Abstract
Bevacizumab is FDA-approved for patients with recurrent GBM. However, the median duration of response is only 4 months. Potential mechanisms of resistance include upregulated FGF signaling and increased PDGF-mediated pericyte coverage. Nintedanib is an oral, small-molecule tyrosine kinase inhibitor of PDGFR α/β, FGFR 1-3, and VEGFR 1-3 that may overcome resistance to anti-VEGF therapy. This was a two-stage phase II trial in adults with first or second recurrence of GBM, stratified by prior bevacizumab therapy (ClinicalTrials.gov number NCT01380782; 1199.94). The primary endpoint was PFS6 in the bevacizumab-naive arm (Arm A) and PFS3 in the post-bevacizumab arm (Arm B). Up to 10 anaplastic glioma (AG) patients were accrued to each arm in exploratory cohorts. Twenty-two patients enrolled in Arm A and 14 in Arm B. Arm A included 12 GBMs (55 %), 13 patients with one prior regimen (59 %), and median age 54 years (range 28–75). Arm B included 10 GBMs (71 %), one patient with one prior regimen (7 %), and median age 52 years (range 32–70). Median KPS overall was 90 (range 60–100). There were no responses. In Arm A (GBM only), PFS6 was 0 %, median PFS 28 days (95 % CI 27–83), and median OS 6.9 months (3.7–8.1). In Arm B (GBM only), PFS3 was 0 %, median PFS 28 days (22–28), and median OS 2.6 months (1.0–6.9). Among AG patients in each arm, PFS6 was 0 %. Treatment was well tolerated. In conclusion, nintedanib is not active against recurrent high-grade glioma, regardless of prior bevacizumab therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis for patients with recurrent high-grade gliomas (HGG) remains poor despite advances in surgery, radiation therapy, and chemotherapy. Among patients with recurrent glioblastoma (GBM) who receive bevacizumab, the radiographic response rate is 28–38 %, and the 6-month progression-free survival (PFS6) rate is 29–50 % [1, 2]. These figures are superior to those reported in the pre-bevacizumab era, but any impact of bevacizumab on overall survival (OS) in this population remains controversial [3], with most data indicating that median OS is approximately 9 months [1, 2]. Tumors inevitably progress during bevacizumab therapy, and when they do, treatment is rarely effective. Among patients with recurrent HGG who were treated with bevacizumab and cytotoxic chemotherapy, continuation of bevacizumab and changing to another chemotherapy agent at recurrence both resulted in a median PFS of only 6 weeks [4]. Among patients with recurrent GBM treated with bevacizumab monotherapy, adding irinotecan at recurrence offered no advantage [2].
Recent studies have investigated the potential mechanisms of resistance to bevacizumab. An increase in levels of serum basic fibroblast growth factor (bFGF) was observed in patients treated with the vascular endothelial growth factor receptor (VEGFR) inhibitor cediranib [5], which may indicate that the FGF pathway promotes tumor revascularization in the setting of persistent anti-angiogenic therapy. Preclinical data suggest that dual VEGFR and platelet-derived growth factor receptor (PDGFR) inhibition reduces resistance to anti-angiogenic therapy [6], perhaps because PDGF signaling facilitates pericyte-endothelial cell interactions that stabilize the neovasculature [7].
Nintedanib is an oral, small-molecule tyrosine kinase inhibitor of PDGFR α/β, FGFR 1–3, and VEGFR 1–3 that may overcome the problem of resistance to prior anti-VEGF therapy [8]. In order to evaluate this hypothesis, we conducted a phase II, single arm, open label clinical trial in adult patients with first or second recurrence of GBM, stratified by prior treatment with bevacizumab. Before this study was complete, negative results from a similar trial conducted in Denmark were published [9].
Patients and methods
Study design and treatment
This was a phase II, single arm, open label clinical trial of nintedanib in patients with recurrent or progressive GBM (ClinicalTrials.gov number NCT01380782; 1199.94). Participants treated on the trial were stratified into Arm A (bevacizumab-naive) and Arm B (bevacizumab-treated). In Arm A, efficacy was measured by PFS6, and in Arm B, by PFS3. Secondary objectives in both arms included OS, radiographic response rate, time-to-progression, and safety. An exploratory subgroup in each arm had recurrent anaplastic glioma (AG).
All subjects received nintedanib 200 mg twice a day by mouth until disease progression or unacceptable adverse event. Treatment was administered in 28-day cycles. Subjects had weekly blood pressure measurement during Cycles 1 and 2. Physical and neurologic examinations were performed every 4 weeks, and brain MRI scans obtained every 8 weeks. Responses were assessed according to the Response Assessment in Neuro-Oncology (RANO) criteria [10].
Eligibility criteria
The research was approved by the local institutional review boards, and informed consent was provided by all participants. Patients were at least 18 years old with Karnofsky performance status of 60 or higher. They had histologically confirmed high-grade glioma with unequivocal evidence of progression and had received treatment for no more than 2 prior relapses. At least 2–6 weeks had elapsed from previous anti-tumor therapy, depending on the specific agent. For patients in Arm B, at least 3 weeks had elapsed from prior bevacizumab therapy. Patients had no prior therapy with an inhibitor of VEGF, VEGFR, PDGFR, or FGFR, except in Arm B, where previous bevacizumab was permissible. All participants were required to have adequate bone marrow and organ function. Exclusion criteria included use of warfarin or enzyme-inducing anti-epileptic drugs within 14 days, evidence of recent hemorrhage on baseline MRI of the brain, and uncontrolled hypertension, recent surgical procedure, or medical illness that would increase the risk of nintedanib therapy.
Treatment modifications
Adverse events were assessed using the common terminology criteria for adverse events version 4.0. Nintedanib dose modification was required if patients developed evidence of hepatic toxicity or severe or unmanageable hypertension. Dose modification was permitted at the investigator’s discretion for other grade 2 adverse events, and it was required for other grade 3 or higher adverse events. Dose reductions to 150 and 100 mg twice a day were permitted. If a dose reduction below 100 mg twice a day was needed, participants stopped study therapy.
Statistical considerations
Arm A was designed to discriminate between a 36 and 55 % PFS6 rate, with alpha error of 0.075 and beta error 0.2. This arm employed a Simon optimal two-stage design [11], with up to 14 GBM patients accrued in Stage 1. The probability of early termination if the drug was ineffective was 61 %.
Arm B was designed to discriminate between a 20 and 40 % PFS3 rate, with alpha error of 0.075 and beta error 0.2. This arm also employed a Simon optimal two-stage design [11], with up to 11 GBM patients accrued in Stage 1. The probability of early termination if the drug was ineffective was 62 %.
Descriptive statistics were used to summarize results. The Kaplan–Meier technique was used to estimate PFS and OS. Ninety-five percent confidence intervals (95 % CI) were calculated for all point estimates.
Results
Patient characteristics
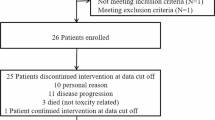
Patient characteristics are presented in Table 1. There were 22 patients in Arm A and 14 in Arm B. The median age was typical for high-grade glioma studies, 54 years in Arm A and 52 years in Arm B. Overall there were 18 men and 18 women, with 7 men and 15 women in Arm A. Median KPS was 90 in both arms. Arm A included 12 GBMs, and Arm B included 10 GBMs. An exploratory subgroup of Arm A included 10 AGs, and an exploratory subgroup of Arm B included 4 AGs. In Arm A, 13 (59 %) patients had been treated for one prior recurrence and 9 (41 %) patients for two prior recurrences. In Arm B, all but one patient had been treated for two prior recurrences.
Adverse events
Treatment was generally well tolerated (Table 2). The most common adverse events included mild diarrhea, nausea, vomiting, abdominal pain, and elevated alanine aminotransferase levels. Serious adverse events (grades 3–5) that were judged at least possibly related to nintedanib included abdominal pain (n = 1), reversible transaminase elevation (n = 8), hypertension (n = 1), hypophosphatemia (n = 3), intracranial hemorrhage (n = 1), colonic perforation (n = 2), and pulmonary embolism (n = 1). There were two deaths during treatment, one due to pulmonary embolism and one due to colonic perforation. Both occurred in Arm A.
Responses and survival
There were no radiographic responses in either arm. In Arm A, 4 GBM patients (33 %) achieved stable disease. In Arm B, one GBM patient (10 %) achieved stable disease. The median and maximum duration of stable disease were 28 days in each arm. Among the patients with GBM in Arm A, PFS6 was 0 %, median PFS 28 days (95 % CI 27–83), and median OS 6.9 months (95 % CI 3.7–8.1). Among the patients with GBM in Arm B, PFS3 was 0 %, median PFS 28 days (95 % CI 22–28), and median OS 2.6 months (95 % CI 1.0–6.9). The survival data are summarized in Fig. 1.
Among AG patients in Arm A, 4 (40 %) patients achieved stable disease. PFS6 was 0 %, median PFS 28 days (95 % CI 27–73), and median OS 11.3 months (95 % CI 2.7–14.6). Among AG patients in Arm B, one (25 %) patient achieved stable disease. PFS3 was 0 %, median PFS 36 days (95 % CI 28–56), and median OS 7.3 months (95 % CI 1.4–18.1).
Discussion
In this phase II study of adults with first or second recurrence of GBM, nintedanib therapy failed to prolong PFS6 in bevacizumab-naive patients or PFS3 in patients whose tumors had progressed despite bevacizumab. No radiographic responses were observed on MRI in either arm. Although the study was not powered to demonstrate benefit in AG patients, these patients also fared poorly. Overall, the study population was typical of recurrent high-grade glioma studies. Participants had unremarkable demographic and performance status characteristics, and the Arm A patients had been treated with no more than 2 prior regimens.
Unfortunately, the results indicate that nintedanib is not active in treating patients with recurrent high-grade glioma, regardless of prior bevacizumab therapy. The findings are consistent with another recent study of nintedanib for recurrent GBM patients in which enrollment was terminated early because of futility [9]. Other small-molecule inhibitors of VEGFR and PDGFR have been studied recently in GBM patients with similarly disappointing findings. Examples include cediranib [12], sunitinib [13], and sorafenib [14, 15].
Nintedanib is the first agent with activity against VEGFR, PDGFR, and FGFR to be studied in recurrent glioma patients. The negative results are unexpected in light of compelling pre-clinical evidence that FGFR signaling has a role in glioma growth and invasion that is both dependent and independent of angiogenesis [16]. There are multiple potential explanations that should be considered. Perhaps most likely is the possibility that the anti-angiogenic effect of nintedanib is insufficiently potent in GBM patients, in comparison for example to bevacizumab. In bevacizumab-treated patients with recurrent GBM, the response rate is in the range of 28–38 % and PFS6 approaches 50 % [1]. Cediranib, an oral inhibitor of VEGFR and PDGFR, has a response rate in the range of 15 % and median PFS of approximately 90 days [12], compared to 0 % and less than 30 days for nintedanib, respectively. This hypothesis might also account for the very low rates of class-related adverse effects seen here, including hypertension, proteinuria, and hemorrhage.
Another reasonable consideration is that PDGFR and FGFR may not be critical mediators of resistance to anti-VEGFR signaling in vivo, or that they are not inhibited with sufficient potency to achieve the needed pharmacodynamic effect. The latter seems doubtful in light of in vitro studies which indicate that the target receptors are all inhibited by nintedanib in low nanomolar concentrations [8]. The recommended dose for phase II monotherapy studies based on two phase I monotherapy studies in patients with advanced cancer is 200 mg twice a day [17, 18], the same dose that was used here. Because of the favorable adverse event profile, few patients in the current study required dose reductions that might have compromised efficacy. The relatively small number of target-related adverse effects observed could suggest that the recommended phase II dose is too low, although this dose has proven effective in patients with recurrent non-small cell lung cancer [19].
Proprietary data indicate that nintedanib does not significantly cross the blood–brain barrier (Investigator’s Brochure U03-1563). It is generally accepted that anti-angiogenic agents may function entirely, or nearly so, in the endothelial compartment outside the blood–brain barrier [20, 21]. Because much of the benefit of anti-VEGF therapies relates to reduced vascular permeability, blood–brain barrier penetration may not offer additional benefit. The same could be said of the anti-VEGFR activity of nintedanib, but it is also plausible that PDGFR and FGFR inhibition must happen at the level of infiltrating glioma cells and not just endothelial cells in order to effectively limit tumor invasion. In addition, nintedanib is a substrate of P-glycoprotein, a drug efflux pump that likely contributes to drug resistance in glioma cells [22].
The observation of two deaths during therapy warrants comment. Both patients who died were in Arm A and succumbed to complications that have been associated with anti-angiogenic therapies, pulmonary embolism [23] and intestinal perforation [24]. The low patient numbers here preclude definitive attribution of the events to nintedanib. The relatively small numbers of patients who experienced more common anti-angiogenic therapy-related adverse events such as hypertension, proteinuria, minor bleeding, and non-catastrophic venous thromboembolism would suggest that the two possibly treatment-related deaths occurred by chance. Both venous thromboembolism and intestinal perforation are known to occur at increased frequency in patients with high-grade glioma [25], the latter particularly among patients who are taking corticosteroids.
Nintedanib is not active in patients with recurrent high-grade gliomas. In the absence of detailed pharmacokinetic and pharmacodynamic evaluations, it is not possible to fully understand the poor outcomes observed in the current trial.
References
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Piccioni DE, Selfridge J, Mody RR, Chowdhury R, Li S, Lalezari S, Wawrzynski J, Quan J, Zurayk M, Chou AP, Sanchez DE, Liau LM, Ellingson BM, Pope WB, Nghiemphu PL, Green RM, Wang HJ, Yong WH, Elashoff R, Cloughesy TF, Lai A (2014) Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol. doi:10.1093/neuonc/nou028
Quant EC, Norden AD, Drappatz J, Muzikansky A, Doherty L, Lafrankie D, Ciampa A, Kesari S, Wen PY (2009) Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 11:550–555
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Erber R, Thurnher A, Katsen A, Groth G, Kerger H, Hammes H, Menger M, Ullrich A, Vajkoczy P (2004) Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 18:338–340
Guo P, Hu B, Gu W, Xu L, Wang D, Huang H, Cavenee W, Cheng S (2003) Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol 162:1083–1093
Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, Garin-Chesa P, Bader G, Zoephel A, Quant J, Heckel A, Rettig WJ (2008) BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 68:4774–4782. doi:10.1158/0008-5472.CAN-07-6307
Muhic A, Poulsen HS, Sorensen M, Grunnet K, Lassen U (2013) Phase II open-label study of nintedanib in patients with recurrent glioblastoma multiforme. J Neurooncol 111:205–212. doi:10.1007/s11060-012-1009-y
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Simon R (1987) How large should a phase II trial of a new drug be? Cancer Treat Rep 71:1079–1085
Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, Mason W, Mikkelsen T, Phuphanich S, Ashby LS, Degroot J, Gattamaneni R, Cher L, Rosenthal M, Payer F, Jurgensmeier JM, Jain RK, Sorensen AG, Xu J, Liu Q, van den Bent M (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218. doi:10.1200/JCO.2012.47.2464
Hutterer M, Nowosielski M, Haybaeck J, Embacher S, Stockhammer F, Gotwald T, Holzner B, Capper D, Preusser M, Marosi C, Oberndorfer S, Moik M, Buchroithner J, Seiz M, Tuettenberg J, Herrlinger U, Wick A, Vajkoczy P, Stockhammer G (2014) A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01–07). Neuro Oncol 16:92–102. doi:10.1093/neuonc/not161
Galanis E, Anderson SK, Lafky JM, Uhm JH, Giannini C, Kumar SK, Kimlinger TK, Northfelt DW, Flynn PJ, Jaeckle KA, Kaufmann TJ, Buckner JC (2013) Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res 19:4816–4823. doi:10.1158/1078-0432.CCR-13-0708
Reardon DA, Vredenburgh JJ, Desjardins A, Peters K, Gururangan S, Sampson JH, Marcello J, Herndon JE 2nd, McLendon RE, Janney D, Friedman AH, Bigner DD, Friedman HS (2011) Effect of CYP3A-inducing anti-epileptics on sorafenib exposure: results of a phase II study of sorafenib plus daily temozolomide in adults with recurrent glioblastoma. J Neurooncol 101:57–66. doi:10.1007/s11060-010-0217-6
Auguste P, Gursel DB, Lemiere S, Reimers D, Cuevas P, Carceller F, Di Santo JP, Bikfalvi A (2001) Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res 61:1717–1726
Okamoto I, Kaneda H, Satoh T, Okamoto W, Miyazaki M, Morinaga R, Ueda S, Terashima M, Tsuya A, Sarashina A, Konishi K, Arao T, Nishio K, Kaiser R, Nakagawa K (2010) Phase I safety, pharmacokinetic, and biomarker study of BIBF 1120, an oral triple tyrosine kinase inhibitor in patients with advanced solid tumors. Mol Cancer Ther 9:2825–2833. doi:10.1158/1535-7163.MCT-10-0379
Mross K, Stefanic M, Gmehling D, Frost A, Baas F, Unger C, Strecker R, Henning J, Gaschler-Markefski B, Stopfer P, de Rossi L, Kaiser R (2010) Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors. Clin Cancer Res 16:311–319. doi:10.1158/1078-0432.CCR-09-0694
Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S, Group LU-LS (2014) Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 15:143–155. doi:10.1016/S1470-2045(13)70586-2
Lampson LA (2011) Monoclonal antibodies in neuro-oncology: getting past the blood-brain barrier. mAbs 3:153–160
Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, Leenders WP, van Furth WR (2009) Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer 9:444. doi:10.1186/1471-2407-9-444
Veringa SJ, Biesmans D, van Vuurden DG, Jansen MH, Wedekind LE, Horsman I, Wesseling P, Vandertop WP, Noske DP, Kaspers GJ, Hulleman E (2013) In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS ONE 8:e61512. doi:10.1371/journal.pone.0061512
Perry JR (2012) Thromboembolic disease in patients with high-grade glioma. Neuro Oncol 14(Suppl 4):iv73–80
Norden AD, Drappatz J, Ciampa AS, Doherty L, LaFrankie DC, Kesari S, Wen PY (2009) Colon perforation during antiangiogenic therapy for malignant glioma. Neuro Oncol 11:92–95. doi:10.1215/15228517-2008-071
Drappatz J, Schiff D, Kesari S, Norden AD, Wen PY (2007) Medical management of brain tumor patients. Neurol Clin 25:1035–1071. doi:10.1016/j.ncl.2007.07.015
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norden, A.D., Schiff, D., Ahluwalia, M.S. et al. Phase II trial of triple tyrosine kinase receptor inhibitor nintedanib in recurrent high-grade gliomas. J Neurooncol 121, 297–302 (2015). https://doi.org/10.1007/s11060-014-1631-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1631-y