Summary
Objectives Sorafenib is a multi-tyrosine kinase inhibitor of Raf kinase, VEGFR, and PDGFR. Angiogenesis is important for growth and progression of SCLC. This trial was conducted to evaluate whether the combination of cisplatin and etoposide plus concurrent and sequential sorafenib could prolong survival in patients with previously untreated SCLC. Methods Previously untreated patients with extensive stage SCLC were treated with cisplatin and etoposide days 1, 2, 3 for four cycles, concurrent with sorafenib 200 mg orally bid starting day 1 cycle 1. Patients with no disease progression after four cycles continued sorafenib 400 mg orally bid as maintenance for maximum of 12 months. The primary endpoint was 1 year survival with response rate and safety as secondary endpoints. Results A total of 18 patients were enrolled with 17 evaluable patients. One patient had a complete response, seven patients had a partial response (overall response rate of 47 %) and one patient had stable disease. Overall median survival was 7.4 months and 1 year survival was 25 %. The most common treatment-related adverse events included fatigue, anorexia, rash, diarrhea, neutropenia and weight loss. Grade 5 GI bleeding, pulmonary hemorrhage and neutropenia occurred in one pt (6 %) each. Accrual was halted on the basis of safety profile as well as preliminary efficacy data. Conclusions The combination of platinum based chemotherapy and sorafenib has significant toxicity at current dose levels and is associated with disappointing efficacy data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common cause of cancer related mortality in United Sates and caused an estimated 156,940 deaths in 2011 [1]. Small-cell lung cancer (SCLC) accounts for approximately 15 % of all lung cancer cases, and of these 70 % are diagnosed with extensive stage (ES-SCLC) disease at the time of initial presentation. SCLC is characterized by rapid tumor proliferation, early development of widespread metastases and a median survival of less than 3 months in untreated patients [2]. Combination chemotherapy is the mainstay of treatment, with cisplatin and etoposide being the most commonly used regimen [3]. Although chemotherapy produces encouraging response rates of 60 %–70 % in ES-SCLC, all patients experience relapse. Prognosis at relapse is poor and response to second-line chemotherapy is low [4]. Unfortunately, long term survivors are rare and despite the use of a variety of strategies, there has been only a modest improvement in survival of these patients over the last several decades [5]. Therefore, there is a need for evaluation of novel agents to improve disease outcomes.
The role of angiogenesis in growth of solid tumors is well known; several antiangiogenic therapies have improved outcomes for different tumor types including non small cell lung cancer (NSCLC), colon cancer, renal cell cancer and glioblastoma [6]. Pre-clinical evidence suggests that angiogenesis is critical to the growth and sustenance of SCLC. Lucchi et al. reported that high microvessel density and vascular endothelial growth factor (VEGF) protein expression correlated with poor clinical outcome in patients with limited stage SCLC undergoing surgical resection followed by adjuvant chemotherapy [7]. High pre-treatment serum levels of VEGF and basic fibroblast growth factor (b-FGF) predict poor prognosis in SCLC [8]. Patients with a lower pretreatment circulating VEGF levels were more likely to respond to chemotherapy compared to those with higher levels of VEGF [9]. The results from these studies suggest that VEGF may be linked to overall poor outcome in SCLC. Therefore, inhibition of VEGF represents a rational therapeutic strategy for evaluation in SCLC.
Sorafenib exhibits broad spectrum oral anti-tumor activity and targets the RAF/MEK/ERK pathway involved in cellular proliferation as well as receptor tyrosine kinases involved in angiogenesis (VEGFR-1, VEGFR-2, VEGFR-3, PDGFR and RET) [10]. Sorafenib demonstrated antitumor activity in various cancer cell lines including lung cancer by inhibiting proliferation and inducing apoptosis. In preclinical human cancer models sorafenib reduced tumor growth by inhibiting angiogenesis and directly inducing tumor cell apoptosis [11]. Sorafenib has been approved to treat advanced renal cell carcinoma and hepatocellular carcinoma based on improved survival in Phase III clinical trials [12, 13]. Sorafenib has also been tested in multiple clinical trials in advanced NSCLC either as single agent or in combination with other biological or cytotoxic agents [11]. On the basis of the potential role of angiogenesis in growth of SCLC, we conducted a phase II study of sorafenib in combination with first line standard chemotherpy (etoposide plus cisplatin) and as maintenance therapy in extensive-stage SCLC. The primary goal of this study was to evaluate the combination of standard chemotherapy plus concurrent and sequential sorafenib with respect to 1 year overall survival in patients with previously untreated SCLC.
Patients and methods
This trial enrolled patients between August 2008 and November 2011. Participating centers included University Hospitals Seidman Cancer Center, Cleveland Clinic Taussig Cancer Institute and New York-Presbyterian Hospital, Columbia University. All participating centers were required to have institutional review board approval for the study, and all patients gave written informed consent to participate in this study in accordance with institutional and federal guidelines. Investigational agent, sorafenib was provided by Bayer-Onyx HealthCare Pharmaceuticals.
Eligibility criteria
Patients with histologically or cytologically confirmed extensive-stage SCLC and no prior chemotherapy were eligible. All patients enrolled in the study were age 18 years or older and had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2. Other eligibility criteria included: adequate organ function, defined as absolute neutrophil count ≥1,500/mm3, platelet count ≥100 × 109/L, hemoglobin ≥9.0 g/dl, serum total bilirubin ≤ 1.5 × the upper limit of normal (ULN), serum ALT and AST ≤ 2.5 × the ULN (≤5 times ULN for patients with liver involvement) and serum creatinine ≤ 1.5 × ULN. Patients must also have an INR < 1.5 or a PT/PTT within normal limits. Those patients receiving anti-coagulation therapy, the INR was stable before entrance into the trial.
Exclusion criteria included: major surgery within 4 weeks of treatment, clinically significant cardiovascular disease, untreated brain metastases, uncontrolled hypertension, thrombotic or embolic events within the past 6 months, pulmonary hemorrhage ≥ common terminology criteria for adverse events (CTCAE) Grade 2 within 4 weeks of first dose of study drug, any other hemorrhage/bleeding event ≥ CTCAE Grade 3 within 4 weeks of first dose of study drug, serious non-healing wound, history of bleeding diathesis or coagulopathy, HIV or chronic HBV or HCV infection and active clinical serious infection. Other standard general medical exclusions also applied.
Treatment
Previously untreated pts with extensive stage SCLC were treated with cisplatin 60 mg/m2 intravenously (IV) on day 1 and etoposide 120 mg/m2 IV on days 1, 2, 3, every 21 days for four cycles, concurrent with sorafenib 200 mg orally twice daily starting on day 1 cycle 1. Use of granulocyte colony stimulating factors was allowed at cycle 2 and beyond or if patients developed febrile neutropenia. Pts with no disease progression after four cycles continued sorafenib 400 mg orally twice daily as maintenance for maximum of 12 months or until unacceptable toxicity, disease progression or withdrawal from the study. Radiographic studies were performed every two cycles of therapy to assess response. The Response Evaluation Criteria in Solid Tumors (RECIST) were used to assess response to the treatment. Toxicity was graded by the National Cancer Institute Common Terminology Criteria version 3.0.
Dose modifications for toxicity
Chemotherapy dose reductions were permitted for febrile neutropenia, thrombocytopenic bleeding, creatinine greater than 2.0 and less than 3.0 mg/dL, with creatinine clearance greater than 60 mL/min, and cisplatin-related neurotoxicity. In the event of significant toxicity (grade 3 non-hematological or grade 4 hematological) related to sorafenib during chemotherapy (relation based on investigator’s assessment), the dose of sorafenib was held until the toxicity resolved to ≤ grade 1. At that time, drug was resumed at 200 mg daily until completion of cycle. Subsequent cycles of chemotherapy used the reduced dose of sorafenib. If further dose reduction was required, sorafenib 200 mg every other day could be given. If more than two dose reductions were required, sorafenib was held until the maintenance phase at which time full dose sorafenib started. During maintenance phase, sorafenib dose reduction for significant toxicity to 400 mg daily and then 400 mg every other day was permitted. For symptomatic Grade 2 or Grade 3 hypertension, sorafenib was held until symptoms resolved and then restarted at one dose lower. Guidelines for management of hypertension and hand foot skin reactions were included in the study protocol. A maximum of two dose reductions were permitted for each patient. Delay in initiation of therapy of longer than 3 weeks due to toxicity resulted in removal from the study.
Baseline and treatment assessments
Baseline evaluations included history and physical examination, assessment of ECOG PS, complete blood count with differential (CBC), serum chemistry, vital signs, serum pregnancy test for women of child bearing potential and international normalized ratio/activated partial thromboplastin time for patients on warfarin. Radiographic studies including computerized axial tomography (CT) scans were performed within 4 weeks prior to study for sites of measurable disease or as clinically indicated. Tumor status was assessed every 8 weeks using RECIST criteria.
Statistical analysis
The primary goal of this study was to determine 1 year overall survival (OS) after treatment with cisplatin/etoposide plus concurrent and sequential sorafenib in patients with previously untreated SCLC. Secondary endpoints included assessment of toxicity, progression free survival (PFS), and response rate. Extensive stage SCLC patients who have been treated with chemotherapy have a 1-year survival rate of 35 to 40 % [3]. This was used as the historical control. A 60 % 1 year survival was expected with the addition of sorafenib. With type I error of 0.05, a power of 80 %, 18 months of accrual and a follow-up period of 12 months the estimated sample size needed for the study was 28 patients based on one-sided exponential MLE test. Progression free survival and overall survival were estimated using the Kaplan-Meier method by comparing 1 year overall survival of patients enrolled in this study to historical controls using 95 % confidence intervals. Patients who received at least one dose of the study drug were considered evaluable for both toxicity and response.
Results
Patient characteristics
A total of 18 patients were enrolled into the trial from three sites between August 2008 and November 2011. Patient characteristics are listed in Table 1. The median age was 63 years (range 45–82) and 53 % of patients were male. ECOG performance status was 1 in 11 patients (65 %) and 2 in 6 patients (35 %). Seventeen patients were evaluable for safety and efficacy; one patient did not receive the treatment regimen.
Dose delivery
One dose reduction in chemotherapy was required in 6/18 (33 %) patients during the chemotherapy induction period. Sorafenib dose reductions and treatment pauses occurred in all patients (100 %) during the concurrent chemotherapy and sorafenib part of the study. Five patients (28 %) were able to receive maintenance sorafenib. In the maintenance phase, sorafenib was given at full dose without a requirement for dose reduction. This suggests that single agent sorafenib is well tolerated in SCLC patients; however when combined with chemotherapy enhanced toxicity was observed.
Efficacy
Objective (complete plus partial) response rate (ORR) was 47 %, with 1 complete and 7 partial responses. One patient achieved stable disease (SD), while all others either had objective disease progression or clinical decline.
Time to event measures
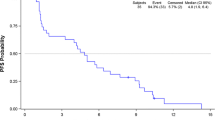
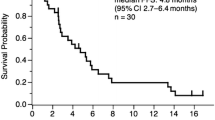
Survival outcomes for 16 of the 18 enrolled patients are summarized in Table 2 with Kaplan-Meier estimates of overall and progression-free survival presented in Fig. 1, respectively. Median overall survival was 7.4 months (95 % confidence interval: 2.5–10.2) (Fig. 1) and median progression-free survival was 5.1 months (95 % confidence interval: 1.2–8.2). Survival estimates at 1 year were 25 % for both overall and progression-free survival.
Toxicity
Treatment related toxicity is summarized in Table 3. The most common treatment-related adverse events included neutropenia, fatigue, anorexia, rash, diarrhea and mucositis. Grade 4 events included neutropenia in eight patients (48 %), pneumonitis, thrombocytopenia and hypocalcemia in one patient (6 %) each. Significant Grade 3 events included hyponatremia in three patients (18 %), supraventricular tachycardia and hypokalemia in two patients (12 %) each. Grade 5 gastrointestinal bleeding, pulmonary hemorrhage and neutropenic fever occurred in one patient (6 %) each. Accrual was halted to this study on the basis of excessive treatment related deaths (17 %) that would be expected with chemotherapy alone.
Discussion
Inhibition of angiogenesis has been successful in improving the efficacy of chemotherapy in variety of solid organ malignancies [6]. Preclinical data supports the use of VEGF tyrosine kinase inhibitor in combination with chemotherapy in mouse models of SCLC [14, 15]. Sorafenib is a multikinase inhibitor that targets VEGF, Raf and PDGF receptor tyrosine kinase signaling and has broad preclinical and clinical activity [16]. To our knowledge this is the first study of sorafenib in combination with first line cytotoxic chemotherapy for extensive stage SCLC. Previously sorafenib was evaluated in a phase II study in relapsed/refractory patients with extensive stage SCLC after first line platinum based chemotherapy [17]. The primary endpoint was to evaluate objective response rate. Patients were treated with oral sorafenib 400 mg twice daily for a 28-day cycle. Patients were stratified into platinum-sensitive or platinum-refractory groups. There were four partial responses seen among the 38 patients on the platinum-sensitive group and one partial response among the 45 patients in the platinum-refractory group. Five objective responses seen in this trial provided some evidence for clinical activity of sorafenib in SCLC. It was hypothesized that addition of sorafenib to chemotherapy we’d increase the response rate and keep the resistant clonal population suppressed with maintenance treatment.
This study was terminated after enrolling 18 patients on the basis of excessive toxicity observed and preliminary efficacy data showing that study was unlikely to meet primary end point. In contrast to the preclinical models and single agent activity in relapsed/refractory setting, the addition of sorafenib to first line chemotherapy did not result in significant increase in response rate or survival at 1 year. Moreover, addition of sorafenib significantly increased the incidence of grade 4 and 5 toxicities. Although small number of patients limits the interpretation of the results, concern for the increased toxicity of chemotherapy in combination with VEGF tyrosine kinase inhibitors has also been reported in other studies. In a phase IB study conducted by CALGB (Cancer and Leukemia Group B), sunitinib 25 mg daily on days 1 to 14 was given in combination with standard cisplatin and etoposide. This regimen resulted in prolonged neutropenia and an unacceptable rate of treatment-related mortality despite the use of prophylactic granulocyte growth factors [18].
Several other studies have been conducted utilizing different angiogenesis inhibitors either in combination with first line chemotherapy or as maintenance therapy in patients responding to the first line chemotherapy (Table 4). Cediranib, a potent inhibitor of VEGFR-1, 2, and 3 tyrosine kinases was evaluated in a phase II study in patients with progressive SCLC after one prior platinum-based regimen [19]. Among 25 patients enrolled in this study, nine had stable disease; none had a confirmed partial response. The median PFS and OS were 2 and 6 months respectively, the trial was terminated for not meeting its predefined efficacy goal. A randomized phase II trial evaluating vandetanib (VEGFR-2 and EGFR inhibitor) in SCLC patients after objective response to first-line platinum-based chemotherapy, showed no benefit in terms of OS or PFS, when compared with placebo [20]. Two separate phase II trials have evaluated maintenance sunitinib (inhibitor of platelet-derived growth factor receptor, VEGFR, c-kit, FLT3, and RET) following first line platinum based chemotherapy for patients with extensive-stage SCLC [21, 22]. Results were conflicting, one study demonstrated encouraging 1-year OS of 54 % and median time to progression of 7.6 months with rare grade 3/4 toxicity, whereas other study concluded that sunitinib did not seem to increase efficacy after response to chemotherapy and was associated with poor tolerance due to toxicity.
Bevacizumab (BV), a monoclonal antibody targeting VEGF pathway has been explored in three single-arm phase II studies of BV and platinum-containing chemotherapy regimens in patients with untreated extensive-stage SCLC. In the ECOG (Eastern Cooperative Oncology Group) 3501 study of BV plus cisplatin/etoposide, the ORR was 63.5 %, with median PFS of 4.7 months and median OS of 10.9 months [23]. In the CALGB 30306 study of BV plus cisplatin/irinotecan, ORR was 75 %, with median PFS of 7.0 month and median OS of 11.6 months [24]. In the LUN90 (Irinotecan, Carboplatin, Bevacizumab in the Treatment of Patients With Extensive Stage Small Cell Lung Cancer) study, ORR was 84 %, with median time to progression of 9.1 months and median OS of 12.1 months [25]. Based on the encouraging efficacy observed in these single arm phase II studies, a randomized, double-blind, placebo-controlled phase II study SALUTE (A Study of Bevacizumab in Previously Untreated Extensive-Stage Small Cell Lung Cancer) was conducted, which showed improved PFS (5.5 months) but no improvement in OS (9.4 months) [26].
Incidence of adverse events (AEs) in trials with combination of chemotherapy and BV in SCLC were similar to expected AEs known to be associated with chemotherapy and BV containing regimens in other solid tumors. Although rationale of combining chemotherapy with VEGFR tyrosine kinase inhibitors appears promising, it has been associated with significant toxicities across the tumor types. A randomized study of cediranib in combination with carboplatin and paclitaxel in NSCLC was halted due to excessive toxicity observed in the combination arm [27]. Combination of sorafenib 400 mg orally twice a day with capecitabine in patients with HER2-negative advanced breast cancer was effective but resulted in unacceptable toxicity for many patients [28]. Although our study was designed with reduced dose of sorafenib (200 mg orally twice a day) for the concurrent administration with chemotherapy, it still resulted in unacceptable toxicity.
Given the disappointing results from the multiple clinical trials of anti-angiogenic agents in SCLC, it is necessary to identify predictive biomarkers that may lead to optimal utilization of these agents. In the ECOG 3501 study, patients who had high baseline vascular cell adhesion molecule (VCAM) levels in plasma, had a higher risk of progression or death [23]. Since this was a single-arm study, it could not be concluded whether this was a prognostic marker or predictive for combination of Bevacizumab with chemotherapy. BATTLE trial in NSCLC showed that patients with k-ras mutation in the tumor derived significant benefit from treatment with sorafenib [29]; however this was not confirmed in recent phase III MISSION trial, where k-ras mutation status was not predictive of sorafenib efficacy in NSCLC patients receiving sorafenib as third or fourth line treatment [30].
In conclusion, the combination of cisplatin, etoposide and sorafenib has significant toxicity and is unlikely to be superior to standard treatment with chemotherapy alone. Further investigation of sorafenib concurrent with chemotherapy at current dose levels in the SCLC population is not recommended. There is need for identification of biomarkers to select the patient population likely to benefit with angiogenesis inhibitors in the treatment of SCLC.
References
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236
Seifter EJ, Ihde DC (1988) Therapy of small cell lung cancer: a perspective on two decades of clinical research. Semin Oncol 15:278–299
Hanna N, Bunn PA Jr, Langer C et al (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24:2038–2043
Schiller JH, Adak S, Cella D, DeVore RF 3rd, Johnson DH (2001) Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593–a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 19:2114–2122
Govindan R, Page N, Morgensztern D et al (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24:4539–4544
Braghiroli MI, Sabbaga J, Hoff PM (2012) Bevacizumab: overview of the literature. Expert Rev Anticancer Ther 12:567–580
Lucchi M, Mussi A, Fontanini G, Faviana P, Ribechini A, Angeletti CA (2002) Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg 21:1105–1110
Ruotsalainen T, Joensuu H, Mattson K, Salven P (2002) High pretreatment serum concentration of basic fibroblast growth factor is a predictor of poor prognosis in small cell lung cancer. Cancer Epidemiol Biomarkers Prev 11:1492–1495
Salven P, Ruotsalainen T, Mattson K, Joensuu H (1998) High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer 79:144–146
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Zhang J, Gold KA, Kim E (2012) Sorafenib in non-small cell lung cancer. Expert Opin Investig Drugs 21:1417–1426
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Litz J, Sakuntala Warshamana-Greene G, Sulanke G, Lipson KE, Krystal GW (2004) The multi-targeted kinase inhibitor SU5416 inhibits small cell lung cancer growth and angiogenesis, in part by blocking Kit-mediated VEGF expression. Lung Cancer 46:283–291
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM (2003) SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2:471–488
Ibrahim N, Yu Y, Walsh WR, Yang JL (2012) Molecular targeted therapies for cancer: sorafenib mono-therapy and its combination with other therapies (review). Oncol Rep 27:1303–1311
Gitlitz BJ, Moon J, Glisson BS et al (2010) Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: a Southwest Oncology Group (SWOG 0435) phase II trial. J Thorac Oncol 5:1835–1840
Ready N, Dunphy F, Pang H et al (2010) Combination chemotherapy with sunitinib (IND 74019; NSC 736511) for untreated extensive-stage small cell lung cancer (SCLC): CALGB 30504 phase IB safety results. J Clin Oncol 28:15s (suppl, abstract 7056)
Ramalingam SS, Belani CP, Mack PC et al (2010) Phase II study of Cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute #7097). J Thorac Oncol 5:1279–1284
Arnold AM, Seymour L, Smylie M et al (2007) Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol 25:4278–4284
Schneider BJ, Gadgeel SM, Ramnath N et al (2011) Phase II trial of sunitinib maintenance therapy after platinum-based chemotherapy in patients with extensive-stage small cell lung cancer. J Thorac Oncol 6:1117–1120
Spigel DR, Greco FA, Rubin MS et al (2012) Phase II study of maintenance sunitinib following irinotecan and carboplatin as first-line treatment for patients with extensive-stage small-cell lung cancer. Lung Cancer 7:359–364
Horn L, Dahlberg SE, Sandler AB et al (2009) Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 27:6006–6011
Ready NE, Dudek AZ, Pang HH et al (2011) Cisplatin, irinotecan, and bevacizumab for untreated extensive-stage small-cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol 29:4436–4441
Spigel DR, Greco FA, Zubkus JD et al (2009) Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 4:1555–1560
Spigel DR, Townley PM, Waterhouse DM et al (2011) Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 29:2215–2222
Goss GD, Arnold A, Shepherd FA et al (2010) Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol 28:49–55
Baselga J, Segalla JG, Roche H et al (2012) Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol 30:1484–1491
Kim ES, Herbst RS, Wistuba II et al (2011) The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov 1:44–53
Mok TS, Paz-Ares L, Wu YL et al (2012) Association between tumor EGFR and KRAS mutation status and clinical outcomes in NSCLC patients randomized to sorafenib plus best supportive care (BSC) or BSC alone: subanalysis of the phase III MISSION trial. Ann Oncol 23:9s (suppl, abstract LBA9-PR)
Waterhouse DM, Morgan SK, Spigel DR et al (2010) Phase II study of oral topotecan plus bevacizumab (topo-bev) for second-line treatment of small cell lung cancer. J Clin Oncol 28:15s (suppl, abstract 7055)
Allen JW, Moon J, Gadgeel SM et al (2012) SWOG 0802: A randomized phase II trial of weekly topotecan with and without AVE0005 (aflibercept) in patients with platinum-treated extensive-stage small cell lung cancer (E-SCLC). J Clin Oncol 30:15s (suppl, abstract 7005)
Conflict of interest
Nathan A. Pennell received research funding from Genentech and served as a consultant for Boehringer Ingelheim and Oncogenex. Balazs Halmos received research funding from Daiichi Sankyo; Eli Lilly and Oncothyreon. All other authors declare no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, N., Pennell, N., Nickolich, M. et al. Phase II trial of Sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs 32, 362–368 (2014). https://doi.org/10.1007/s10637-013-0061-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-0061-6