Abstract
We explored whether fin clips and scales can be used as potential non-lethal alternatives to muscle tissue for examining the isotopic composition of asp Leuciscus aspius, a locally threatened freshwater species. Dorsal fin clips, scales and muscle plugs were collected from two asp populations and subsequently analysed for nitrogen and carbon stable isotopes. Both fins and scales were consistently depleted in 15N and enriched in 13C relative to muscle. A linear regression found that the isotope values in asp fins and scales were significantly related to those in the muscle tissue. These results indicate that fins and scales have the potential to be a substitute for muscle in stable isotope studies of asp, thus providing a non-destructive sampling method for this species. Nevertheless, to determine reliable conversion factors between tissues, a subset of individuals covering a sufficiently wide range of body sizes may need to be sacrificed for any given population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naturally occurring stable isotopes, especially those of carbon and nitrogen, are now routinely used in studies of aquatic food webs. The most common applications of stable carbon and nitrogen isotope measurements are to determine trophic interactions among organisms (Clarke et al., 2005; McIntyre et al., 2006; Hayden et al., 2014), to quantify energy flow through ecological communities (Vander Zanden et al., 1999; Finlay et al., 2002; Karlsson & Byström, 2005), to trace nutrient pollution (Schlacher et al., 2005; Anderson & Cabana, 2006; Xu & Zhang, 2012) and to predict contaminant bioaccumulation (Cabana & Rasmussen, 1994; Kidd et al., 2001). For fish, dorsal white muscle has been the tissue traditionally used in stable isotope analyses (Pinnegar & Polunin, 1999). However, sampling of muscle tissue is usually destructive and requires killing the fish. Lethal research sampling causes ethical issues and should be avoided, especially in cases of endangered and locally rare species or populations of exceptional value. Hence, to eliminate unnecessary mortality of sampled animals, the use of non-lethal methods in stable isotope studies of fish has recently received much attention (Kelly et al., 2006; Church et al., 2009; Willis et al., 2013). Particularly, fin clips and scales are the tissues that can be relatively easily obtained by non-lethal sampling, and several studies have shown that isotopic signals of fins and scales were correlated with those of muscle (e.g., Sanderson et al., 2009; Hanisch et al., 2010; Jardine et al., 2011; Fincel et al., 2012; Tronquart et al., 2012). It has also been demonstrated that isotopic offsets between tissues may differ, not only among species but also even among populations and life-history stages of the same species (Kelly et al., 2006; Sinnatamby et al., 2008; Blanco et al., 2009; Graham et al., 2013). However, despite the increasing interest in using non-destructive methods, information on the relationship between isotope signatures of muscle and those of non-lethally sampled tissues is still lacking for many species and populations of concern. To help fill this gap, the present study explored the use of fin clips and scales as a tool for examining isotopic composition of asp Leuciscus aspius (Linnaeus, 1758), a rarely studied species from the family Cyprinidae.
Asp is a specialist piscivore, inhabiting large–medium-sized lowland rivers and large lakes in Eurasia (Kottelat & Freyhof, 2007). The IUCN Red List conservation status of this species is Least Concern (Freyhof & Kottelat, 2008). Nevertheless, asp is included among species protected under the EU Habitats Directive (Natura 2000 network), as it is locally threatened, particularly by alteration of river morphology. In the Czech Republic, asp is not only relatively abundant in many rivers and reservoirs, partially due to artificial enhancement through stocking, but also because it thrives well under the prevalent eutrophic conditions (Vašek et al., 2013). Asp is valued both as a game fish and as a species used to induce top-down control on lower trophic levels (Donabaum et al., 1999; Vašek et al., 2013).
The aim of this study was to examine whether fins and scales can be used as non-lethal alternatives to muscle tissue in carbon and nitrogen stable isotope analyses of asp. The specific objectives were to (1) explore how fins and scales compare with muscle tissue in their isotope signatures, (2) develop appropriate correction factors allowing reciprocal use of different tissues in future stable isotope studies, (3) explore whether differences in isotope signatures between tissues vary with asp length, and (4) determine whether the isotopic composition differs between asp males and females.
Materials and methods
Study area
Asp were collected from Želivka Reservoir (dam coordinates: 49°43′31″N, 15°05′21″E) and Římov Reservoir (dam coordinates: 48°51′00″N, 14°29′28″E), in the Czech Republic. Both reservoirs are of canyon-type character, having elongated morphology and pronounced internal gradients of depth and productivity (Vašek et al., 2016). Želivka Reservoir has a surface area of 1430 ha, length of 31 km and maximum depth of 54 m. Římov Reservoir has a surface area of 210 ha, length of 10 km and maximum depth of 43 m. Both reservoirs contain fish communities dominated by cyprinids [bream Abramis brama (Linnaeus, 1758), roach Rutilus rutilus (Linnaeus, 1758), and bleak Alburnus alburnus (Linnaeus, 1758)] accompanied by perch Perca fluviatilis Linnaeus, 1758 and ruffe Gymnocephalus cernua (Linnaeus, 1758) (Vašek et al., 2016). Želivka Reservoir hosts a viable, wild population of asp (Vejřík et al., 2014) and is therefore protected as a Natura 2000 site (site code CZ0214016). Each year, a portion of this wild population is utilised as brood stock in a local hatchery, which produces pond-reared, 1-year-old asp used for enhancing the populations in other reservoirs. Římov Reservoir has also a naturally reproducing asp population (Blabolil et al., 2016) which is, however, more or less regularly supported by stocking pond-reared fingerlings originating from the wild Želivka’s brood stock.
Sample collection and preparation
Mature asp [aged ≥5+, standard length (SL) range 410–650 mm] of the Želivka Reservoir were collected in April 2013, shortly after they entered their spawning ground at the major tributary. Twenty one individuals (19 males and 2 females), captured with a boat mounted electro-fishing unit, were sacrificed to obtain samples for stable isotope analysis. A total of 27 mostly juvenile and subadult asp (aged between 1+ and 4+, SL range 158–370 mm) were collected in August 2014 at different sites of the Římov Reservoir using gillnets and shore seine nets. All these asp were euthanised, measured and weighed. Otoliths were dissected and used to age the fish. White dorsal muscle tissue, a dorsal fin clip (a tip of the dorsal fin) and 5–10 scales from above the lateral line were collected from each asp. All tissues were kept frozen (−20°C).
Prior to the stable isotope analysis, the muscle and fin samples were oven dried (60°C for 48 h) and pulverised in a mixer mill (Retsch MM 200). Whole fin clips were homogenised, which means that these samples contained all fin tissue elements, including bony rays. Scale samples were submersed in distilled water and gently cleaned from epidermal skin, mucus and adhered material with a scalpel under a binocular microscope. To correct for the potential influence of inorganic carbonates, the scale samples were acidified for 2 min in 1.2 M hydrochloric acid, rinsed five times in distilled water and oven dried (Perga & Gerdeaux, 2003). Only the outermost area of the scale, representing growth of fish during the last 1–2 years, was cut off and analysed. Two–five scales from each individual usually provided a sufficient amount of sample material for stable isotope analysis (~1 mg).
To maximally protect the wild asp population in the Želivka Reservoir, males were primarily sacrificed to obtain muscle samples for stable isotope analysis. As a consequence of this, only fin samples were used to compare the isotope composition between males and females. For this purpose, dorsal fin clips were taken from another 13 females (SL range 510–650 mm) captured on the spawning ground at the major tributary of the Želivka Reservoir in April 2013. After measuring, weighing and fin clipping, these females were released alive. The fin samples were stored and prepared in the same way as described above.
Stable isotope analysis
Stable isotope ratios of carbon and nitrogen and the elemental composition of all samples were determined at the Iso-Analytical Limited (Crewe, Cheshire, UK) using a Europa Scientific 20–20 isotope ratio mass spectrometer coupled with an elemental analyser. Vienna Pee Dee Belemnite and atmospheric N2 were used as the international standards for carbon and nitrogen, respectively. The working standard for our samples was NBS-1577B (powdered bovine liver, δ13CV-PDB = −21.60‰, δ15NAir = 7.65‰). NBS-1557B was calibrated in-house as a secondary reference material and is directly traceable to IAEA-CH-6 (sucrose, δ13CV-PDB = −10.43‰) and IAEA-N-1 (ammonium sulphate, δ15NAir = 0.40‰). Isotope ratios of the tissue samples were expressed in conventional delta notation (δ15N, δ13C) as parts per thousand (‰) differences from the international standard. The analytical error, estimated from replicated runs of the reference material, was less than 0.1 and 0.2‰ for δ13C and δ15N, respectively. Data were not corrected for lipids since elemental carbon–nitrogen (C:N) ratios of all tissues were <4, indicating a low lipid content (Hoffman et al., 2015).
Data analysis
Paired t-tests were run to determine whether fin and scale isotope values differ from those of muscle. Mean C:N ratios were compared among the three tissue types using a one-way repeated measures analysis of variance (ANOVA) with multiple comparisons (Tukey test). The isotopic offsets between tissues, defined as the differences between fin and muscle or scale and muscle isotope values, were compared between Želivka and Římov asp with an independent two-sample t-test. Relationships between isotope values of fins or scales and those of muscle tissue were examined by linear regression analysis. Linear regressions were also applied to test if the isotopic offsets between tissues were influenced by asp body size. Male and female Želivka asp were compared for body size and mass using an independent two-sample t-test. Analysis of covariance (ANCOVA), with sex as the fixed factor and fish SL as the covariate, was performed to examine whether fin clips of male and female Želivka asp differ in nitrogen and carbon stable isotope values and C:N ratios. All statistical tests were carried out with STATISTICA 9.1 (StatSoft, Inc., Tulsa, OK, USA, 2010).
Results
The δ15N and δ13C isotope values in fins and scales of asp were significantly different from those in their muscle tissue (Table 1). Both fins and scales were depleted in 15N and enriched in 13C relative to the muscle, but the magnitude of these differences was generally greater in scales compared with fins (Table 1). The Želivka and Římov asp did not differ in the δ15N offset between fin and muscle (t-test: t 46 = 1.49, P = 0.11) but they significantly differed in the δ15N offset between scale and muscle (t-test: t 46 = −3.41, P < 0.002). The two populations did not differ in the δ13C offset between fin and muscle (t-test: t 46 = 0.45, P = 0.65), nor scale and muscle (t-test: t 46 = −0.93, P = 0.36). The C:N ratios of both Želivka and Římov asp significantly differed among the types of tissue, being highest in fins, intermediate in muscle and lowest in scales (Table 1).
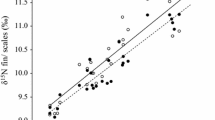
Linear regression demonstrated that muscle δ15N and δ13C values were significantly related to those measured from fins and scales (Fig. 1). Fins and scales of Římov asp were a strong predictor of muscle δ15N (R 2 = 0.81–0.90; Table 2) but a moderate predictor of muscle δ13C (R 2 = 0.57–0.61; Table 2). In Želivka asp, fins were moderate predictors of muscle δ15N and δ13C values (R 2 = 0.42–0.64; Table 2), whereas scales were comparatively poorer predictors of muscle δ15N and δ13C values (R 2 = 0.22–0.31; Table 2).
Linear regressions of δ15N and δ13C values in muscle on those of fins and scales for Želivka (open circles) and Římov (shaded circles) asp. The black lines indicate the regression lines where significant relationships were found (P < 0.05). The grey lines represent the 1:1 reference line at which both tissue types have identical values
No significant trend in isotopic offsets between fin and muscle or scale and muscle with fish body length was usually evident in Želivka asp (linear regressions: P > 0.05; Fig. 2). The only exception was a positive relationship between asp body length and the δ13C scale–muscle offset (R 2 = 0.30, P < 0.05; Fig. 2). In Římov asp, the δ15N offset between fin and muscle was positively related to body length (R 2 = 0.19, P < 0.05; Fig. 2) but the δ15N offset between scale and muscle was unrelated to body length (R 2 = 0.05, P = 0.28; Fig. 2). Further, in Římov asp, the δ13C offsets between fin and muscle and between scale and muscle were both positively affected by body length (R 2 = 0.45 and 0.57, respectively, both P < 0.001; Fig. 2).
Relationships between the δ15N and δ13C fin–muscle or scale–muscle offsets and body sizes of Želivka (open circles) and Římov (shaded circles) asp. The black lines indicate the regression lines where significant linear relationships were found (P < 0.05). The grey lines represent the zero reference line at which both tissue types have identical values
The males from the Želivka Reservoir reached a SL of 505 ± 52 mm and mass of 1.9 ± 0.6 kg (mean ± SD). The females from the same reservoir were significantly larger (t-test: t 32 < −3.96, P < 0.001), and their mean SL and mass were 570 ± 42 mm and 2.8 ± 0.7 kg, respectively. No significant difference between sexes was found in terms of fin δ15N, with mean (±SD) values of 16.0 ± 0.5‰ for males and 15.9 ± 0.4‰ for females (ANCOVA: F 1,31 = 1.75, P = 0.20). However, the fin δ13C did differ significantly between sexes, with the mean values being −22.7 ± 0.6‰ for males and −22.0 ± 0.6‰ for females (ANCOVA: F 1,31 = 4.47, P < 0.05). No difference between sexes was found in the fin C:N ratio (ANCOVA: F 1,31 = 0.48, P = 0.49), with the mean values being 3.7 ± 0.2 and 3.6 ± 0.2 for males and females, respectively.
Discussion
Asp fins and scales were found to be lower in 15N and richer in 13C compared with muscle tissue. Previous studies done on other fish species have also observed that fins and scales were usually depleted in 15N and enriched in 13C relative to muscle (Kelly et al., 2006; Tronquart et al., 2012; Willis et al., 2013; Cano-Rocabayera et al., 2015). In both asp populations, the mean isotopic differences between scales and muscle were considerably larger than the mean isotopic differences between fins and muscle. Unlike fins, asp scales were acid treated to remove inorganic carbonates, which might potentially affect their isotopic composition. However, since Syväranta et al. (2008) and Ventura & Jeppesen (2010) reported that untreated and acid treated cyprinid scales differed only slightly in isotope values, acid treatment is unlikely to be the primary cause of the differing isotope signatures of asp scales and fins. Instead, the differences suggest that isotopic fractionation might vary among the tissues. Such results are supported by the few studies of other fish species that simultaneously examined isotope signatures of muscle, fin and (untreated) scale tissues and revealed a closer similarity in isotopic composition between muscle and fins than between muscle and scales (Fincel et al., 2012; Cano-Rocabayera et al., 2015). These isotopic differences probably reflect the variability in biochemical components of the tissues (Vollaire et al., 2007), particularly the relative abundance of different amino acids (Pinnegar & Polunin 1999; Estrada et al., 2005).
Because of the isotopic differences between the asp tissues, correction factors were necessary to convert the isotope values of fins and scales to those of the muscle. The fin-to-muscle and scale-to-muscle conversion equations were all significant but their coefficients of determination varied quite widely (R 2 = 0.22–0.90). These results are, nevertheless, comparable with other studies that have examined isotopic relationships between tissues in single populations of various fish species (Hanisch et al., 2010; Graham et al., 2013; Cano-Rocabayera et al., 2015). Stronger inter-tissue relationships (R 2 >0.90) have often been observed in studies that combined isotope data from different populations in a single regression (e.g., Sanderson et al., 2009; Syväranta et al., 2010; Inamura et al., 2012; Tronquart et al., 2012). However, such an approach raises the problem of pseudoreplication and should be avoided because it may spuriously enlarge the range of isotope data (via pooling locations with different isotopic baselines) used in regression analysis (Willis et al., 2013).
In the Želivka asp, body length usually had no effect on the isotopic differences between tissues, which may indicate that inter-tissue isotopic fractionations are fairly constant during the adult life-history stage. In contrast, in the Římov asp, the isotopic offsets between tissues were mostly related to body length. In particular, both the δ13C fin–muscle and scale–muscle offsets exhibited an increase of 0.5‰ per 100 mm of SL. Changes in tissue fractionation and turnover rates during a period of rapid growth and ontogenetic diet shifts might be responsible for this body length effect. Alternatively, the observed patterns in δ13C could be caused by lipid content increasing as the size of the asp increased. However, although the C:N ratio (as a proxy for lipid content) of dorsal muscle statistically significantly increased with body length of the Římov asp (linear regression: R 2 = 0.28, P = 0.004), the slope of this regression was extremely small (b = 0.0005), suggesting that the change in C:N ratio alone cannot explain the isotopic differences between the tissues.
The fin δ15N signatures of the Želivka asp did not differ between sexes. This indicates that both males and females were feeding at the same trophic level. The fin C:N ratio also did not differ between males and females, implying a similar nutritional status for both sexes. In contrast, male and female Želivka asp were significantly distinct in the fin δ13C signatures, although the difference was only 0.7‰. To decide whether this sex-related difference was caused by physiological processes connected with gonad maturation, utilisation of prey resources with distinct δ13C signatures or other mechanisms will, however, require further research.
The results of this study suggest that fin and scale tissues, after adjustment, can be a useful non-lethal proxy for muscle in stable isotope studies of asp. Fin clipping is widely used as a standard marking technique in fisheries research (Guy et al., 1996). Moreover, fin clips are frequently sampled for genetic analyses (Wasko et al., 2003) while scales are commonly collected for age and growth determination (DeVries & Frie, 1996). In general, such non-lethally obtained samples can also be used profitably for examining the isotopic composition of fish. In the present study, the strength of inter-tissue relationships differed between the two asp populations. Whereas for juvenile asp of the Římov Reservoir both fins and scales were good predictors of the muscle isotope values, for mature asp of the Želivka Reservoir scales appeared to be a relatively poor predictor compared with fins. Isotopic turnover rates of fish generally decrease with body mass (Vander Zanden et al., 2015) and are slower in less metabolically active tissues (e.g., scales). Nevertheless, the study of Sinnatamby et al. (2008) has demonstrated that muscle and scale tissues equilibrated to new diets at a similar rate in fast-growing Atlantic salmon Salmo salar Linnaeus, 1758. It is therefore reasonable to expect that fast-growing juvenile asp of the Římov Reservoir had rapid turnover rates that differed little among the tissues, and both fins and scales thus correlated well with the muscle. In slow-growing adult asp of the Želivka Reservoir, however, scales might have a considerably lower isotopic turnover, meaning that they integrated consumer diets over a longer time period than muscle or fin tissues. Consequently, in adult fish, the differing tissue turnover rates might have caused the poor agreement observed between scale and muscle isotope values. Overall, for asp in this study, fins seemed to be a better non-lethal alternative to muscle. Also, because the preparation of fin clips for the analysis was much easier than that of scales, future non-lethal sampling for isotope studies of asp, and perhaps also other fish species, should preferably use fin clips. Nevertheless, scales can still be a useful non-lethal tissue for those future works interested in determining long-term feeding ecology.
The present study has revealed that the δ15N scale–muscle offset significantly differed between the asp from Želivka and Římov Reservoirs. Whether this differing isotopic offset refers to local conditions in the two reservoirs or to different body sizes of the fish examined was, however, impossible to determine. Nevertheless, some previous studies done on other species have shown that isotopic fractionation between tissues may vary among populations of a single species (Kelly et al., 2006; Blanco et al., 2009; Graham et al., 2013). Caution should therefore be taken when applying the correction factors developed in this study to asp populations in other systems, where environmental conditions may be considerably different. For each population of interest, when possible, it is best to sacrifice a subset of individuals in order to assess relationships among tissues and determine population-specific conversion factors (Willis et al., 2013). Such development of conversion factors may need to incorporate a sufficient range of body sizes representative of the given population. In summary, although some individuals must be sacrificed for calibration, the use of fin clips and scales in stable isotope studies certainly has the potential to dramatically reduce the number of lethally sampled fish.
References
Anderson, C. & G. Cabana, 2006. Does δ15N in river food webs reflect the intensity and origin of N loads from the watershed? Science of the Total Environment 367: 968–978.
Blabolil, P., D. Ricard, J. Peterka, M. Říha, T. Jůza, M. Vašek, M. Prchalová, M. Čech, M. Muška, J. Seďa, T. Mrkvička, D. S. Boukal & J. Kubečka, 2016. Predicting asp and pikeperch recruitment in a riverine reservoir. Fisheries Research 173: 45–52.
Blanco, A., S. Deudero & A. Box, 2009. Muscle and scale isotopic offset of three fish species in the Mediterranean Sea: dentex dentex, Argyrosomus regius and Xyrichtys novacula. Rapid Communications in Mass Spectrometry 23: 2321–2328.
Cabana, G. & J. B. Rasmussen, 1994. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 372: 255–257.
Cano-Rocabayera, O., A. Maceda-Veiga & A. de Sostoa, 2015. Fish fins and scales as non-lethally sampled tissues for stable isotope analysis in five fish species of north-eastern Spain. Environmental Biology of Fishes 98: 925–932.
Church, M. R., J. L. Ebersole, K. M. Rensmeyer, R. B. Couture, F. T. Barrows & D. L. G. Noakes, 2009. Mucus: a new tissue fraction for rapid determination of fish diet switching using stable isotope analysis. Canadian Journal of Fisheries and Aquatic Sciences 66: 1–5.
Clarke, L. R., D. T. Vidergar & D. H. Bennett, 2005. Stable isotopes and gut content show diet overlap among native and introduced piscivores in a large oligotrophic lake. Ecology of Freshwater Fish 14: 267–277.
DeVries, D. R. & R. V. Frie, 1996. Determination of age and growth. In Murphy, B. R. & D. W. Willis (eds), Fisheries Techniques, 2nd ed. American Fisheries Society, Bethesda: 483–512.
Donabaum, K., M. Schagerl & M. T. Dokulil, 1999. Integrated management to restore macrophyte domination. Hydrobiologia 395(396): 87–97.
Estrada, J. A., M. Lutcavage & S. R. Thorrold, 2005. Diet and trophic position of Atlantic bluefin tuna (Thunnus thynnus) inferred from stable carbon and nitrogen isotope analysis. Marine Biology 147: 37–45.
Fincel, M. J., J. A. VanDeHey & S. R. Chipps, 2012. Non-lethal sampling of walleye for stable isotope analysis: a comparison of three tissues. Fisheries Management and Ecology 19: 283–292.
Finlay, J. C., S. Khandwala & M. E. Power, 2002. Spatial scales of carbon flow in a river food web. Ecology 83: 1845–1859.
Freyhof, J. & M. Kottelat, 2008. Aspius aspius. In The IUCN Red List of Threatened Species 2008: e.T2178A9311209 [available on doi at 10.2305/IUCN.UK.2008.RLTS.T2178A9311209.en]. Downloaded on 21 March 2016.
Graham, C. T., S. S. C. Harrison & C. Harrod, 2013. Development of non-lethal sampling of carbon and nitrogen stable isotope ratios in salmonids: effects of lipid and inorganic components of fins. Isotopes in Environmental and Health Studies 49: 555–566.
Guy, C. S., H. L. Blankenship & L. A. Nielsen, 1996. Tagging and marking. In Murphy, B. R. & D. W. Willis (eds), Fisheries Techniques, 2nd ed. American Fisheries Society, Bethesda: 353–383.
Hanisch, J. R., W. M. Tonn, C. A. Paszkowski & G. J. Scrimgeour, 2010. δ13C and δ15N signatures in muscle and fin tissues: nonlethal sampling methods for stable isotope analysis of salmonids. North American Journal of Fisheries Management 30: 1–11.
Hayden, B., C. Harrod & K. K. Kahilainen, 2014. Lake morphometry and resource polymorphism determine niche segregation between cool- and cold-water-adapted fish. Ecology 95: 538–552.
Hoffman, J. C., M. E. Sierszen & A. M. Cotter, 2015. Fish tissue lipid-C:N relationships for correcting δ13C values and estimating lipid content in aquatic food-web studies. Rapid Communications in Mass Spectrometry 29: 2069–2077.
Inamura, O., J. Zhang & M. Minagawa, 2012. δ13C and δ15N values in scales of Micropterus salmoides largemouth bass as a freshwater environmental indicator. Rapid Communications in Mass Spectrometry 26: 17–24.
Jardine, T. D., R. J. Hunt, B. J. Pusey & S. E. Bunn, 2011. A non-lethal sampling method for stable carbon and nitrogen isotope studies of tropical fishes. Marine and Freshwater Research 62: 83–90.
Karlsson, J. & P. Byström, 2005. Littoral energy mobilization dominates energy supply for top consumers in subarctic lakes. Limnology and Oceanography 50: 538–543.
Kelly, M. H., W. G. Hagar, T. D. Jardine & R. A. Cunjak, 2006. Nonlethal sampling of sunfish and slimy sculpin for stable isotope analysis: how scale and fin tissue compare with muscle tissue. North American Journal of Fisheries Management 26: 921–925.
Kidd, K. A., H. A. Bootsma, R. H. Hesslein, D. C. G. Muir & R. E. Hecky, 2001. Biomagnification of DDT through the benthic and pelagic food webs of Lake Malawi, East Africa: importance of trophic level and carbon source. Environmental Science and Technology 35: 14–20.
Kottelat, M. & J. Freyhof, 2007. Handbook of European Freshwater Fishes. Publications Kottelat, Cornol.
McIntyre, J. K., D. A. Beauchamp, M. M. Mazur & N. C. Overman, 2006. Ontogenetic trophic interactions and benthopelagic coupling in Lake Washington: evidence from stable isotopes and diet analysis. Transactions of the American Fisheries Society 135: 1312–1328.
Perga, M. E. & D. Gerdeaux, 2003. Using the δ13C and δ15N of whitefish scales for retrospective ecological studies: changes in isotope signatures during the restoration of Lake Geneva, 1980–2001. Journal of Fish Biology 63: 1197–1207.
Pinnegar, J. K. & N. V. C. Polunin, 1999. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225–231.
Sanderson, B. L., C. D. Tran, H. J. Coe, V. Pelekis, E. A. Steel & W. L. Reichert, 2009. Nonlethal sampling of fish caudal fins yields valuable stable isotope data for threatened and endangered fishes. Transactions of the American Fisheries Society 138: 1166–1177.
Schlacher, T. A., B. Liddell, T. F. Gaston & M. Schlacher-Hoenlinger, 2005. Fish track wastewater pollution to estuaries. Oecologia 144: 570–584.
Sinnatamby, R. N., J. B. Dempson & M. Power, 2008. A comparison of muscle- and scale-derived δ13C and δ15N across three life-history stages of Atlantic salmon, Salmo salar. Rapid Communications in Mass Spectrometry 22: 2773–2778.
Syväranta, J., S. Vesala, M. Rask, J. Ruuhijärvi & R. I. Jones, 2008. Evaluating the utility of stable isotope analyses of archived freshwater sample materials. Hydrobiologia 600: 121–130.
Syväranta, J., J. Cucherousset, D. Kopp, A. Crivelli, R. Céréghino & F. Santoul, 2010. Dietary breadth and trophic position of introduced European catfish Silurus glanis in the River Tarn (Garonne River Basin), southwest France. Aquatic Biology 8: 137–144.
Tronquart, N. H., L. Mazeas, L. Reuilly-Manenti, A. Zahm & J. Belliard, 2012. Fish fins as non-lethal surrogates for muscle tissues in freshwater food web studies using stable isotopes. Rapid Communications in Mass Spectrometry 26: 1603–1608.
Vander Zanden, M. J., J. M. Casselman & J. B. Rasmussen, 1999. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401: 464–467.
Vander Zanden, M. J., M. K. Clayton, E. K. Moody, C. T. Solomon & B. C. Weidel, 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS One 10: e0116182.
Vašek, M., M. Prchalová, J. Peterka, H. A. M. Ketelaars, A. J. Wagenvoort, M. Čech, V. Draštík, M. Říha, T. Jůza, M. Kratochvíl, T. Mrkvička, P. Blabolil, D. S. Boukal, J. Duras & J. Kubečka, 2013. The utility of predatory fish in biomanipulation of deep reservoirs. Ecological Engineering 52: 104–111.
Vašek, M., M. Prchalová, M. Říha, P. Blabolil, M. Čech, V. Draštík, J. Frouzová, T. Jůza, M. Kratochvíl, M. Muška, J. Peterka, Z. Sajdlová, M. Šmejkal, M. Tušer, L. Vejřík, P. Znachor, T. Mrkvička, J. Seďa & J. Kubečka, 2016. Fish community response to the longitudinal environmental gradient in Czech deep-valley reservoirs: implications for ecological monitoring and management. Ecological Indicators 63: 219–230.
Vejřík, L., E. Bouše, I. Matějíčková, D. Ricard & J. Kubečka, 2014. A Survey of Reproductive Biology and Population Size of Asp (Leuciscus aspius) in the Švihov (Želivka) Reservoir During the Period 2008–2014. Technical report. Biology Centre of the Czech Academy of Sciences, Institute of Hydrobiology, České Budějovice (in Czech).
Ventura, M. & E. Jeppesen, 2010. Evaluating the need for acid treatment prior to δ13C and δ15N analysis of freshwater fish scales: effects of varying scale mineral content, lake productivity and CO2 concentration. Hydrobiologia 644: 245–259.
Vollaire, Y., D. Banas, M. Thomas & H. Roche, 2007. Stable isotope variability in tissues of the Eurasian perch Perca fluviatilis. Comparative Biochemistry and Physiology, Part A 148: 504–509.
Wasko, A. P., C. Martins, C. Oliveira & F. Foresti, 2003. Non-destructive genetic sampling in fish. An improved method for DNA extraction from fish fins and scales. Hereditas 138: 161–165.
Willis, T. J., C. J. Sweeting, S. J. Bury, S. J. Handley, J. C. S. Brown, D. J. Freeman, D. G. Cairney & M. J. Page, 2013. Matching and mismatching stable isotope (δ13C and δ15N) ratios in fin and muscle tissue among fish species: a critical review. Marine Biology 160: 1633–1644.
Xu, J. & M. Zhang, 2012. Primary consumers as bioindicator of nitrogen pollution in lake planktonic and benthic food webs. Ecological Indicators 14: 189–196.
Acknowledgments
The authors would like to thank members of the FISHECU team (www.fishecu.cz) for their assistance during field sampling, Kateřina Soukalová for determining the ages of the asps, Tomáš Mrkvička for statistical advice, and Mary J. Morris for checking the English. Two anonymous reviewers provided useful comments on an earlier draft of this paper. The study was financially supported by the Czech Science Foundation (15-01625S), and the Project CEKOPOT (CZ.1.07/2.3.00/20.0204) co-financed by the European Social Fund and the State Budget of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: M. Power
Rights and permissions
About this article
Cite this article
Vašek, M., Vejřík, L., Vejříková, I. et al. Development of non-lethal monitoring of stable isotopes in asp (Leuciscus aspius): a comparison of muscle, fin and scale tissues. Hydrobiologia 785, 327–335 (2017). https://doi.org/10.1007/s10750-016-2940-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2940-2