Abstract
Detailed information of fish diets is required if we are to understand complex interactions between species and successfully manage resources at an ecosystem level. We compiled diet information from 76 species of fish targeted by recreational and commercial fishers in North West Australia. Based on 81 independent studies we demonstrate that species targeted by the fishery are all carnivores, however the type of prey they consume and their trophic level is variable (3.31–4.49) and trophic range of some species spans different trophic levels (e.g. Lethrinus nebulosus, 3.46–4.35). These findings infer that in highly diverse systems, such as coral reefs, trophic cascades instigated by fishing must be investigated at the species, rather than functional or trophic level. Moreover, as prey availability is likely to vary spatially and temporally, diet must be quantified locally to assess ecosystem level impacts of fishing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to their high commercial value and catchability, large predatory fishes are often selectively harvested in the recreational and commercial fisheries of the world (Pauly et al. 1998; Jennings et al. 2001). The suite of life history characteristics that typifies the majority of these large predatory fish, i.e. being long-lived, slow-growing and with advanced ages at maturity (Reynolds et al. 2005), infers low rates of intrinsic population growth. As a consequence these species are extremely vulnerable to over exploitation (Myers et al. 1997; Denney et al. 2002). In evidence of this inherent vulnerability, recent estimates have shown that populations of large predatory fish have been declining at a rapid pace worldwide (Friedlander and DeMartini 2002; Myers and Worm 2003; Baum et al. 2003; Myers et al. 2007).
While the consequences of removing predators from marine ecosystems are not well understood (Hughes et al. 2003; Hawkins and Roberts 2004), their depletion in fish assemblages has been linked to a lowering of mean trophic level (Pauly et al. 1998; Jennings et al. 2002; Pinnegar et al. 2002; Nicholson and Jennings 2004; Essington et al. 2006), and the alteration of trophic guild structure (Link and Garrison 2002; Allen and Clarke 2007; Watson et al. 2007). The removal of top predatory fish is also likely to have significant indirect effects on marine ecosystems, influencing a range of ecological processes that might include predation, competition and herbivory (Babcock et al. 1999; Pinnegar et al. 2000; Willis and Anderson 2003).
Understanding the potential impacts of declines in biomass of large predatory fish is essential for the management of marine ecosystems and the fisheries that they sustain. Ecosystem models are a common method for exploring the wider effects of such top-predator declines (Pauly et al. 2000a), but these rely on the model creator having a detailed knowledge of trophic linkages within the particular system being examined. Dietary composition data is an important component of this required knowledge, yet these data are often only available for a very limited range of species in a given area or ecosystem. The strong push towards the modeling of marine ecosystems in recent times, particularly in data-poor regions, has often led to a heavy reliance on data obtained from outside the local area. For highly specialized feeders whose diet does not vary spatially information for such models may be gathered from several different locations. However, for species with a generalist diet the trophic level will reflect availability of prey and is likely to vary both spatially and temporally. Identifying generalists and specialists and using appropriate dietary information is therefore an important facet of building credible ecosystem models.
In this paper, we summarize all available dietary data for a range of recreationally and commercially important fish species from the inshore waters of North West Australia. Trawl and trap fisheries along this coast target lethrinids, lutjanids and serranids, producing ~3,000 t of finfish, worth 12 million AUD, annually (Fletcher and Santoro 2008). There are also significant fisheries for mackerel (predominantly Scomberomorus spp.), a gillnet fishery that targets barramundi (Lates calcarifer) and threadfin salmon (e.g. Eleutheronema tetradactylum), a shark fishery (predominantly Carcharhinus spp.) and an expanding recreational fishery. Clearly the focus in this region is on fish from higher trophic levels and, unlike many other tropical fisheries, herbivorous fish species are generally not targeted. Indirect effects of fishing are therefore not confounded by removal of herbivorous prey species and may be more apparent.
Based on the compilation and comparison of multiple studies, conducted in locations around the world, we assess how diet of fisheries targeted finfish may vary spatially. We also identify the fish families most commonly preyed upon, providing an indication of what species may benefit from removal of predators and how this may affect the ecosystem. This information will facilitate a better understanding of the indirect effects of fishing, highlighting gaps in our current knowledge of fish diets and provide direction for future research in this region.
Methods
Diet of fish recreationally and/or commercially targeted in the inshore waters of N W Australia was assessed using gut content information collected from locations around the world. A paucity of local data precluded exclusive use of data collected from N W Australia, however by taking this global approach we were able to use variation in trophic level as an indicator of plasticity in species diet. A list of key targeted fish species was formed using data obtained from recreational catch surveys (Sumner et al. 2002; Williamson et al. 2006), and from several years of commercial catch statistics (Fletcher and Head 2006; Fletcher and Santoro 2007, 2008), for the northern Gascoyne (including Ningaloo Marine Park), Pilbara and Kimberley bioregions. This list was expanded to include predatory fish species, which may be competitors to the main target species of the aforementioned fisheries and could thus benefit from their removal.
Systematic, computerized literature searches were conducted to find all available dietary data on these species, irrespective of where the study was conducted. Abstracting and indexing services, such as Aquatic Sciences and Fisheries Abstracts (ASFA), Biological Abstracts (BIOSYS), Web of Science (WoS) and Google Scholar were searched for peer-reviewed articles, and grey literature, including books, reports and unpublished theses. Unpublished raw data was also utilized for some fish species.
Information provided from the literature was tabulated by species, study area, sample size (number of stomachs containing food items), length range of specimens, method used for diet expression, and dietary composition. Dietary information was summarized using 11 food item categories: teleosts, chondrichthyans, cephalopods, molluscs (other than cephalopods), crustaceans, echinoderms, annelids, other invertebrates, birds, reptiles, and mammals. These represented the major taxa ingested. Overall dietary composition for each species was calculated using a weighted average that takes into account the number of fish sampled by each study. The proportion of each prey item category P j to the overall diet was calculated as;
where P ij is the proportion of prey category j in study i, Ni is the number of stomachs with food used to calculate Pij in study i, n is the number of studies, j is the number of prey categories, and \( \sum {{P_j}} = 1 \).
Fractional trophic levels were calculated for each individual study using the 11 dietary categories and by substituting into the following equation the average values for each dietary category as described in the TrophLab manual (Eq. 2, Pauly et al. 2000b);
where TROPH j is the fractional trophic level of prey j as defined by TrophLab (Pauly et al. 2000b), DCij represents the fraction of j in the diet of i, and n is the total number of prey species. Trophic levels for species were calculated for each study, overall TROPHs were then calculated for a species using a weighted average that takes into account the sample size in each study.
The examination of trophic level variation within and between fish species was limited to those species for which there were at least three independent studies, each involving the examination of 10 or more fish. Trophic level of species was only assessed from studies where weight or volume of dietary items were expressed as a proportion of total gut contents, as these values were typically similar within species. We excluded from calculations those studies where only presence or percentage occurrence of dietary items was recorded. Studies involving the examination of juvenile diet only, or where juveniles may have contributed substantially to the sample, were also excluded. We also examined if the number of studies on a particular species diet influence tropic breadth by assessing the strength of the relationship between the number of independent studies and TROPH range. The number of studies was not strongly correlated to diet breadth (F1,14 = 0.24, P = 0.63, r2 = 0.01), suggesting sample size did not unduly influence our results. Similarly, diet breadth was not related to the mean distance between study locations from which dietary information was collated (F1,14 = 0.49, P = 0.49, r2 < 0.03), suggesting geographic range of studies did not influence results.
To assess which fish are susceptible to predation by fisheries targeted species the teleost component of piscivorous fish diets was examined separately. Only those species for which teleosts accounted for >50% of the overall diet were considered piscivores, as prevalence of fish in guts suggests these are the predators most likely to remove fish prey and affect top down control of community structure. Prey information was collated at the family level and the frequency of occurrence of teleost families in the diets of major piscivores was determined. Pelagic and demersal predators and prey were identified to provide a rough gauge of how well these two systems are linked. The percentage of pelagic and benthic prey in the diet of target species was used as an indicator of where predators predominantly feed. Pelagic species were defined as those belonging to groups 35–38 of the FAO International Standard Statistical Classification of Aquatic Animals and Plants (ISSCAAP). This includes the sharks, scombrids, carangids and species which spend most of their time swimming in open water. Demersal species were defined as those belonging to ISSCAAP groups 31 and 34. These included serranids, lutjanids and lethrinids and species that live in close association with the benthos, typically swimming near to, or sheltering within, benthic structures.
Results
Eighty-one dietary studies were included in the present study to calculate standardized dietary compositions of 76 fish species from the inshore waters of N W Australia (Table 1). Although most of these species are an important component of the recreational and commercial catch in Western Australia, there has been very little study into the diets of these species locally. Only five studies were found to have been conducted in Western Australian waters, with these containing quantitative (volumetric or weight) dietary data on 10 of the 76 species examined. A further 20 studies, conducted in Queensland and the Northern Territory, provided quantitative dietary data on 44 species. Globally, 28 studies were found to contribute quantitative dietary data on 51 of the 78 species included in the present study. While quantitative dietary data were available for the majority of fishery target species in N W Australia, there were some recreationally and/or commercially important species for which data was not found. These included: Epinephelus areolatus, E. bilobatus, E. microdon, E. multinotatus, E. rivulatus, Carangoides gymnostethus, Lutjanus lemniscatus, L. lutjanus, Lethrinus hutchinsi, Grammatocynus bicarinatus and Acanthopagrus palmaris. For some species dietary information is available, however these studies have only recorded prey presence in guts rather than providing a quantitative assessment of how this prey contributes to total weight or volume of ingested matter. Most notable are the 13 studies on tiger shark (Galeocerdo cuvier) diet, none of which provide data on volume or weight of prey ingested (Table 1).
All of the 76 species for which dietary data was obtained were carnivorous, the mean fractional trophic levels ranging from 2.92 for Amniataba caudavittata up to 4.64 for Sphyrna mokarran (Table 1). The majority of these fishes, (44 species), could be classified as piscivores, as teleosts represented >50% of volume or weight ingested. Piscivorous families targeted by fishers included the Platycephalidae, Serranidae, Rachycentridae, Carangidae and Lutjanidae, whilst non-targeted piscivores included Muraenidae and Synodontidae. The Sillaginidae, Haemulidae, Lethrinidae, Sparidae, Sciaenidae and Mullidae are also targeted by many fishers, but are mostly invertivores, predominantly feeding on crustaceans and molluscs.
Pelagic species had a slightly higher mean (± standard error) fractional trophic level (4.39 ± 0.05) than demersal species (3.91 ± 0.10). A T-test finding the difference to be significant (t18 = 4.16, p < 0.01). Pelagic fish such as barracuda, tuna and mackerel tended to feed on pelagic prey such as carangids, clupeids and mugilids. However, sharks that forage over both benthic and pelagic environments fed on both pelagic and benthic prey. Predators such as grouper, lethrinids and some of the lutjanids, that are typically associated with the benthos, fed mainly on fish that are associated with the benthos (Table 2). A notable exception to this pattern was a high percentage of clupeids in the diet of the Lutjanus bohar, which, although classified as a benthic predator is often observed swimming well above the substratum. The lizardfish, Saurida undosquamis, which is typically associated with the benthos, also had a high percentage of clupeids in the diet.
Members of the family Clupeidae were the most frequently consumed teleost prey, occurring in the diet of 21/27 species (Fig. 1). Clupeids were present in both demersal and pelagic piscivores, representing a prominent component of the diet of eight demersal and 13 pelagic species. Mugilidae, Carangidae, Monocanthidae and Antherinidae were all prey of five or more pelagic predators, whilst Nemipteridae, Mullidae and Pomacentridae were typically preyed upon by benthic predators. Fish from the families Leiognathidae, Engraulidae, Gerriedae, Labridae and Haemulidae were a prominent component of both pelagic and benthic piscivore diets (Fig. 1).
Fish families most frequently preyed on by fisheries targeted species. Histogram shows the frequency at which teleost prey families contributed to the teleost component of the diets of species for which teleosts accounted for >50% of the overall diet. Black and grey shading represents the proportion of demersal or pelagic piscivores, respectively, preying upon a particular teleost family
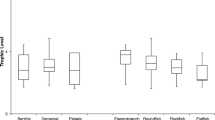
Dietary assessment of fish varied considerably between studies. The smallest range in mean fractional trophic levels were observed for the pelagic species S. barracuda, Euthynnus affinis and Carcharhinus tilstoni, while the largest amount of variation was observed for the demersal Lethrinus nebulosus and the reef-associated pelagic Caranx ignobilis (Fig. 2). Dietary assessment of both L. nebulosus and C. ignobilis spanned trophic levels 3 and 4, emphasizing the extent of plasticity in their diet. Moreover, comparison of species within genera revealed that trophic ranges do not always overlap (Fig. 2). For example there was no overlap in the trophic values of Lutjanus vitta and L. fulviflamma inferring that these congeners are trophically distinct.
Trophic range of species targeted by fishers in North West Australia. Box-whisker plots show fractional trophic levels (TROPHs) for those species for which there were between three and five independent studies, each involving the examination of 10 or more samples, and that expressed dietary composition volumetrically or by weight as a proportion of total gut contents. Boxes extend out to the minimum and maximum values of the data. The mean and median are displayed within the box as a black dot and a vertical line, respectively
Discussion
Effective ecosystem based management is founded upon a sound knowledge of how ecosystem components interact (Levin and Lubchenco 2008). In particular, dietary information is critical for modeling trophic pathways and assessing the implications of predator removal from a system. For example, fishing typically removes large bodied predators from the system, but details of predator diet are required before cascading consequences can be investigated. We have demonstrated that prey consumed by fisheries targeted species varies between species and that not all fish targeted by N W Australian fishers are piscivores. For example, the blacktip shark, Carcharhinus tilstoni, an important component of the northern shark fishery (Fletcher and Santoro 2008), feed almost exclusively on other fish, yet the whiting Silago sihama, which is targeted by recreational fishers (Williamson et al. 2006), feeds exclusively on invertebrates. Moreover, there is considerable intra-specific variation in the diet of some species. This plasticity in diet is best demonstrated by the Spangled Emperor, Lethrinus nebulosus, which is targeted by both recreational and commercial fishers (Sumner et al. 2002; Fletcher and Santoro 2008). At two locations L. nebulosus fed predominantly on invertebrates and only small volumes (<5%) of fish (Westera 2003; Kulbicki et al. 2005). But at another location fish represented >80% of the weight of ingested matter (Salini et al. 1994). Differences in density of prey items may account for a small amount of variation when diet is assessed by weight and volume, however the large disparity in diet from these studies suggests L. nebulosus will feed on a variety of prey resources. Importantly, diet clearly varies among and within species targeted by N W Australian fishers and the impact of fishing on prey will depend on the species and quantity of fish removed.
Furthermore, for species whose diet is spatially variable it may be inappropriate to assess indirect effects of fishing based on dietary information collected from other locations. Diet for many fish may also change ontogenetically (e.g., Davis 1985; St John 1999; Cocheret de la Moriniere et al. 2003) or seasonally (e.g., Kingsford 1992; Arendt et al. 2001; Griffiths et al. 2009). These spatial, ontogenetic and temporal changes reflect shifts in fish feeding capacity, energetic demands and availability of prey. As a consequence, any trophic cascade instigated by fishing will be dependant on the size, age and type of fish species removed. This is especially true in ecosystems as complex as coral reefs, where there is a greater diversity of species and carnivores are often opportunistic generalists (Russ 1991).
There is a general paucity of local dietary information for fisheries target species in N W Australia. There are only five studies containing quantitative dietary data on 10 recreationally and/or commercially important fish species in this region. Furthermore, while information on many other fisheries target species could be obtained from studies conducted outside of this region, there are a few species for which no quantitative dietary data is currently available. These include: Lethrinus hutchinsi, Epinephelus multinotatus and Epinephelus rivulatus, which form an important component of the recreational and commercial fisheries of Western Australia (Sumner et al. 2002; Williamson et al. 2006; Fletcher and Santoro 2008).
The dietary data obtained during the present study indicates that the majority of recreationally and commercially targeted fishes in N W Australia are carnivorous, with many of these being large predatory fishes with high mean fractional trophic levels. The vulnerability of top predatory fishes to fishing is well documented in the scientific literature, with many Northern Hemisphere fisheries reporting significant declines in the mean trophic level of their catches (Pauly et al. 1998; Pinnegar et al. 2002; Essington et al. 2006). While there has been a much shorter history of fishing in Western Australian waters, the expansive nature of the coastline of this state means that any regional trophic declines may not be observed in the catch data while fishers are still finding and fishing new grounds. Monitoring and management of fish assemblages in N W Australia will therefore require comprehensive spatial and temporal assessments of both fish populations and fishing effort.
The removal of predators from marine ecosystems through exploitation is thought to result in elevated prey abundance, which, in turn, can influence primary productivity (Pace et al. 1999; Pinnegar et al. 2000; Shurin et al. 2002). While there is some evidence for trophic cascades on coral reefs due to the removal of predatory fish (McClanahan et al. 2002; Graham et al. 2003; Dulvy et al. 2004), such events have been more commonly reported for less diverse ecosystems, such as lakes and rocky reefs (Pace et al. 1999). High species diversity on coral reefs is thought to confer a measure of redundancy that maintains ecological processes when individual species decline (McNaughton 1977; King and Pimm 1983; Tilman et al. 1996; Naeem 1998). Indeed variation in response to environmental drivers within trophic groups can often mask trophic cascades that occur at species level (Wilson et al. 2008). Consequently, even in complex systems flow on effects from exploitation of marine resources may exist and it is vital to examine trophic interactions at a species level.
Importantly there may be some key species that have relatively distinct ecological roles and may not always be fully redundant, interchangeable or replaceable (Rowan et al. 1997). For example, it is often hypothesized that removal of predators could increase the abundance of herbivores, reducing the standing biomass of algae. However on the reefs of N W Australia herbivorous fish such as kyphosids, scarids, siganids and acanthurids were not identified as an important component in the diet of predatory fish targeted by fishers. These large bodied herbivores were present in only five of the species targeted by fishers and represented <5% of ingested material in any one species. Consequently tropic cascades are unlikely to be instigated by increased predation of large bodied herbivores. Similarly, links between large herbivorous fish and predators in the Caribbean are weak (Mumby et al. 2006) and increased predation of herbivores in marine protected areas does not overwhelm the ecological benefits of increased herbivory afforded by these reserves (Mumby et al. 2007). Indirect effects of fishing on herbivory on N W Australian reefs may however be altered by removal of fish that feed on juveniles, increasing the survival rates of post-settlement fish. Mortality during this early life history stage is high and changes in predation may have a profound impact on adult populations and associated processes (Webster 2003). Moreover, some predators target invertebrates, including herbivorous echinoderms, which may alter rates of herbivory and erosion. On the reefs of N W Australia, fishers target lethrinids and labrids, which often feed on echinoderms. If predation by these fish controls echinoderm populations fishing may reduce predator abundance resulting in the release of echinoderm prey. For example, where fishing is prohibited at Ningaloo reef, abundance of some lethrinids is greater, density of herbivorous urchins is lower and algal communities differ, implying fishing has instigated trophic cascades (Westera 2003). Similarly, reduced fishing pressure in Kenyan reserves increased the abundance of the triggerfish, Balistapus undulatus, and reduced the abundance of urchins (McClanahan 2000). Removal of invertebrate feeding species by fishers has also been associated with increased abundance and outbreaks of the coral feeding starfish, Acanthaster planci (Dulvy et al. 2004; Sweatman 2008), which have caused extensive declines in coral cover (Chesher 1969; Randall 1973; Pratchett et al. 2009). These studies indicate trophic cascades can occur on tropical reefs and further investigations are required to validate and understand trophic links and possible cascades caused by fishing in NW Australia.
Clupeids, carangids, engraulids and mugilids, which spend most of their time in the water column, were the primary prey of scombrids which were classified as pelagic predators. This suggests pelagic fisheries are not tightly linked to benthic prey. Several benthic predators (e.g., Saurida undosquamis, Lutjanus bohar) did however ingest pelagic prey, and carcharinids that move between these two habitats fed on both pelagic and benthic prey, suggesting there are some links between these environments. Moreover, most benthic marine animals have a bipartite life history, where early life stages are spent in the pelagic environment. Predation of “larval” fish by pelagic species may be an important determinant of juvenile abundance, as large pelagic species, such as greater barracuda, (Sphyraena barracuda), can have large numbers of larval fish in their stomachs (Wilson pers. obs.). Careful assessment of predation on pre-settlement stages of demersal species by pelagic fish is therefore required.
Overall N W Australian fishers currently target carnivores which feed on both fish and invertebrates. Large herbivorous fish are not a major component of these fishes diet and there is little evidence to suggest fishing will alter herbivory by fish. Fishing may however result in the release of invertebrate prey, which could alter herbivory and other processes attributable to invertebrates. To fully appreciate the indirect effects of fishing further work is required to elucidate functional roles and trophic links of fish and invertebrates on N W Australian reefs. Clearly assessing and predicting the cascading effects of fishing on coral reefs requires collection of dietary information from relevant species at appropriate temporal and spatial scales. This information must then be combined with information on predator/prey abundance and consumption rates of predators to asses the magnitude of trophic fluxes. Current trophic knowledge of reef fish diet is largely based on a handful of studies that collected large numbers of species at specific locations and carried out gut content analyses on these fish (e.g., Hiatt and Strasburg 1960; Randall 1967; Kulbicki et al. 2005). A concerted effort to build on the information collected in these studies is required if we are to understand trophic interactions on reefs. This should involve a combination of stable isotope, fatty acid and gut content analyses to provide diet assessments of high taxonomic resolution over extended time frames.
References
Allen JI, Clarke KR (2007) Effects of demersal trawling on ecosystem functioning in the North Sea: a modelling study. Mar Ecol Prog Ser 336:63–75
Al-Zibdah M, Odat N (2007) Fishery status, growth, reproduction biology and feeding habit of two scombrid fish from the Gulf of Aqaba, Red Sea. Leb Sci J 8:3–20
Arendt MD, Olney JE, Lucy JA (2001) Stomach content analysis of cobia, Rachycentron canadum, from lower Chesapeake Bay. Fish Bull 99:665–670
Babcock RC, Kelly S, Shears NT, Walker JW, Willis TJ (1999) Changes in community structure in temperate marine reserves. Mar Ecol Prog Ser 125:125–134
Bachok Z, Mansor MI, Noordin RM (2004) Diet composition and food habits of demersal and pelagic marine fishes from Terengganu waters, east coast of Peninsular Malaysia. NAGA 27:41–47
Barnett A, Bellwood DR, Hoey AS (2006) Trophic ecomorphology of cardinalfish. Mar Ecol Prog Ser 322:249–257
Bass AJ, D’Aubrey JD, Kistnasamy N (1973) Sharks of the east coast of southern Africa. 1. The genus Carcharhinus (Carcharhinidae). S Afr Ass Mar Biol Res, Oceanogr Res Inst. Invest Rep 33:1–168
Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA (2003) Collapse and conservation of shark populations in the northwest Atlantic. Science 299:389–392
Begg GA, Hopper GA (1997) Feeding patterns of school mackerel (Scomberomorus queenslandicus) and spotted mackerel (S. munroi) in Queensland east-coast waters. Mar Freshwat Res 48:565–571
Bell JC, Nichols JT (1921) Notes on the food of Carolina sharks. Copeia 1921:17–20
Beukers-Stewart BD, Jones GP (2004) The influence of prey abundance on the feeding ecology of two piscivorous species of coral reef fish. J Exp Mar Biol Ecol 299:155–184
Blaber SJM, Cyrus DP (1983) The biology of Carangidae (Teleostei) in Natal estuaries. J Fish Biol 22:173–188
Blaber SJM, Milton DA, Rawlinson NJF (1990) Diets of lagoon fishes of the Solomon Islands: predators of tuna baitfish and trophic effects of baitfishing on the subsistence fishery. Fish Res 8:263–286
Blaber SJM, Brewer DT, Salini JP (1994) Diet and dentition in tropical ariid catfishes from Australia. Environ Biol Fishes 40:159–174
Brewer DT, Warburton K (1992) Selection of prey from a seagrass/mangrove environment by golden lined whiting, Sillago analis (Whitley). J Fish Biol 40:257–271
Brewer DT, Blaber SJM, Salini JP, Farmer MJ (1995) Feeding ecology of predatory fishes from Groote Eylandt in the Gulf of Carpentaria, Australia, with special reference to predation on penaeid prawns. Estuar Coast Shelf Sci 40:577–600
Castillo JL, Uribe JA, Bonfil R, Vélez R, Mendizabal D, de Anda D, Mena R, Márquez JF (1992) Estado actual de la pesquería de tiburón en México. Instituto Nacional de la Pesca, México
Castro JI (1996) The biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bull Mar Sci 59:508–522
Chesher RH (1969) Destruction of Pacific corals by the sea star Acanthaster planci. Science 18:280–283
Cliff G (1995) Sharks caught in the protective gill nets off Kwazulu-Natal, South Africa. 8. The great hammerhead shark (Sphyrna mokarran) (Ruppell). S Afr J Mar Sci 15:105–114
Cocheret de la Moriniere E, Pollu BJA, Nagelkerken I, Hemminga MA, Huiskes AHL, Van der Velde G (2003) Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: stable isotopes and gut content analysis. Mar Ecol Prog Ser 246:279–289
Connell SD (1998) Patterns of piscivory by resident predatory reef fish at One Tree Reef, Great Barrier Reef. Mar Freshwat Res 49:25–30
Davis TLO (1985) The food of barramundi, Lates calcarifer (Bloch), in coastal and inland waters of Van Dieman Gulf and the Gulf of Carpentaria, Australia. J Fish Biol 26:669–682
DeCrosta MA, Taylor LR Jr, Parrish JD (1984) Age determination, growth, and energetics of three species of carcharhinid sharks in Hawaii. In: Grigg RW, Tanoue KY (eds) Proceedings of the second symposium on resource investigations in the northwest Hawaiian Islands, vol. 2, May 25–27, 1983. University of Hawaii Sea Grant MR-84-01, pp 75–95
Denney NH, Jennings S, Reynolds JD (2002) Life-history correlates of maximum population growth rates in marine fishes. Proc R Soc Lond B Biol Sci 269:2229–2237
Dodrill JW (1977) A hook and line survey of the sharks found within five hundred meters of shore along Melbourne Beach, Brevard County, Florida. Masters Thesis, Florida Institute of Technology, Florida
Dudley SFJ, Cliff G (1993) Sharks caught in the protective gill nets of Natal, South Africa. 7. The blacktip shark (Carcharhinus limbatus) (Valenciennes). S Afr J Mar Sci 13:237–254
Dulvy NK, Freckleton RP, Polunin NVC (2004) Coral reef cascades and the indirect effects of predator removal by exploitation. Ecol Lett 7:410–416
Essington TE, Beaudreau AH, Wiedenmann J (2006) Fishing through marine food Webs. Proc Natl Acad Sci USA 103:3171–3175
Fletcher WJ, Head F (2006) State of the Fisheries Report 2005/06. Department of Fisheries, Western Australia
Fletcher WJ, Santoro K (2007) State of the Fisheries Report 2006/07. Department of Fisheries, Western Australia
Fletcher WJ, Santoro K (2008) State of the Fisheries Report 2007/08. Department of Fisheries, Western Australia
Franks JS, Garber NM, Warren JR (1996) Stomach contents of juvenile cobia, Rachycentron canadum, from the northern Gulf of Mexico. Fish Bull 94:374–380
Friedlander AM, DeMartini EE (2002) Contrasts in density, size and biomass of reef fishes between the northwestern and the main Hawaiian Islands: the effects of fishing down apex predators. Mar Ecol Prog Ser 230:253–264
Galván-Magana F, Nienhuis HJ, Klimley AP (1989) Seasonal abundance and feeding habits of sharks of the lower Gulf of California, Mexico. Calif Fish Game 75:74–84
Goeden GB (1978) A Monograph of the Coral Trout Plectropomus leopardus (Lacepede). Qld Fish Serv Res Bull 1:1–42
Graham NAJ, Evans RD, Russ GR (2003) The effects of marine reserve protection on the trophic relationships of reef fishes on the Great Barrier Reef. Environ Conserv 30:200–208
Griffiths SP, Kuhnert PM, Fry GF, Manson FJ (2009) Temporal and size-related variation in the diet, consumption rate, and daily ration of mackerel tuna (Euthynnus affinis) in neritic waters of eastern Australia. ICES J Mar Sci 66:720–733
Gunn JS, Milward NE (1985) The food, feeding habits and feeding structures of the whiting species Sillago sihama (Forsskal) and Sillago analis Whitley from Townsville, north Queensland, Australia. J Fish Biol 26:411–427
Hajisamae S, Yeesin P, Ibrahim S (2006) Feeding ecology of two sillaginid fishes and trophic interrelations with other co-existing species in the southern part of South China Sea. Environ Biol Fishes 76:167–176
Harmelin-Vivien ML, Bouchon C (1976) Feeding behaviour of some carnivorous fishes (Serranidae and Scorpaenidae) from Tulear (Madagascar). Mar Biol 37:329–340
Hawkins JP, Roberts CM (2004) Effects of artisanal fishing on Caribbean coral reefs. Conserv Biol 18:215–226
Haywood MDE, Heales DS, Kenyon RA, Loneragan NR, Vance DJ (1998) Predation of juvenile tiger prawns in a tropical Australian estuary. Mar Ecol Prog Ser 162:201–214
Heithaus MR (2001) The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environ Biol Fishes 61:25–36
Hiatt RW, Strasburg DW (1960) Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol Monogr 30:65–127
Hobson ES (1974) Feeding relationships of teleostean fishes on coral reefs in Kona, Hawaii. Fish Bull 72:915–1031
Hueter RE (1994) Bycatch and catch-release mortality of small sharks and associated fishes in the estuarine nursery grounds of Tampa Bay and Charlotte Harbor. Final Report to FDEP
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jenkins GP, Milward NE, Hartwick RF (1984) Food of larvae of Spanish mackerels, genus Scomberomorus (Teleostei: Scombridae), in shelf waters of the Great Barrier Reef. Aust J Mar Freshw Res 35:477–482
Jennings S, Kaiser MJ, Reynolds JD (2001) Marine fisheries ecology. Blackwell Science, Oxford
Jennings S, Greenstreet SPR, Hill L, Piet GJ, Pinnegar JK, Warr KJ (2002) Long-term trends in the trophic structure of the North Sea fish community: evidence from stable-isotope analysis, size-spectra and community metrics. Mar Biol 141:1085–1097
Kamukuru AT, Mgaya YD (2004) The food and feeding habits of blackspot snapper, Lutjanus fulviflamma (Pisces: Lutjanidae) in shallow waters of Mafia Island, Tanzania. Afr J Ecol 42:49–58
King AW, Pimm SI (1983) Complexity, diversity, and stability: a reconciliation of theoretical and empirical results. Am Nat 122:229–239
Kingsford MJ (1992) Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 11:193–198
Knapp FT (1951) Food habits of the Sergeantfish, Rachycentron canadus. Copeia 1951:101–102
Kulbicki M, Bozec Y, Labrosse P, Letourneur Y, Mou-Tham G, Wantiez L (2005) Diet composition of carnivorous fishes from coral reef lagoons of New Caledonia. Aquat Living Resour 18:231–250
Lek E (2004) Diets of three carnivorous fish species in marine waters of the west coast of Australia. Honours thesis. Murdoch University, Perth, Western Australia
Levin SA, Lubchenco J (2008) Resilience, robustness, and marine ecosystem-based management. Bioscience 58:27–32
Link JS, Garrison LP (2002) Trophic ecology of Atlantic cod Gadus morhua on the northeast US continental shelf. Mar Ecol Prog Ser 227:109–123
Lowe CG, Wetherbee BM, Crow GL, Tester AL (1996) Ontogenetic dietary shifts and feeding behavior of the tiger shark, Galeocerdo cuvier, in Hawaiian waters. Environ Biol Fishes 47:203–211
Lyle JM (1987) Observations on the biology of Carcharhinus cautus (Whitley), C. melanopterus (Quoy and Gaimard) and C. fitzroyensis (Whitley) from Northern Australia. Aust J Mar Freshw Res 38:701–710
Marais JFK (1984) Feeding ecology of major carnivorous fish from four Eastern Cape estuaries. S Afr J Zool 19:210–223
Marnane MJ, Bellwood DR (2002) Diet and nocturnal foraging in cardinalfishes (Apogonidae) at One Tree Reef, Great Barrier Reef, Australia. Mar Ecol Prog Ser 231:261–268
McClanahan TR (2000) Recovery of a coral reef keystone predator, Balistapus undulatus, in East African marine parks. Biol Cons 94:191–198
McClanahan TR, Polunin NVC, Done TJ (2002) Ecological states and the resilience of coral reefs. Conserv Ecol 6:18
McNaughton SJ (1977) Diversity and stability of ecological communities: a comment on the role of empiricism in ecology. Am Nat 111:515–525
Meyer CG, Holland KN, Wetherbee BM, Lowe CG (2001) Diet, resource partitioning and gear vulnerability of Hawaiian jacks captured in fishing tournaments. Fish Res 53:105–113
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Box S, Stoffle RW, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101
Mumby PJ, Harborne AR, Williams J, Kappel CV, Brumbaugh DR, Micheli F, Holmes KE, Dahlgren CP, Paris CB, Blackwell P (2007) Trophic cascades facilitates coral recruitment in a marine reserve. Proc Natl Acad Sci USA 104:8362–8367
Myers RA, Worm B (2003) Rapid worldwide depletion of predatory fish communities. Nature 423:280–283
Myers RA, Mertz G, Fowlow PS (1997) Maximum population growth rates and recovery times for Atlantic cod, Gadus morhua. Fish Bull 95:762–772
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850
Naeem S (1998) Species redundancy and ecosystem reliability. Conserv Biol 12:39–45
Nakamura Y, Horimonchi M, Nakai T, Sano M (2003) Food habits of fishes in a seagrass bed on a fringing reef at Iriomote Island, southern Japan. Ichthyol Res 50:15–22
Nicholson MD, Jennings S (2004) Testing candidate indicators to support ecosystem-based management: the power of monitoring surveys to detect temporal trends in fish community metrics. ICES J Mar Sci 61:35–42
Pace LP, Cole JJ, Carpenter SR, Kitchell JF (1999) Trophic cascades revealed in diverse ecosystems. Trends Ecol Evol 14:483–488
Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F Jr (1998) Fishing down marine food webs. Science 279:860–863
Pauly D, Christensen V, Walters C (2000a) Ecopath, Ecosim, and Ecospace as tools for evaluating ecosystem impact of fisheries. ICES J Mar Sci 57:697–706
Pauly D, Froese R, Sa-a PS, Palomares ML, Christensen V, Rius J (2000b) TrophLab manual. ICLARM, Manila
Phelan MJ, Gribble NA, Garrett RN (2008) Fishery biology and management of Protonibea diacanthus (Sciaenidae) aggregations in far northern Cape York Peninsula waters. Cont Shelf Res 28:2143–2151
Pinnegar JK, Polunin NVC, Francour P, Badalamenti F, Chemello R, Harmelin-Vivien M-L, Hereu B, Milazzo M, Zabala M, D’Anna G, Pipitone C (2000) Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ Conserv 27:179–200
Pinnegar JK, Jennings S, O’Brien CM, Polunin NVC (2002) Long-term changes in the Celtic Sea fish community and fish market price distribution. J Appl Ecol 39:377–390
Platell ME, Ang HP, Hesp SA, Potter IC (2007) Comparisons between the influences of habitat, body size and season on the dietary composition of the sparid Acanthopagrus latus in a large marine embayment. Estuar Coast Shelf Sci 72:626–634
Pratchett MS, Schenk TJ, Baine M, Syms C, Baird AH (2009) Selective coral mortality associated with outbreaks of Acanthaster planci L. in Bootless Bay, Papua New Guinea. Mar Environ Res 67:230–236
Randall JE (1967) Food habits of reef fishes of the West Indies. Stud Trop Oceanog 5:665–847
Randall RH (1973) Distribution of corals after Acanthaster planci (L.) infestation at Tanguisson Point, Guam. Micronesica 3:213–222
Randall JE (1992) Review of the biology of the tiger shark (Galeocerdo cuvier). Aust J Mar Freshw Res 43:21–31
Randall JE, Brock VE (1960) Observations on the ecology of epinepheline and lutjanid fishes of the Society Islands, with emphasis on food habits. Trans Am Fish Soc 89:9–16
Randall JE, Head SM, Sanders APL (1978) Food habits of the giant humphead wrasse, Cheilinus undulates (Labridae). Environ Biol Fishes 3:235–238
Rao KVS (1981) Food and feeding of lizard fishes (Saurida spp.) from north western part of Bay of Bengal. Indian J Fish 28:47–64
Reynolds JD, Dulvy NK, Goodwin NB, Hutchings JA (2005) Biology of extinction risk in marine fishes. Proc R Soc Lond, B Biol Sci 272:2337–2344
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Russ GR (1991) Coral reef fisheries: effects and yields. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 601–635
Salini JP, Blaber SJM, Brewer DT (1990) Diets of piscivorous fishes in a tropical Australian estuary, with special reference to predation on penaeid prawns. Mar Biol 105:363–374
Salini JP, Blaber SJM, Brewer DT (1992) Diets of sharks from estuaries and adjacent waters of the north-eastern Gulf of Carpentaria, Australia. Aust J Mar Freshw Res 43:87–96
Salini JP, Blaber SJM, Brewer DT (1994) Diets of trawled predatory fish of the Gulf of Carpentaria, Australia, with particular reference to predation on prawns. Aust J Mar Freshw Res 45:397–411
Salini JP, Brewer DT, Blaber SJM (1998) Dietary studies on the predatory fishes of the Norman River Estuary, with particular reference to penaeid prawns. Estuar Coast Shelf Sci 46:837–847
Sano M, Shimizu M, Nose Y (1984) Food habits of teleostean reef fishes in Okinawa Island, southern Japan. Univ Museum, Univ Tokyo Bull 25
Shpigel M, Fishelson L (1989) Food habits and prey selection of three species of groupers from the genus Cephalopholis (Serranidae: Teleostei). Environ Biol Fishes 24:67–73
Shurin JB, Borer ET, Seabloom EW, Anderson K, Blanchette CA, Broitman B, Cooper SD, Halpern BS (2002) A cross-ecosystem comparison of the strength of trophic cascades. Ecol Lett 5:785–791
Simpfendorfer C (1992) Biology of tiger sharks (Galeocerdo cuvier) caught by the Queensland shark meshing program off Townsville, Australia. Aust J Mar Freshw Res 43:21–31
Simpfendorfer CA, Milward NE (1993) Utilisation of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrnidae. Environ Biol Fishes 37:337–345
Simpfendorfer CA, Goodreid AB, McAuley RB (2001) Size, sex and geographic variation in the diet of the tiger shark, Galeocerdo cuvier, from Western Australian waters. Environ Biol Fishes 61:37–46
St John J (1999) Ontogenetic changes in the diet of the coral reef grouper Plectropomus leopardus (Serranidae): patterns in taxa, size and habitat of prey. Mar Ecol Prog Ser 180:233–246
St John J, Russ GR, Brown IW, Squire LC (2001) The diet of the large coral reef serranid Plectropomus leopardus in two fishing zones on the Great Barrier Reef, Australia. Fish Bull 99:180–192
Stanger JD (1974) A study of the growth, feeding, and reproduction of the threadfin, Eleutheronema tetradactylum (Shaw). Honours thesis. James Cook University, Townsville, Queensland
Stevens JD (1984a) Biological observations on sharks caught by sport fishermen off New South Wales. Aust J Mar Freshw Res 35:573–590
Stevens JD (1984b) Life-history and ecology of sharks at Aldabra Atoll, Indian Ocean. Proc R Soc Lond, B Biol Sci 222:79–106
Stevens JD, Lyle JM (1989) Biology of three hammerhead sharks (Eusphyra blochii, Sphyrna mokarran, and S. lewini), from northern Australia. Aust J Mar Freshw Res 40:129–146
Stevens JD, McLoughlin KJ (1991) Distribution, size, and sex composition, reproductive biology and diet of sharks from northern Australia. Aust J Mar Freshw Res 40:129–146
Stevens JD, Wiley PD (1986) Biology of two commercially important carcharhinid sharks from northern Australia. Aust J Mar Freshw Res 37:671–688
Sumner NR, Williamson PC, Malseed BE (2002) A 12-month survey of recreational fishing in the Gascoyne bioregion of Western Australia during 1998–99. Fisheries Research Report No. 139, Department of Fisheries, Western Australia
Sumpton W, Greenwood J (1990) Pre- and post-flood feeding ecology of four species of juvenile fish from the Logan-Albert estuarine system, Moreton Bay, Queensland. Aust J Mar Freshw Res 41:795–806
Sweatman HPA (1993) Tropical snapper (Lutjanidae) that is Piscivorous at settlement. Copeia 1993:1137–1139
Sweatman HPA (2008) No-take reserves protect coral reefs from predatory starfish. Curr Biol 18:598–599
Tavares RJ (1997) Alimentación de Carcharhinus limbatus (Valenciennes, 1839) (Pisces: Carcharhinidae) y datos sobre la dieta de otras especies de tiburones presentes en el Parque Nacional Archipiélago Los Roques. Trabajo Espacial de Grado, Universidad de Lisboa
Taylor LR, Naftel G (1978) Preliminary investigations of shark predation on the Hawaiian monk seal at Pearl and Hermes Reef and French Frigate Shoals. U.S. Marine Mammal Commission Report No. MMC-77/07
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grasslands. Nature 367:363–365
Walker MH (1978) Food and feeding habits of Lethrinus chrysostomus Richardson (Pisces: Perciformes) and other Lethrinidae on the Great Barrier Reef. Aust J Mar Freshw Res 29:623–630
Wass RC (1971) A comparative study of the life history, distribution and ecology of the sandbar shark and the gray reef shark in Hawaii. Ph.D. Thesis. Univiversity of Hawaii, Honolulu
Watson DL, Harvey ES, Kendrick GA, Nardi K, Anderson MJ (2007) Protection from fishing alters the species composition of fish assemblages in a temperate-tropical transition zone. Mar Biol 152:1197–1206
Webster MS (2003) Temporal density dependence and population regulation in a marine fish. Ecology 84:623–628
Westera MB (2003) The effect of recreational fishing on targeted fishes and trophic structure, in a coral reef marine park. PhD thesis, Edith Cowan University, Perth, Western Australia
Wetherbee BM, Crow GL, Lowe CG (1997) Distribution, reproduction and diet of the gray reef shark Carcharhinus amblyrhynchos in Hawaii. Mar Ecol Prog Ser 151:181–189
Williamson PC, Sumner NR, Malseed BE (2006) A 12-month survey of recreational fishing in the Pilbara region of Western Australia during 1999–2000. Fisheries Research Report No. 153, Department of Fisheries, Western Australia
Willis TJ, Anderson MJ (2003) Structure of cryptic reef fish assemblages: relationships with habitat characteristics and predator density. Mar Ecol Prog Ser 257:209–221
Wilson SK, Fisher R, Pratchett MS, Graham NAJ, Dulvy NK, Turner RA, Cakacaka A, Polunin NVC, Rushton SP (2008) Exploitation and habitat degradation as agents of change within coral reef fish communities. Glob Chang Biol 14:2796–2809
Wright A, Dalzell PJ, Richards AH (1986) Some aspects of the biology of the red bass. Lutjanus bohar (Forsskal), from the Tigak Islands, Papua New Guinea. J Fish Biol 28:533–544
Yamashita Y, Mochizuki K, Piamthipmanus M (1991) Gut contents analysis of fishes sampled from the Gulf of Thailand. Bull Japan Soc Fish Oceanogr 55:197–207
Acknowledgements
We are grateful to K Sainsbury, C Bulman and E Cortes for providing unpublished details of fish diets. Comments from two anonymous reviewers and discussions with C Simpson and T Holmes greatly improved early versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farmer, B.M., Wilson, S.K. Diet of finfish targeted by fishers in North West Australia and the implications for trophic cascades. Environ Biol Fish 91, 71–85 (2011). https://doi.org/10.1007/s10641-010-9761-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-010-9761-3