Summary
VEGF signaling through VEGFR-2 is the major factor in glioblastoma angiogenesis. CT-322, a pegylated protein engineered from the 10th type III human fibronectin domain, binds the VEGFR-2 extracellular domain with high specificity and affinity to block VEGF-induced VEGFR-2 signaling. This study evaluated CT-322 in an open-label run-in/phase 2 setting to assess its efficacy and safety in recurrent glioblastoma. Eligible patients had 1st, 2nd or 3rd recurrence of glioblastoma with measurable tumor on MRI and no prior anti-angiogenic therapy. The initial CT-322 dose was 1 mg/kg IV weekly, with plans to escalate subsequent patients to 2 mg/kg weekly if tolerated; within each CT-322 dose cohort, patients were randomized to ±irinotecan IV semiweekly. The primary endpoint was 6-month progression-free survival (PFS-6). Sixty-three patients with a median age of 56 were treated, the majority at first recurrence. One-third experienced serious adverse events, of which four were at least possibly related to study treatment (two intracranial hemorrhages and two infusion reactions). Twenty-nine percent of subjects developed treatment-emergent hypertension. The PFS-6 rate in the CT-322 monotherapy groups was 18.6 and 0.0 % in the 1 and 2 mg/kg treatment groups, respectively; results from the 2 mg/kg group indicated that the null hypothesis that PFS-6 ≤12 % could not be rejected. The study was terminated prior to reaching the planned enrollment for all treatment groups because data from the completed CT-322 2 mg/kg monotherapy treatment arm revealed insufficient efficacy. Despite biological activity and a tolerable side effect profile, CT-322 failed to meet the prespecified threshold for efficacy in recurrent glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-grade gliomas comprise a class of diffuse central nervous system (CNS) tumors that includes glioblastoma (GBM) as well as anaplastic gliomas. There are approximately 16,500 new cases of high-grade glioma and more than 13,000 glioma deaths annually in the United States [1]. Glioblastoma accounts for approximately 75 % of high-grade glioma patients [2]. Standard initial treatment for GBM includes surgical resection to the maximal extent possible consistent with neurological preservation, followed by concurrent radiotherapy combined with chemotherapy consisting of temozolomide, and then continued adjuvant temozolomide alone. Even with this therapy, however, the median survival in clinical trial patients is only 14.6 months, and the 1- and 2-year survivals are 61 and 27 %, respectively [3].
There is sound biological basis for the use of anti-angiogenesis agents in highly vascularized VEGF-driven tumors such as GBM. At the time this trial was launched, preliminary clinical results with bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), and other anti-VEGF agents, such as AZD2171 and aflibercept, also known as VEGF-Trap, were promising [4–8].
Fibronectins are naturally occurring proteins that bind integrins via RGD receptor recognition. CT-322 is a fibronectin tenth type 3 domain fragment modified with 19 amino acid mutations to redirect binding loops to the extracellular domain of vascular endothelial growth factor receptor-2 (VEGFR-2). A high affinity blocker of VEGFR-2, it blocks all known ligands of this critical tumor angiogenesis receptor, including VEGF-A, VEGF-C, and VEGF-D and is pegylated to extend its plasma half-life. In contrast to VEGFR tyrosine kinase inhibitors like sunitinib and sorafenib, it blocks the extracellular domain of the receptor (Fig. 1). The maximum tolerated dose (MTD) on weekly schedule from phase 1 study is 2 mg/kg [9]. Bevacizumab does not bind to or block the action of VEGF-C and VEGF-D, and the extent to which these ligands may be involved in driving recurrent GBM suggested a potential for differential activity with CT-322.
Given the clear medical need for improved treatment of recurrent GBM, the biological and clinical anti-angiogenesis precedents previously described, CT-322’s potential to curb tumor progression by preventing angiogenesis, and CT-322’s favorable adverse event (AE) profile when dosed up to the Phase 1 determined maximum tolerated dose (MTD), this phase II trial of CT-322 in recurrent GBM was undertaken. Because it is not yet clear how anti-angiogenic monotherapy with an anti-VEGF agent such as bevacizumab compares with a combination approach utilizing both anti-angiogenic and cytotoxic agents, and given the early results reported for recurrent GBM with the combination of bevacizumab and irinotecan [4], this study examined both CT-322 monotherapy and CT-322 in combination with irinotecan.
Materials and methods
This was a Phase 2, two-part, open-label study in patients with recurrent GBM for whom no standard therapy was available. The study was approved by the Institutional Review Board of each participating institution. Part 1 was the safety lead-in portion of the study, and Part 2 was the efficacy portion. Part 1 assessed the initial safety of administering CT-322 with and without irinotecan to patients with recurrent GBM in cohorts of up to four patients (Table 1). Safe was defined as: ≤1 patient in a dose and schedule cohort of 4 evaluable patients experienced an unacceptable toxicity event. Part 2 assessed the anti-tumor efficacy of CT-322 with and without irinotecan. Planned accrual for each Part 2 cohort was 21 patients. The initial CT-322 dose for Part 2 was 1 mg/kg weekly. While Part 2 was ongoing, clinical data from a phase 1 of CT-322 study became available indicating that the maximum tolerated dose of CT-322 was 2 mg/kg weekly [9]. Consequently, the protocol was amended with enrollment into the initial randomized arms of Part 2 (Arms A and B) suspended, and a 2 mg/kg safety lead-in commenced with enrollment into Cohorts 3 and 4. Patients previously assigned to receive 1 mg/kg CT-322 (Arms A and B) continued to receive their assigned treatment regimen without dose escalation. The irinotecan dose was 125 mg/m2 intravenously every 2 weeks for patients not receiving enzyme-inducing anti-epileptic drugs (EIAEDs) and 340 mg/m2 every 2 weeks for patients receiving EIAEDs. During screening, all patients were studied for the presence of the UDP-glucuronosyltransferase 1A1 (UGT1A1)*28 polymorphism. In order to minimize the risk of severe neutropenia, patients who were homozygous for this polymorphism were only assigned to a CT-322 monotherapy arm.
Eligible patients were at least 18 years of age with a histologically confirmed diagnosis of a recurrent/progressive GBM and presenting in first, second, or third relapse. Patients had to have evidence of bi-dimensionally measurable recurrent or residual tumor on contrast-enhanced magnetic resonance imaging (MRI) and the ability to undergo serial contrast-enhanced MR evaluations. Karnofsky performance status (KPS) ≥70 % and adequate bone marrow, liver, and renal function were requisite. Exclusion criteria included prior anti-VEGF therapy, CNS hemorrhage > grade 1, and full therapeutic anticoagulation. Response was assessed with MRI performed after the first 4-week cycle of therapy and repeated at 8-week intervals; confirmation of partial or complete response required confirmatory evaluation with MRI 4 weeks later.
The randomized population included all patients enrolled in Part 2 of the study (ie, Treatment Arms A-D). Patients who were randomized in Part 2 were stratified for the following: primarily refractory disease during first therapy or first recurrence versus second or third recurrence; KPS 70 % or 80 % versus 90 % or 100 %; age <50 years versus ≥50 years. Patients were analyzed according to the treatment they were assigned to receive. The ITT population included all patients enrolled in Part 1 and 2 who received any dose or part of a dose of CT-322 with or without irinotecan. All patients in the ITT sample were analyzed according to the treatment assigned, in either the safety lead-in (Part 1, Cohorts 1–4) or the randomized portion (Part 2, Treatment Arms A-D), resulting in classification into 1 of 4 treatment groups: CT-322 (1 mg/kg), CT-322 (1 mg/kg) + irinotecan, CT-322 (2 mg/kg), and CT-322 (2 mg/kg) + irinotecan. Efficacy analyses were performed on the ITT population, and each treatment group was independently evaluated for efficacy. For each treatment group, the primary null hypothesis that PFS-6 ≤12 % (p0) was tested against the alternative hypothesis that PFS-6 ≥35 % (p1) using a Kaplan-Meier estimation. The 1-sided α (type I error) was 0.025 and power (1-β) was 80 %. No alpha adjustment was made for multiplicity.
The primary efficacy endpoint was the rate of PFS-6. Secondary endpoints included PFS-12, percent of patients alive at 6 months and at 12 months, duration of PFS, duration of OS, and ORR. Assessment of objective tumor response and progression followed the Macdonald response criteria and was based on bi-dimensional tumor measurements, clinical neurological assessment, and steroid dosing. Results of an independent radiological and clinical oncology panel (IRC) constituted the primary analysis.
The safety population included all patients who received any dose or part of a dose of CT-322 with or without irinotecan. Safety variables included AEs, laboratory results, vital signs, ECGs, and echocardiograms/MUGA, and were summarized and presented by regimen.
The study was terminated prior to reaching the planned enrollment for all treatment groups because data from the completed CT-322 2 mg/kg monotherapy treatment arm revealed insufficient efficacy; consequently, the sponsor decided to terminate the study for strategic reasons. At that time, there were no safety concerns relative to CT-322 or CT-322 in combination with irinotecan.
Results
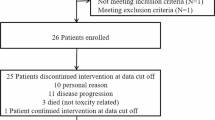
Sixty-six patients were enrolled between October 2007 and September 2010. Of these, two patients were not allocated to treatment arms because they were found to be ineligible upon central eligibility review; a third patient withdrew consent after assignment to the CT-322 arm but before initiating treatment. Thus, the study population for safety and intent-to-treat analyses was 63 patients. Patient demographics and baseline disease/treatment characteristics are recorded in Table 2; all patients had received prior radiation and chemotherapy and were naïve to anti-VEGF therapy.
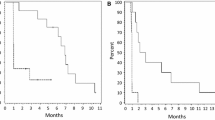
The primary efficacy endpoint was rate of PFS-6. Based on independent review committee (IRC) assessment, the rate of PFS-6 in the combination therapy groups was 64.3 and 42.1 % in the CT-322 1 mg/kg + irinotecan and CT-322 2 mg/kg + irinotecan treatment groups, respectively, and in the monotherapy groups was 18.6 and 0.0 % in the CT-322 1 and CT-322 2 mg/kg treatment groups, respectively. Objective response rates were 0 % in both combination therapy groups, 3.8 % in the CT-322 2 mg/kg monotherapy group, and 14.3 % in the CT-322 1 mg/kg monotherapy group. The PFS-6 of 0 % in the CT-322 2 mg/kg monotherapy group, the only group that reached the planned enrollment, indicated that the null hypothesis that PFS-6 ≤12 % could not be rejected for this treatment group (Table 3).
The majority of patients (93.7 % of safety population) experienced at least 1 treatment-emergent AE (TEAE) during the study; the most frequently reported TEAEs were fatigue, diarrhea, nausea and headache (Table 4). Overall, 58.7 % of patients experienced at least one grade 3 or higher TEAE. A total of 46 of 63 patients died. Of these, 6 (13.0 %) patients died within 30 days after the end of study treatment. One death was considered related to study treatment by the investigator (intracranial hemorrhage).
A total of 21 (33.3 %) patients experienced serious AEs (SAEs) during the study. Four patients overall experienced SAEs considered possibly, probably, or definitely related to study treatment: 2 patients with cerebral hemorrhage deemed possibly related, and 2 patients each with 1 event of infusion-related reaction, both definitely related.
The only TEAE leading to discontinuation for more than 1 patient was infusion-related reaction (2 patients). Four patients discontinued protocol therapy due to TEAEs that were considered possibly related to study treatment; 1 of these patients was in the CT-322 1 mg/kg treatment group (thrombocytopenia), 2 were in the CT-322 2 mg/kg treatment group (headache and intracranial hemorrhage, and intracranial hemorrhage), and 1 was in the CT-322 2 mg/kg +irinotecan treatment group (fatigue). Overall, 18 (28.6 %) patients experienced treatment-emergent hypertension. Other TEAEs of special interest were 3 events each of non-CNS hemorrhage and increased lipase; 2 infusion-related reactions as previously described; and 1 event each of venous thrombosis in a limb, proteinuria, and duodenal ulcer perforation. No events of left ventricular systolic dysfunction, reversible posterior leukoencephalopathy, pancreatitis, hypersensitivity, or impaired wound healing occurred. No trend was noted with respect to treatment group. Except for low absolute lymphocyte count (grade 3, observed in 12 patients overall [19 %]), few patients had severe abnormalities in hematology and serum chemistry laboratory parameters while on study treatment.
Discussion
Recurrent or progressive glioblastoma following standard therapy with radiotherapy and temozolomide represents a major unmet need. Prior to bevacizumab, partial response rates in this setting with standard and investigational agents were well under 10 %, with PFS-6 rates under 15 % [10]. Bevacizumab, which received FDA approval in May 2009 while the current study was well underway, produces partial responses in approximately one-quarter of patients with median progression-free survival on the order of 4 to 5 months [5, 6]. Despite the markedly improved response rates that bevacizumab produces over previous therapies, its impact on overall survival remains uncertain and likely very modest at best. Thus, more effective therapies are urgently needed, and targeting of the VEGF/VEGFR pathway remains of interest.
Scaffold-based molecules such as adnectins combine properties of small molecules and antibodies with affinity and specificity similar to antibodies [11]. Improved tissue penetration and low immunogenicity are other potential advantages of adnectins [9]. CT-322 is a pegylated adnectin that binds to human VEGFR2 with an affinity of 11 nM and has preclinical antitumor activity [12]. When the current study was initiated, an ongoing phase I study had demonstrated safety with CT-322 in weekly doses of 1 mg/kg IV; that study subsequently found an MTD of 2 mg/kg [9], leading to amendment of the recurrent glioblastoma study protocol to pursue this dose level.
CT-322 demonstrated a tolerable side effect profile. Concerns of precipitating intratumoral hemorrhage, which sometimes occurs spontaneously in glioblastoma, delayed the initial study of anti-VEGF therapy in this VEGF-driven tumor. The extent to which VEGF/R blockade increases this is unknown, although in general the risk with bevacizumab appears low and many of the bevacizumab-associated hemorrhages occur in the setting of anticoagulation [13]. Three patients in the current study developed intracranial hemorrhage. Treatment-emergent hypertension, seen in 29 %, is a class effect of VEGF/R-targeting agents [14] and was manageable in this study population. Overall, with the exception of infusion reactions, the observed adverse effects were those expected from the class of angiogenesis inhibitors and no other trends in adverse events appear related to treatment with the adnectin.
Although CT-322 induced some radiographic responses and demonstrated an anti-edema effect (Fig. 2), response rates were low and the PFS-6 rate among the 26 patients in the 2 mg/kg monotherapy arm was 0 %. While this study predated RANO criteria, utilization of these updated response criteria would not have led to superior response rates [15]. Thus, CT-322 can be considered an ineffective agent in recurrent glioblastoma. The reasons are uncertain; possible explanations include: 1) CT-322 does not effectively block the VEGFR-2 receptor clinically, 2) blocking the VEGF-2 receptor is not an effective way of altering VEGF-driven angiogenesis in recurrent glioblastoma, or 3) blocking VEGF-driven angiogenesis in any form is not an effective way of treating glioblastoma. Dynamic contrast-enhanced MRIs were performed in this study at baseline, 24 h after the first dose of CT-322, and on cycle 2, day 1 prior to administration of CT-322. Data from the subset of patients available for analysis [16] showed a modest albeit statistically insignificant reduction in Ktrans at 24 h that did not persist at the trough concentration time point, supporting the possibility that pharmacokinetics contributed to lack of efficacy.
This study’s findings confirm that for glioblastomas, targeting VEGFR2 has proven more challenging than targeting VEGF. As with cediranib [17] and cabozantinib [18] in recurrent glioblastoma, despite encouraging evidence that this new construct could inhibit angiogenic signals mediated through VEGFR2, CT-322 failed to meet the prespecified hurdle for efficacy in the absence of any clear safety signal. Similarly, a randomized phase 2 study of paclitaxel/carboplatin plus either bevacizumab or CT-322 in non-small cell lung cancer suggested that despite anti-angiogenic activity CT-322 did not improve outcomes and might be inferior [19]. Further comparisons of VEGFVEGFR-targeting agents may help delineate the differences in targeting the receptor or the ligand for optimal impact in glioblastoma and other malignancies.
Ian Walters was employed by and owned stock in Bristol-Myers-Squibb at the time of the study, and Bruce Silver was a paid consultant to Bristol-Myers-Squibb and Adnexus. The remaining authors report no conflict of interest.
References
Ostrom QT, Gittleman H, Farah P et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(Suppl 2):ii1–ii56
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(Suppl 5):v1–v49
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Batchelor TT, Sorensen AG, di Tomaso E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11(1):83–95
Gomez-Manzano C, Holash J, Fueyo J et al (2008) VEGF Trap induces antiglioma effect at different stages of disease. Neuro Oncol 10(6):940–945
Tolcher AW, Sweeney CJ, Papadopoulos K et al (2011) Phase I and pharmacokinetic study of CT-322 (BMS-844203), a targeted Adnectin inhibitor of VEGFR-2 based on a domain of human fibronectin. Clin Cancer Res 17(2):363–371
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17(8):2572–2578
Weidle UH, Auer J, Brinkmann U, Georges G, Tiefenthaler G (2013) The emerging role of new protein scaffold-based agents for treatment of cancer. Cancer Genomics Proteomics 10(4):155–168
Mamluk R, Carvajal IM, Morse BA et al (2010) Anti-tumor effect of CT-322 as an adnectin inhibitor of vascular endothelial growth factor receptor-2. MAbs 2(2):199–208
Letarte N, Bressler LR, Villano JL (2013) Bevacizumab and central nervous system (CNS) hemorrhage. Cancer Chemother Pharmacol 71(6):1561–1565
Armstrong TS, Wen PY, Gilbert MR, Schiff D (2012) Management of treatment-associated toxicites of anti-angiogenic therapy in patients with brain tumors. Neuro Oncol 14(10):1203–1214
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Schiff D, Reardon DA, Kesari S et al (2010) Phase II study of CT-322, a targeted biologic inhibitor of VEGFR-2 based on a domain of human fibronectin, in recurrent glioblastoma (rGBM). J Clin Oncol 28(No 15 suppl):2011
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31(26):3212–3218
de Groot JF, Prados M, Urquart T et al (2009) A phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse. J Clin Oncol 27(15s): Abstract 2047
Paschold EH, Mazieres J, Lena H et al (2012) A randomized, double-blinded, phase II study of paclitaxel/carboplatin (PC) plus CT-322 versus PC plus bevacizumab (Bev) as first-line treatment for advanced nonsquamous non-small cell lung cancer (NSCLC). J Clin Oncol 30(No 15_suppl (May 20 Supplement)): Abstract 7584
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schiff, D., Kesari, S., de Groot, J. et al. Phase 2 study of CT-322, a targeted biologic inhibitor of VEGFR-2 based on a domain of human fibronectin, in recurrent glioblastoma. Invest New Drugs 33, 247–253 (2015). https://doi.org/10.1007/s10637-014-0186-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0186-2