales, arise as potential non-lethal alternatives for muscle in δ13C and δ15N isotopes in fish with some additional advantages such as easy preservation, transportation and even low lipid content (Grey et al. 2009; Inamura et al. 2012). In addition, fins and scales are typically the target tissues in studies of genetics and ageing in high valuable fish species (Metcalf and Swearer 2005), suggesting that fish health is not affected seriously by fin clipping and the removal of some scales. For stable isotope analysis, fins and muscle tissues are reportedly to have the most similar isotopic signatures but making straightforward equivalences is generally discouraged (Hobson and Clark 1992; Kelly et al. 2006; Blanco et al. 2009). The isotopic values of non-lethally sampled tissues could be equivalent to those of muscle by using correction factors (e.g., constant enrichment or depletion in a given isotopic signature) (Blanco et al. 2009; Fincel et al. 2012). However, such correction factors are unlikely to be of widespread use because the isotopic composition of fish tissues can differ between species, populations and sampling sites (Kelly et al. 2006; Fincel et al. 2012; Willis et al. 2013).
The present study aimed to test whether fins and scales of two native (Barbus haasi, Squalius laietanus), and three introduced fish species (Rutilus rutilus, Lepomis gibbosus and Alburnus alburnus) collected in rivers from north-eastern Spain can be used as non-lethal alternatives of muscle tissue in carbon and nitrogen stable isotope analysis. Tronquart et al. (2012) have recently suggested that fin tissue presents equivalent isotopic signatures with muscle in roach, pumpkinseed and a closely related species to Ebro chub (formerly S.cephalus) in temperate European rivers. Therefore, we expected to observe the same isotopic relationship between these tissues in our set of species. As tissue isotopic relationships are influenced by tissue constituents, we also predicted the strength of the isotopic relationship between muscle and fins would be higher than between muscle and scale tissues. Finally, we discussed the suitability of the correction factors for determining muscle’s isotopic signature from that of the non-lethally sampled tissues in these species.
Material and methods
Fish sampling and tissue collection
Sampling was performed on two consecutive days in September 2008 as part of a pilot study to determine parasite baseline of fish inhabiting three Mediterranean rivers in north-eastern Spain. Fish were caught with a portable electro fishing unit which generated up to 400 V and 2 A pulsed DC in Sant Joan de Vilatorrada (E:400340, N:4622900) in Llobregat basin with the exception of B. haasi collected in Súria, (E:396054, N:4631395), and S. laietanus in Sabadell (E:424071, N:4603425) in Llobregat and Besòs basins, respectively (Fig. 1). Each fish was then euthanised using an overdose of MS-222 (3-aminobenzoic acid ethyl ester; Sigma-Aldrich®), identified individually in plastic bags and kept in ice in isothermal bags during surveys and then frozen upon arrival at the laboratory. Later, fish were unfrozen, measured to the nearest mm (fork length, FL) and weighed to 0.01 g (total body wet weight, W). We also collected a sample of white muscle tissue (~2 cm2) from each fish and 10–30 scales from mid dorsal section below the dorsal fin and a caudal fin clip (~0.5 cm2). Fin and scales were then rinsed with distilled water, shaken, and observed under a stereomicroscope to guarantee organic debris removal.
Sample processing for stable isotope analysis
All samples were dried at 60 °C for 48 h and grounded to fine powder. To minimize the effect of the high lipid content of muscle compared to fins or scales on δ 13C, muscle samples underwent lipid-extraction with three consecutive 10 min baths in chloroform-methanol (CHCl3 - CH3OH; 2:1) and ultrasonic irradiation at three consecutive series discarding the supernatant at each bath. Alternatively, δ 13C values in muscle could be also reliably standardised by the application of a mathematical correction (Satterfield and Finney 2002; Post et al. 2007). Although chloroform-methanol extraction is widely applied to remove the lipid content, it may modify δ 15N signatures (Post et al. 2007). This bias, however, can be considered irrelevant to a certain extent because it is within the range of the analytical error (δ 15N = 0.3 ‰) for continuous flow techniques used in stable isotope analysis (Pinnegar and Polunin 1999; Post et al. 2007). Sub-samples of the powdered muscle (~0.30 mg), scale (~0.35 mg) and caudal fin clip (~0.50 mg) were then homogenised, weighed to the nearest μg, and placed into ultraclean tin capsules. For the determination of isotopic composition, samples were oxidised in a Flash EA1112 and TC/EA coupled to a stable isotope mass spectrometer Delta C through a Conflo III interface (ThermoFinnigan). Analytical accuracy was controlled using replicate assays of certified standards indicating an analytical error of ±0.1 ‰ and ±0.3 ‰ for δ 13C and δ 15N, respectively. The isotopic ratio mass spectrometry facility at University of Barcelona applies international standards (IAEA CH7, IAEA CH6 and USGS 40 for C, IAEA N1, IAEA N2 and IAEA NO3 for N), which are analysed every 12 samples to calibrate the system and compensate for any drift over time. Isotope ratios are expressed conventionally as δ values in ppt (‰) according to the following equation: δX = [(R sample/R standard) – 1] 1,000, where X (‰) is 13C, 15 N, and R are the corresponding ratios 13C/12C and 15 N/14 N, related to the standard values: R standard for 13C is Pee Dee Belemnite (PDB), for 15 N is atmospheric nitrogen (AIR).
Statistical analysis
Using parametric statistics as data achieved normality and homogeneity of variances after arcsine square-root transformation based on Shapiro-Wilk and Levene’s tests, mean δ 13C and δ 15N signatures were compared between muscle, fins and scales collected from each fish species using a one-way repeated measures ANOVA. As there is no proper function in R for post-hoc (pairwise comparisons) after this type of ANOVA, we applied a paired t-test and then corrected the p-values using the procedure described by Benjamini and Hochberg (1995) (“p.adjust” function in R) (see García 2004 for details). To further explore the suitability of fin and scales as surrogates for muscle tissue in δ 13C and δ 15N analysis, we also determined the isotopic relationship between these tissues using the standardised major axis (SMA) linear regressions (‘lmodel2’ function in R). Compared to ordinary least squares regressions, SMA regression enables a more accurate calculation of the regression parameters assuming natural variability and measurement error in the two variables analysed (Legendre 1998). All analyses were performed in R v.2.15.2 (R Development Core Team 2012) using the libraries ‘stats’ and ‘lmodel2’, and assuming the alternative hypothesis at P <0.05.
Results
Mean δ 13C and δ 15N values significantly differed between the three tissues analysed in each of the five fish species: bleak (n = 25; δ 13C: F = 107.6, P < 0.001; δ 15N: F = 103.9, P < 0.001), pumpkinseed (n = 22; δ 13C: F = 51.2, P < 0.001; δ 15N: F = 34.0, P < 0.001), roach (n = 12; δ 13C: F = 162.8, P < 0.001; δ 15N: F = 81.7, P < 0.001), Ebro chub (n = 27; δ 13C: F = 44.5, P < 0.001; δ 15N: F = 40.7, P < 0.001) and Iberian redfin barbel (n = 10; δ 13C: F = 49.7, P < 0.001; δ 15N: F = 70.3, P < 0.001) (Table 1). For all the species, our results showed a variety of scenarios depending on the target isotope, tissue and species, although most species consistently showed significant differences between muscle and non-lethally sampled tissues (Table 1). The differences can be accounted for the fact that fins and scales were on average depleted in 15 N (│Δ15N│ = 0.37 ± 0.84; │Δ15N│ = 1.76 ± 0.75, respectively) and enriched in 13C (│Δ13C│ = 0.15 ± 0.84; │Δ13C│ = 2.31 ± 0.56, respectively) as compared to muscle tissue (Table 1).
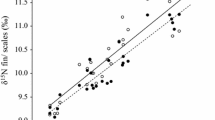
These results were mostly concordant with the determination of tissue-isotope relationships calculated with the standardised major regression analysis. Of the five fish species examined, roach, pumpkinseed and Ebro chub showed a significant relationship between the isotopic values of the muscle and those of scales and fin tissues (Fig. 2, Table 2). Ebro chub showed the strongest relationship between the isotopic signatures of the three tissues examined (Fig. 2e, Table 2). However, the estimated explained variation in the tissue-isotope pairs for the remaining species was moderate (R 2 = 0.59–0.75) and the slopes of the linear regressions (b) deviated somewhat from 1 either in fins or scale tissues, although this value was included in all 95 % confidence intervals with the exception of δ13C in roach’s fin (Table 2). A weak and marginally significant relationship was observed in bleak for δ 13C in scale tissue, and none of the tissue-isotopic relationships was significant for Iberian red-fin barbel (Table 2).
SMA linear regressions between muscle δ 15N and δ 13C values and fin (x F ) and scale (x S ) tissues in: a bleak (Alburnus alburnus); b Iberian redfin barbel (Barbus haasi); c pumpkinseed (Lepomis gibbosus); d roach (Rutilus rutilus); and e Ebro chub (Squalius laietanus). Dotted lines and filled circles represent fin, while dashed lines and empty triangles represent scales. Note that only in significant relationships (from Table 2) the fitted line is shown
Discussion
The refinement of scientific procedures goes towards the use of non-lethally sampled tissues for stable isotope analysis. Nevertheless, our study suggests caution when making straightforward equivalences between the isotopic composition of the muscle and that of fins and scales. As previously reported (e.g. Jardine et al. 2005; Kelly et al. 2006; Hanisch et al. 2010; Tronquart et al. 2012), the highest isotopic similarity in non-lethally sampled tissues with fish muscle was observed for most species in fins (│Δ15N│ ≤ 0.8‰; │∆13C│ ≤ 0.7‰) compared to scale tissue (│Δ15N│ ≥ 1.3‰; │∆13C│ ≥ 2.1‰). These authors also reported that fins or scales were generally a moderate predictor of the isotopic composition of muscle tissue (R2 = 0.33–0.99) as observed in the current study for most species with the exception of Ebro chub (R2 = 0.59–0.97). Tronquart et al. (2012), however, reported a higher explained variation and slope values for roach, pumpkinseed and chub than those found in the current study. Such differences could be attributed to the different regression methods applied to both data-sets since the ordinary least square (OLS) regression was used in Tronquart et al. (2012). As the application of OLS regression to our data set would have produced even lower R2 and slope values than those obtained from the SMA regression (Legendre 1998), this fact cannot explain the differences observed between both set of results. There are, however, other factors that were not considered here, such as differences in the water quality between rivers or the inter-population variability in the physiological response of fish to environmental conditions (Blanco et al. 2009; Willis et al. 2013), which may have influenced the isotopic relationships between tissues.
The discrepancies observed in the isotopic relationships among different tissue samples might reflect different isotopic fractionation related to tissue constituents (Pinnegar and Polunin 1999; Vollaire et al. 2007). As lipids were extracted, the observed depletion in 13C in muscle tissue is unlikely to be lipid content related. The presence of carbonates (i.e. fin rays or scales) could be responsible for the enrichment in 13C we observed in the current study either in fins or scales related to muscle, although this effect (│∆13C│ < 1‰) seems to have irrelevant biological repercussion (Hanisch et al. 2010; Ventura and Jeppesen 2010; Inamura et al. 2012). An additional explanation to the high 13C in scales might be that glycine is the predominant amino acid of scales’ collagen and it is enriched by 8‰ δ13C compared to other amino acids (Hare et al. 1991; Sinnatamby et al. 2008). Finally, differences in the isotopic composition between tissues found in the current study could be attributed to differences in turn-over among fish tissues since muscle tissue has a faster metabolic rate than fins and scales, and then the former is likely to reflect better short-term changes in fish diet (Sinnatamby et al. 2008).
In conclusion, this study demonstrates variation in tissue-isotopic relationships across this particular set of species. For the species where the differences observed in the isotopic composition of fins and muscle is low and remain constant (│∆15 N│ < 3‰ and │∆13C│ < 1‰), such differences are likely to have a low influence in the determination of animals’ trophic position or foraging grounds, although they might affect more sensitive analysis such as the determination of animals’ nutritional status (Post et al. 2007). The application of the linear regressions equations could improve the accuracy of tissue-isotopic conversions but the moderate explained variation (R 2 = 0.59–0.75) observed in the current study suggests that the resulting bias in tissue-isotopic conversions cannot be considered negligible for most species. Further studies should examine the likely mechanisms behind the observed isotopic composition of these species’ tissues as well as the consequences of fin clipping or scale removal for fish well-being to convert fins and scales into full suitable non-lethally sampled tissues for stable isotope analysis.
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate – a practical and powerful approach to multiple testing. J Roy Stat Soc 57:289–300. doi:10.2307/2346101
Blanco A, Deudero S, Box A (2009) Muscle and scale isotopic offset of three fish species in the Mediterranean Sea: Dentex dentex, Argyrosomus regius and Xyrichtys novacula. Rapid Commun Mass Spectrom 23:2321–2328. doi:10.1002/rcm.4154
DeNiro MJ, Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197:261–263. doi:10.1126/science.327543
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. http://www.R-project.org.
Fincel MJ, VanDeHey JA, Chipps SR (2012) Non-lethal sampling of walleye for stable isotope analysis: a comparison of three tissues. Fish Manag Ecol 19:283–292. doi:10.1111/j.1365-2400.2011.00830.x
García LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657–663. doi:10.1111/j.0030-1299.2004.13046.x
Grey J, Graham CT, Britton JR, Harrod C (2009) Stable isotope analysis of archived roach (Rutilus rutilus) scales for retrospective study of shallow lake responses to nutrient reduction. Freshw Biol 54:1663–1670. doi:10.1111/j.1365-2427.2009.02215.x
Hanisch JR, Tonn WM, Paszkowski CA, Scrimgeour GJ (2010) δ 13C and δ 15N signatures in muscle and fin tissues: nonlethal sampling methods for stable isotope analysis of salmonids. N Am J Fish Manag 30:1–11. doi:10.1577/M09-048.1
Hare PE, Fogel ML, Stafford TW, Mitchell AD, Hoering TC (1991) The isotopic composition of carbon and nitrogen in individual amino acids isolated from modern and fossil proteins. J Archaeol Sci 18:277–291. doi:10.1016/0305-4403(91)90066-X
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94:189–197. doi:10.2307/1368808
Inamura O, Zhang J, Minagawa M (2012) δ 13C and δ 15N values in scales of Micropterus salmoides largemouth bass as a freshwater environmental indicator. Rapid commun mass sp 26:17–24. doi:10.1002/rcm.5288
Jardine TD, Gray MA, McWilliam SM, Cunjak RA (2005) Stable isotope variability in tissues of temperate stream fishes. Trans Am Fish Soc 134:1103–1110. doi:10.1577/T04-124.1
Kelly MH, Hagar WG, Jardine TD, Cunjak RA (2006) Nonlethal sampling of sunfish and slimy sculpin for stable isotope analysis: how scale and fin tissue compare with muscle tissue. N Am J Fish Manag 26:921–925. doi:10.1577/M05-084.1
Legendre, P. (1998). Model II Regression User’s Guide, R edition. R Vignette. http://cran.r-project.org/web/packages/lmodel2/vignettes/mod2user.pdf. Accessed 30 August 2013
Maceda-Veiga A (2013) Towards the conservation of freshwater fish: Iberian Rivers as an example of threats and management practices. Rev Fish Biol Fish 23:1–22. doi:10.1007/s11160-012-9275-5
Metcalf SJ, Swearer SE (2005) Non-destructive ageing in Notolabrus tetricus using dorsal spines with an emphasis on the benefits for protected, endangered and fished species. J Fish Biol 66:1740–1747. doi:10.1111/j.0022-1112.2005.00704.x
Pinnegar JK, Polunin NVC (1999) Differential fractionation of δ 13C and δ 15N among fish tissues: implications for the study of trophic interactions. Funct Ecol 13:225–231. doi:10.1046/j.1365-2435.1999.00301.x
Post DM (2002) Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83:703-718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. doi:10.1007/s00442-006-0630-x
Satterfield FR, Finney BP (2002) Stable isotope analysis of Pacific salmon: insight into trophic status and oceanographic conditions over the last 30 years. Prog Oceanogr 53:231–246. doi:10.1016/S0079-6611(02)00032-0
Sinnatamby RN, Dempson JB, Power M (2008) A comparison of muscle- and scale-derived δ 13C and δ 15N across three life-history stages of Atlantic salmon, Salmo salar. Rapid commun mass sp 22:2773–2778. doi:10.1002/rcm.3674
Tronquart NH, Mazeas L, Reuilly‐Manenti L, Zahm A, Belliard J (2012) Fish fins as non‐lethal surrogates for muscle tissues in freshwater food web studies using stable isotopes. Rapid Commun Mass Spectrom 26:1603–1608. doi:10.1002/rcm.6265
Vander Zanden MJ, Cabana G, Rasmussen JB (1997) Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ 15N) and literature dietary data. Can J Fish Aquat Sci 54:1142–1158. doi:10.1139/f97-016
Vander Zanden MJ, Shuter BJ, Lester N, Rasmussen JB (1999) Patterns of food chain length in lakes: a stable isotope study. Am Nat 154:406–416. doi:10.1086/303250
Ventura M, Jeppesen E (2010) Evaluating the need for acid treatment prior to δ 13C and δ 15N analysis of freshwater fish scales: effects of varying scale mineral content, lake productivity and CO2 concentration. Hydrobiologia 644:245–259. doi:10.1007/s10750-010-0121-2
Vollaire Y, Banas D, Thomas M, Roche H (2007) Stable isotope variability in tissues of the Eurasian perch Perca fluviatilis. Comp Biochem Physiol 148:504–509. doi:10.1016/j.cbpa.2007.06.419
Willis TJ, Sweeting CJ, Bury SJ, Handley SJ, Brown JC, Freeman DJ, Cairney DG, Page MJ (2013) Matching and mismatching stable isotope (δ 13C and δ 15N) ratios in fin and muscle tissue among fish species: a critical review. Mar Biol 160:1633–1644. doi:10.1007/s00227-013-2216-6
Acknowledgments
This work was supported by FURIMED-2, the Catalan Water Agency (ACA) and the Sabadell Town Council (“Parc Fluvial del Ripoll”). The study was authorized by the Autonomous Government of Catalonia (Generalitat de Catalunya) and fish were euthanized following the standard protocol established by the animal welfare service at the University of Barcelona. In addition, AMV and ADS performed a training course on the use of animals in research provided by the University of Barcelona. We thank M. Monroy, A. Canyelles and C. Cardoso for field work and P. Rubio for her assistance in stable isotope analyses at the “Serveis Cientifico-Tècnics” at the University of Barcelona, and M. Stremska for English revision. AMV was funded by a Marie Curie Fellow (EC-Funded ‘Para-Tox’ project n° 327941).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cano-Rocabayera, O., Maceda-Veiga, A. & de Sostoa, A. Fish fins and scales as non-lethally sampled tissues for stable isotope analysis in five fish species of north – eastern Spain. Environ Biol Fish 98, 925–932 (2015). https://doi.org/10.1007/s10641-014-0328-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0328-6