Summary
Purpose The study evaluated the potential effect of dacomitinib, a small molecule epidermal growth factor receptor (EGFR) inhibitor, on the electrocardiogram (ECG) parameters in adult patients with advanced non-small cell lung cancer enrolled in a multicenter, open-label, phase 2 study. Methods Patients received dacomitinib for six doses of 45 mg every 12 h in a 7-day lead-in cycle (cycle 0), then 60 mg every 12 h for six doses in a 14-day cycle (cycle 1). Clock time-matched triplicate ECGs were performed at 0, 2, 4, 6, 8 and 10 h on day 1 (baseline) and day 4 of cycle 0, and prior to dose on days 1 and 4 of cycle 1. The QT interval was corrected for heart rate using Fridericia’s correction (QTcF) and a study specific correction factor (QTcS). Results Thirty-two patients in the study comprised the QTc-evaluable population. Dacomitinib had no effect on the heart rate. The upper limits of the 95% confidence interval (CI) for the mean change from baseline in QTcF and QTcS were < 10 ms at all time points. A lack of relationship between plasma concentrations of dacomitinib or total active moiety on QTcF and QTcS was evidenced. All upper 90% CIs of the PR intervals were < 200 ms, although a small mean increase from baseline (2.7–6.6 ms) was observed. Conclusions There was a lack of a clinically relevant effect of dacomitinib on ECG parameters at dacomitinib concentrations comparable to those obtained at its highest therapeutic dosing regimen of 45 mg once daily. ClinicalTrials.gov identifier: NCT01858389.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dacomitinib is a selective, adenosine triphosphate–competitive, irreversible, small-molecule inhibitor of the ErbB human epidermal growth factor receptor (HER) family of receptor tyrosine kinases, including epidermal growth factor receptor (EGFR) or HER1, HER2, HER4 and their oncogenic variants (i.e. EGFR with exon 19 deletions or exon 21 L858R mutation) [1]. When used as a first-line treatment in patients with EGFR mutation–positive non-small cell lung cancer (NSCLC), dacomitinib was found to statistically significantly improve progression-free survival [2] and overall survival [3] versus gefitinib, a first-generation EGFR tyrosine kinase inhibitor, in a randomized, open-label, phase 3 trial (ARCHER 1050). On the basis of the results from ARCHER 1050, dacomitinib was approved for the first-line treatment of patients with metastatic NSCLC containing EGFR exon 19 deletion or exon 21 L858R substitution [4].
The most common adverse events associated with dacomitinib were diarrhea, paronychia, dermatitis acneiform, and stomatitis; there were no cardiovascular adverse events of note [2]. However, a number of pharmacologic agents, including tyrosine kinase inhibitors such as sunitinib, vemurafenib, imatinib, and erlotinib, have been reported to delay cardiac repolarization and result in prolongation of the QT interval as assessed by electrocardiograms (ECGs) [5]. Prolongation of the QT interval is associated with potentially serious and life-threatening conditions, including ventricular arrhythmias, seizures, and sudden cardiac death. Therefore, the International Conference on Harmonisation guideline E14 recommends a rigorous evaluation of new drugs for potential effects on cardiac repolarization [6] by a thorough QT/corrected QT interval (QTc) clinical study also called a TQT study. A TQT study is typically conducted in healthy volunteers and is expected to cover the maximum therapeutic exposures expected in the patient population. However, conducting a TQT study for anticancer agents in healthy volunteers is associated with unwarranted risks of exposure, given that multiple dosing is needed to achieve steady-state plasma concentrations of the drug. For this reason, a TQT study in healthy volunteers was not considered for dacomitinib.
In cases where a TQT study is not possible in healthy volunteers, an alternative approach to evaluate the effect of the drug on the QT interval includes robust ECG monitoring and exposure-response analysis of data collected in an adequate number of patients with predefined criteria [6, 7]. Study A7471047 (ClinicalTrials.gov identifier: NCT01858389) was a multicenter, open-label, phase 2 study evaluating pulsatile intermittent dosing of dacomitinib in adult patients with advanced NSCLC [8]. Dacomitinib was initially dosed at 45 mg every 12 h for 6 doses, which was expected to result in plasma drug concentrations similar to the steady-state concentrations achieved with the therapeutic dose of 45 mg dacomitinib once daily [2, 3, 9]. The study included 2 cohorts of patients (cohort A, patients with EGFR T790 M–positive tumors, and cohort B, patients with EGFR T790 M–negative tumors that were otherwise molecularly unselected). The primary objective of the study was to evaluate efficacy as represented by best overall response per Response Evaluation Criteria in Solid Tumors, version 1.1. A rigorous evaluation of the potential effect of dacomitinib on QTc prolongation was included as a key secondary objective. Intensive on-treatment and baseline ECG assessments with clock-matched time points were made, along with collection of pharmacokinetic (PK) data of dacomitinib and its metabolite with similar pharmacologic activity (PF-05199265) at each on-treatment ECG assessment time point. This design was to enable the assessments of any potential QT interval prolongation and potential abnormality of other ECG parameters, and to characterize the concentration-QTc (and other ECG parameters, if needed) relationship of dacomitinib.
Materials and methods
Study design, patients, and treatment
The study design, patient population and primary results of this open-label, phase 2 study of intermittent oral dacomitinib were previously published [8]. Briefly, eligible patients had advanced NSCLC either with (cohort A) or without (cohort B) T790 M mutations. Patients with uncontrolled or significant cardiovascular disease, including a history of myocardial infarction, uncontrolled angina, congestive heart failure, ventricular arrhythmias, prolonged corrected QT interval, second- or third-degree heart block, and uncontrolled hypertension were excluded from the study. A single 12-lead ECG was performed at screening to determine eligibility.
This study was conducted under the ethical principles of the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice Guidelines. The final protocol, any amendments, and informed consent documentations were approved by the institutional review boards and/or independent ethics committees at each investigational center participating in the study. All patients provided written informed consent.
Treatment began with a 7-day lead-in cycle (cycle 0), during which patients received dacomitinib 45 mg without food every 12 h for 6 doses. Following the completion of cycle 0, cycle 1 began the following week with patients receiving dacomitinib 60 mg every 12 h for 6 doses, and cycles were repeated every 14 days. Intrapatient dose escalation beyond 60 mg was considered for patients in cohort A, provided they did not have substantial toxicity after 2 cycles on the same dose, and was done in 15-mg intervals following sponsor approval. Dose interruptions followed by dose reductions in 15-mg intervals to a decreased dose of 30 mg occurred in response to toxicity. Dose reductions below 30 mg required sponsor approval. Patients maintained medication diaries to track treatment adherence. Treatment was discontinued for progression of disease, unacceptable adverse effects, nonadherence, or withdrawal of informed consent.
ECG assessments
A centralized ECG collection system provided by Biomedical Systems (St Louis, Missouri, USA) was used in this study. Standardized ECG machines (Mortara ELI 150c, Mortara Instrument Inc., Milwaukee, Wisconsin, USA) with CAMI 7 software were supplied by Biomedical Systems to the study sites. All ECGs were performed using a 12-lead tracing, and all collected ECG data were sent to a central laboratory for review and adjudication carried out by an over-reading cardiologist. All ECGs for a given patient were to be read by a single cardiologist to maintain consistency.
Twelve-lead ECGs were performed in triplicate ~2 min apart at time 0, 2, 4, 6, 8, and 10 h on the morning of cycle 0, day 1 (C0D1; baseline; Fig. 1), and at clock-matched time points (predose [0 h] and 2, 4, 6, 8, and 10 h post dose) on C0D4 when dacomitinib plasma concentrations were expected to achieve steady-state concentrations following the therapeutic dose of 45 mg dacomitinib once daily. Triplicate ECGs were also collected prior to the first dose on C1D1 (0 h) and the sixth dose on C1D4 (0 h) and at these same time points during subsequent cycles if the dose was escalated. All ECGs were performed before blood pressure and pulse rate assessments and any type of blood draws. Meals were consumed at approximately the same time on C0D1 and C0D4. Breakfast was consumed at least 2 h prior to the first ECG on ECG assessment days. Lunch and dinner were also consumed at consistent times (recommended snacks were to be taken as needed). ECG measurements included PR interval, QT interval, RR interval, and QRS complex.
PK assessments
Blood samples were collected immediately following each ECG assessment during C0D4 for assessment of the PK of dacomitinib and its metabolite (PF-05199265; Fig. 1). Blood samples for PK assessments to match ECG collections were also collected prior to dosing on day 1 and day 4 of subsequent cycles if the dacomitinib dose was escalated (Fig. 1). Plasma concentrations of dacomitinib and its metabolite PF-05199265 were determined using a validated high-performance liquid chromatography tandem mass spectrometry assay [10]. The linearity of the calibration curve for dacomitinib was in the range of 1.0 to 200 ng/mL, with a lower limit of quantification of 1.00 ng/mL. The linearity of the calibration curve for PF-05199265 was in the range of 0.1 to 20 ng/mL, with a lower limit of quantification of 0.1 ng/mL.
Statistical analysis
Sample size determination
The sample size determination for the QTc evaluation was based on a noninferiority hypothesis testing framework. From the previous studies with data collected from >200 patients treated with dacomitinib, the estimated standard deviation of change from baseline of QTc (∆QTc) was 17.1 ms (Pfizer Inc., unpublished observations). Hence, a minimum of 31 dacomitinib-treated patients, evaluable for QTc, were required for a noninferiority margin of 20 ms, assuming 90% power, an overall 1-sided significance level of 0.05, and mean ∆QTc up to 8 ms with a standard deviation of 17.1 ms. If the upper bounds of the 1-sided 95% confidence intervals (CIs) of ∆QTc for all 6 QTc sampling time points are below 20 ms, the postbaseline dose QTc interval was to be considered noninferior to the baseline QTc interval; the QTc effect of dacomitinib was to be concluded not unacceptable. To account for an estimated 10% nonevaluability rate for QTc, ~35 patients were to be enrolled in the study, with a minimum of 31 patients evaluable for QTc. Additional patients could be enrolled in the study if required to further characterize QTc effects under the noninferiority specification with 90% power and an overall significance level of 0.05.
The as-treated population comprised all patients who received at least 1 dose of dacomitinib. The QTc-evaluable population comprised patients in the as-treated population who had all 6 ECG assessments performed at C0D1 (baseline) and C0D4. The PK analysis population comprised patients in the as-treated population who had at least 1 plasma concentration measurement of dacomitinib or its metabolite, PF-05199265.
PK analysis
For patients in the QTc-evaluable population, observed concentrations of dacomitinib, PF-05199265, and the total active moiety (dacomitinib plus PF-05269927) were summarized using descriptive statistics by nominal postdose sample collection times and visit. The concentrations of total active moiety were expressed as dacomitinib-equivalent units (ng/mL) by calculating as [concentration of dacomitinib (ng/mL)] + [concentration of PF-05199265 (ng/mL)] × molecular weight of dacomitinib (469.4)/molecular weight of PF-05199265 (455.9).
ECG analysis
The average (arithmetic mean) of triplicate ECG measurements at each time point for each patient was used for all summary statistics, data presentations, and analyses. If 1 or 2 of the triplicate ECG measurements were missing, the average of the remaining 2 measurements or the single measurement was used in the analysis. Any data obtained from ECGs repeated for safety reasons after the nominal time points were not averaged along with the preceding triplicates.
To eliminate the dependence of the QT interval on heart rate, 3 correction methods were evaluated including the QT interval corrected for heart rate using Bazett’s formula (QTcB; QT/RR0.5), QT interval corrected for heart rate using Fridericia’s formula (QTcF; QT/RR0.33), and QT interval corrected for heart rate using a study-specific correction method (QTcS; QT/RRS, where S is the slope of linear regression between unaveraged singlet values of the natural log of QT and RR intervals). QTcF was chosen prospectively as the primary end point for QTc analysis.
ECGs recorded on C0D1 were used as the baseline. For each patient at each time point (predose and 2, 4, 6, 8, and 10 h), the ΔQTc (QTcB, QTcF, and/or QTcS) and the change from baseline in other ECG parameters (heart rate, PR interval, and QRS complex) were calculated by subtraction of the time-matched baseline value (C0D1) at a particular time point from the appropriately matched postbaseline value (C0D4 and C1D4). These individual changes from baseline were summarized using descriptive statistics. Maximum change from baseline values and absolute values for ECG parameters (QTcF, QTcB, QTcS, PR interval, and QRS complex) were summarized by the number and percentage of patients using the following categories: <450, 450 to <480, 480 to <500, and ≥ 500 ms for QTc; <30, 30 to <60, and ≥ 60 ms for ΔQTc; ≥25% PR interval changes from baseline when the absolute baseline value was >200 ms; and ≥ 25% QRS complex changes from baseline when the absolute baseline value was >110 ms. The ECG analyses were repeated separately for the as-treated population and QTc-evaluable population.
For the QTc-evaluable population, a random-effects model with the nominal time point as a fixed effect and the patient as a random effect was used to estimate the mean change in QTc from baseline at each postbaseline nominal time point. The 90% CI for the changes from baseline in QTc (equivalent to the boundaries of the upper 1-sided 95% CI) was provided at each postbaseline nominal time point. Only those patients who received all scheduled doses of dacomitinib and had all ECGs performed on days 1 to 4 of cycle 0 were eligible for this assessment.
Exposure-response analysis
Patients in the as-treated population were included in the exposure-response analysis. The arithmetic mean of triplicate ECG measurements and corresponding dacomitinib and total active moiety concentrations from time-matched PK samples were used for the calculation of exposure-response relationships for each ECG parameter. For baseline ECG measurements collected on C0D1, the dacomitinib or metabolite concentration was set to 0. Observations with missing ECG and/or concentration data were excluded from the analysis.
A linear mixed-effects model was used to assess the relationships of the RR interval, QTc, and PR interval with dacomitinib concentration and the total active moiety concentration (further details are in Online Resource 1: Supplementary Methods). The arithmetic mean of the observed triplicate QTc values were used in the analyses. Nominal time was included as a factor variable on the intercept to remove the potential effect of circadian rhythm on the RR interval, QTc, and PR interval, as shown in the following model equations, Eq. 1, Eq. 2, and Eq. 3.
j indexes the measurement time for the ith patient. The intercept parameters θ1, θ2, θ3, θ4, θ5, and θ6 represent the mean RR interval or QTc in the absence of drug (concentration = 0 for baseline data) at nominal collection times 0, 2, 4, 6, 8, and 10 h, respectively; θ7 represents the population mean slope; Ƞi(1) and Ƞi(2) (ETAs) represent individual-specific random effects, which were assumed to be normally distributed with mean 0 and variance-covariance matrix ω (OMEGA); and εij (epsilon) represents the residual random variable with mean 0 and variance Σ2 (SIGMA).
Covariates, including age and sex, were tested in these models using a stepwise covariate model building procedure, and those determined to be significant were incorporated to develop the final model. The adequacy of the models developed was assessed by generating diagnostic and goodness-of-fit plots. A visual predictive check for the final model was generated to evaluate whether the model provided an accurate description of the data.
Model developments, diagnostics including graphical analysis, preprocessing, and postprocessing of data were performed using NONlinear Mixed-Effects Modeling (NONMEM®), version 7.2.0 (ICON Development Solutions, Ellicott City, MD, USA), Perl-speaks-NONMEM®, version 4.2.0, R (version 3.2.2, R Foundation for Statistical Computing, Vienna, Austria), and RStudio (version 0.99.489).
Results
Patients
A total of 41 patients were enrolled (16 patients in cohort A and 25 patients in cohort B), of whom 38 received ≥1 dose of dacomitinib and comprised the as-treated population. The PK analysis population comprised 37 patients, and the QTc-evaluable population comprised 32 patients. In the QTc-evaluable population, most patients were female (19/32, 59%) and Asian (14/32, 44%) or white (12/32, 38%), and the overall median age was 61 years, ranging from 31 to 83 years (Table 1). The baseline demographic characteristics of the as-treated and QTc-evaluable populations were comparable (Table 1).
As previously reported [8], the median duration on treatment for patients was 82 days (range 11–382 days) and 39 days (11–431 days) for cohorts A and B, respectively. The majority of patients (9 of 16) in cohort A were dose escalated. The maximum dose escalation was to 75 mg every 12 h in 5 patients, 90 mg every 12 h in 3 patients and 105 mg every 12 h in 1 patient.
Plasma concentrations of dacomitinib, metabolite, and total active moiety
Of the 32 patients in the QTc-evaluable population, 31 had plasma concentrations of dacomitinib, PF-05199265, and total active moiety (Table 2). Mean maximum plasma concentration of dacomitinib (86.6 ng/mL) occurred at 6 h post dose on C0D4 after 45 mg dacomitinib every 12 h. The range of plasma concentrations and maximum plasma concentrations observed in this study were comparable to steady-state concentrations achieved after dosing with the highest recommended therapeutic dose of dacomitinib at 45 mg once daily [4, 9, 11].
ECG analysis
The study population-specific QT correction factor, estimated to be 0.38, was identified to be the most appropriate correction factor for the exposure-response analysis on the basis of visual inspection of the QTc versus RR interval plots and the slope values generated for the 3 correction methods (Online Resource 2: Supplementary Table S1 and Online Resource 3: Supplementary Fig. S1). QTcS along with QTcF (the prespecified primary end point) were used for QTc analysis data interpretation and conclusion. The results of the QTcB analysis were included for completeness.
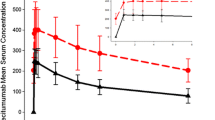
Results of the random-effects models used to estimate mean change in time-matched ECG parameters in the QTc-evaluable population are shown in Table 3, and least squares mean (LSM) change in time-matched QTcF, QTcS and QTcB intervals from baseline over time are shown in Fig. 2a–c. The LSM changes from baseline ranged from −0.4 to 4.4 ms for QTcF, −3.1 to 2.6 ms for QTcB, and − 3.5 to 2.4 ms for QTcS for all observations collected on C0D4, C1D1, and C1D4 (Table 3). The upper limits of the 1-sided 95% CI for the LSM change from baseline for QTcF and QTcS were below 10 ms for all of these observations. The largest LSM increase from baseline in QTcF (4.4 ms) and QTcS (0.4 ms) over 10 h of observation on C0D4 occurred 2 h post dose. The LSM changes from baseline ranged from 2.7 to 6.6 ms for PR interval, 0 to 2.0 ms for QRS complex, and − 4.3 to −0.8 bpm for heart rate for all observations collected on C0D4, C1D1, and C1D4 (Table 3). Summary statistics for ECG data for all patients in the QTc-Evaluable population and in the as-treated population are included in Online Resource 2: Supplementary Table S2).
Change from baseline a QTcF, b QTcS and c QTcB over time on cycle 0, day 4 and all of cycle 1 in the QTc-evaluable population. QT and RR interval values were defined at each individual time point value and then combined to derive the triplicate average values for the time point. The mean change from baseline in QTc and corresponding 90% confidence intervals (error bars) are indicated for each time point. Baseline was defined as the ECGs recorded on cycle 0, day 1. C, cycle; D, day; ECG, echocardiogram; QTcB, QT interval corrected using Bazett’s formula; QTcF, QT interval corrected using Fridericia’s formula; QTcS, QT interval corrected using a study-specific formula
The categorical analysis of QTc data in the QTc-evaluable population is shown in Table 4. No patients had a maximum postdose QTcF or QTcS interval value ≥500 ms or a maximum increase in QTcS of ≥60 ms from baseline, and 1 patient had a maximum increase in QTcF of ≥60 ms from baseline. No clinically meaningful changes in the duration of the QRS complex were observed, and no patient had a maximum increase in PR interval that was ≥25% from baseline and an absolute value that exceeded 200 ms (Table 4). Results for ECG analysis in the as-treated population were similar to those in the QTc-evaluable population (Online Resource 2: Supplementary Table S3). There were no clinically significant findings related to ECG changes at any time point in the as-treated population.
Exposure-response analysis
Data for the exposure-response analysis included a total of 1638 matched PK-ECG pairs from 38 patients at dacomitinib doses ranging from 30 mg every 12 h up to 105 mg every 12 h for a maximum of 6 doses of each cycle. At baseline (on C0D1), the average RR interval, QTcF, and QTcS were in the range of 786 to 826 ms, 404 to 407 ms, and 420 to 423 ms, respectively, across the nominal time points at baseline. On treatment (C0D4 up to C8D4), the average RR interval, QTcF, and QTcS were in the range of 803 to 867 ms, 405 to 412 ms, and 419 to 423 ms, respectively. In this analysis data set, the average on-treatment concentrations of dacomitinib, PF-05199265, and total active moiety were 79.6 ng/mL (range, 2.26 to 285 ng/mL), 7.20 ng/mL (range, 0.17 to 87.0 ng/mL), and 87.0 ng/mL (range, 2.46 to 298 ng/mL), respectively.
Effect of dacomitinib on heart rate
The relationship between the dacomitinib concentration and the RR interval was analyzed using a linear mixed-effects model with nominal time as a factor variable on the intercept as shown previously in Eq. 1. The slope estimate for the concentration-RR interval relationship was 0.14 ms per ng/mL (90% CI, 0.013, 0.267). The results projected a slight decrease in heart rate (4 beat per minute) from baseline at the highest mean concentration of 105 ng/ml observed on C1D4 after 60 mg dacomitinib every 12 h, indicating that dacomitinib had no clinically relevant effect on the heart rate.
Exposure-response relationship for QTc
A linear mixed-effects model with an intercept and slope was used to model the QTcF and QTcS against plasma concentrations of dacomitinib or total active moiety. No covariates (age, sex) were identified to be significant by stepwise covariate modeling and therefore no covariates were included in the final models. The slope estimates of the final models were 0.003 ms per ng/mL (90% CI, −0.015, 0.021) and 0.007 ms per ng/mL (90% CI, −0.013, 0.026) for QTcS vs plasma concentration (Online Resource 3: Supplementary Fig. S2A) and QTcF vs plasma concentration (Online Resource 3: Supplementary Fig. S2B), respectively. As the 95% CI included zero for both QTc assessments (QTcS and QTcF), the results of the exposure-response analyses suggested a lack of relationship between QTc and dacomitinib plasma concentration. Similar results were observed for the relationship of total active moiety concentration versus QTc (QTcS and QTcF).
Exposure-response relationship for PR interval
The relationship between the concentration of dacomitinib or total active moiety and the PR interval was analyzed using a linear mixed-effects model with nominal time as a factor variable on the intercept as shown in Eq. 3. The slope estimate of the final model was 0.044 ms per ng/mL (90% CI, 0.030, 0.058) and 0.040 ms per ng/mL (90% CI, 0.027, 0.053) for PR interval-dacomitinib concentration and PR interval-total active moiety, respectively. Diagnostic plots and visual predictive check plots showed no apparent model mis-specifications, indicating that the model was adequate to describe the observed data. The model-based mean and 2-sided 90% CI change from baseline in PR interval at the highest observed steady-state mean maximum concentration of 105 ng/ml was predicted to be 4.6 ms (90% CI, 3.1, 6.0), suggesting that dacomitinib had no clinically relevant effect on PR interval prolongation at the recommended therapeutic dosing regimen.
Discussion
The QTc evaluation in this study was conducted as an alternative assessment to a thorough QT study to investigate the potential effects of dacomitinib on cardiac repolarization at equivalent plasma concentrations of dacomitinib and its active metabolite PF-05199265 to the therapeutic dose of 45 mg once daily. Conducting a thorough QT study in oncology is challenging because dosing of anticancer agents in healthy individuals is not always feasible. Although single dose studies following a washout have been conducted with dacomitinib in healthy volunteers, an expected 5–6 fold accumulation of plasma dacomitinib concentrations at steady state following once daily dosing in clinical use precluded evaluation following a single oral dose. Therefore, a thorough QT study in healthy individuals was not considered because of the unknown risks of unwarranted exposure in this population.
Dacomitinib plasma concentrations with 45 mg once daily are expected to reach steady state within 14 days. To control for variability due to time lapse from baseline and on-treatment ECG measurements and to minimize patient non-evaluability due to dose reductions, dosing interruptions, or permanent treatment discontinuations prior to reaching steady state, ECG evaluations were conducted in this study following an every 12 h dose schedule. Using this every 12 h dosing approach, therapeutic concentrations of dacomitinib were quickly achieved with six 45-mg doses given every 12 h in the lead-in cycle (cycle 0). Slightly higher concentrations were achieved following six 60-mg doses given every 12 h in cycle 1 of treatment. Further, for patients in cohort A who were eligible for dose escalation, six doses up to 105 mg every 12 h in cycles 3–8 were administered. This enabled evaluation of ECGs at plasma concentrations higher than those expected following the recommended therapeutic dose of 45 mg once daily.
The random-effects analysis of the QTc data in this study showed that the upper bounds of the 1-sided 95% CI for the mean time-matched change from baseline for QTcF, QTcS, and QTcB were < 10 ms at all 6 time points on C0D4 in the QTc-evaluable population, indicating that a large QTc effect (i.e. >20 ms), which is considered as a clinically relevant effect, could be excluded at steady-state concentrations of dacomitinib following 45 mg once daily dosing. No patients had a maximum postdose QTcF or QTcS value ≥500 ms or a maximum increase in QTcS of ≥60 ms from baseline. One patient had a maximum increase in QTcF of ≥60 ms from baseline. Collectively, these results indicate a lack of a clinically relevant effect of dacomitinib treatment on QTc.
Further evidence of the lack of a QT interval prolongation effect of dacomitinib was demonstrated by exposure-response (concentration-QTc) modeling. The linear mixed-effects analysis was adequate to describe the relationship between dacomitinib concentrations and QTc, PR interval, and RR interval. To account for the potential effect of circadian rhythm on the QT interval, nominal time was incorporated as a factor variable on the intercept in the model. The results of concentration-QTc analyses demonstrate a lack of relationship between plasma concentrations of dacomitinib or the total active moiety on QTc (QTcF and QTcS).
The random-effect analysis demonstrated that there was a small increase of LSM in the PR interval from baseline (range, 2.7 to 6.6 ms) observed across all prespecified post-dose time points on C0D4 through C1D4 in the QTc-evaluable population (Table 3). However, the highest absolute value of the mean postbaseline PR interval, which occurred at both 2 and 10 h post dose on C0D4, was 170.3 ms with 95% CIs of 161.5, 170.0 and 160.8, 179.8, respectively (Pfizer Inc., unpublished observations). The highest upper bound of the 95% CI of the PR interval for all time points was <200 ms, indicating there was little risk for an occurrence of the first-degree atrioventricular block. The concentration-PR interval analysis demonstrated a slight positive relationship between plasma concentrations of dacomitinib on the PR interval. The model-based predicted increase in PR interval from baseline at the highest mean maximum concentration of dacomitinib was small (4.6 ms), suggesting a lack of clinically relevant drug-induced increase in the PR interval.
In conclusion, these results indicate a lack of a clinically relevant effect of dacomitinib treatment on the QTc, heart rate, or PR interval at concentrations comparable to those obtained when dacomitinib is administered at its recommended therapeutic dosing regimen of 45 mg once daily.
References
Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong KK, Janne PA (2007) PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 67(24):11924–11932. https://doi.org/10.1158/0008-5472.CAN-07-1885
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18(11):1454–1466. https://doi.org/10.1016/S1470-2045(17)30608-3
Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Lee M, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Wu YL (2018) Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 36(22):2244–2250. https://doi.org/10.1200/JCO.2018.78.7994
VIZIMPRO® (dacomitinib) tablets: US prescribing information (2018) Pfizer, Inc.; 2018
Kloth JS, Pagani A, Verboom MC, Malovini A, Napolitano C, Kruit WH, Sleijfer S, Steeghs N, Zambelli A, Mathijssen RH (2015) Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer 112(6):1011–1016. https://doi.org/10.1038/bjc.2015.82
Sager PT, Nebout T, Darpo B (2005) ICH E14: a new regulatory guidance on the clinical evaluation of QT/QTc internal prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Drug Inf J 39(4):387–394. https://doi.org/10.1177/009286150503900407
Darpo B, Sarapa N, Garnett C, Benson C, Dota C, Ferber G, Jarugula V, Johannesen L, Keirns J, Krudys K, Ortemann-Renon C, Riley S, Rogers-Subramaniam D, Stockbridge N (2014) The IQ-CSRC prospective clinical phase 1 study: “can early QT assessment using exposure response analysis replace the thorough QT study?”. Ann Noninvasive Electrocardiol 19(1):70–81. https://doi.org/10.1111/anec.12128
Yu HA, Ahn MJ, Cho BC, Gerber DE, Natale RB, Socinski MA, Giri N, Quinn S, Sbar E, Zhang H, Giaccone G (2017) Phase 2 study of intermittent pulse dacomitinib in patients with advanced non-small cell lung cancers. Lung Cancer 112:195–199. https://doi.org/10.1016/j.lungcan.2017.08.017
Janne PA, Ou SH, Kim DW, Oxnard GR, Martins R, Kris MG, Dunphy F, Nishio M, O'Connell J, Paweletz C, Taylor I, Zhang H, Goldberg Z, Mok T (2014) Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol 15(13):1433–1441. https://doi.org/10.1016/S1470-2045(14)70461-9
Ruiz-Garcia A, Masters JC, Mendes da Costa L, LaBadie RR, Liang Y, Ni G, Ellery CA, Boutros T, Goldberg Z, Bello CL (2016) Effect of food or proton pump inhibitor treatment on the bioavailability of dacomitinib in healthy volunteers. J Clin Pharmacol 56(2):223–230. https://doi.org/10.1002/jcph.588
Janne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, Guo F, Wong S, Liang J, Letrent S, Millham R, Taylor I, Eckhardt SG, Schellens JH (2011) Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 17(5):1131–1139. https://doi.org/10.1158/1078-0432.CCR-10-1220
Acknowledgments
The authors acknowledge all of the A7471047 patients and their families, the investigators and the study sites staff.
Editorial support was provided by Michelle Daniels, inScience Communications, Springer Healthcare (Philadelphia, PA, USA) and funded by Pfizer.
Funding
This study was sponsored by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
WT, KW and KP are employees of and own stock in Pfizer Inc. NG and SQ were employees of Pfizer Inc. during the analysis of the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 299 kb)
Rights and permissions
About this article
Cite this article
Tan, W., Giri, N., Quinn, S. et al. Evaluation of the potential effect of dacomitinib, an EGFR tyrosine kinase inhibitor, on ECG parameters in patients with advanced non-small cell lung cancer. Invest New Drugs 38, 874–884 (2020). https://doi.org/10.1007/s10637-019-00887-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00887-0