Abstract
Purpose
To assess the efficacy and toxicity of S-1 and bevacizumab combination therapy for patients previously treated for advanced non-squamous non-small cell lung cancer (NSCLC).
Methods
This was a prospective, multi-center, single-arm phase II study. Patients with non-squamous NSCLC who had experienced progression after cytotoxic chemotherapy were enrolled. Oral S-1 was administered on days 1–14 of a 21-day cycle, and bevacizumab (15 mg/kg) was given intravenously on day 1. Patients received S-1 adjusted on the basis of their creatinine clearance and body surface area. The primary endpoint was response rate (RR); secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety.
Results
We enrolled 30 patients. One patient had never received platinum-based therapy. Five patients had activating mutations of the epidermal growth factor receptor gene, of whom four had received tyrosine kinase inhibitors before this study. The RR was 6.7% [95% confidence interval (CI) 1.8–21.3%], and the disease control rate (DCR) was 80% (95% CI 62.7–90.5%). Median PFS was 4.8 months (95% CI 2.7–6.4 months], and median OS was 13.8 months (95% CI 8.4 months–not applicable). Patients did not experience any Grade 4 toxicity or treatment-related death. Grade 3 hematologic toxicity (anemia) occurred in one patient (3.3%). The main Grade 3 non-hematologic toxicities were anorexia (10%), infection (10%), and diarrhea (6.7%).
Conclusion
The addition of bevacizumab to S-1 was tolerable, but not beneficial for patients with previously treated non-squamous NSCLC. We do not recommend further study of this regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is currently the leading cause of cancer-related mortality worldwide, resulting in more than 25% of all cancer deaths [1]. Non-small cell lung cancer (NSCLC) accounts for a high proportion, approximately 85–90%, of lung cancer cases [2]. Many patients with NSCLC have advanced disease at diagnosis. The combination of platinum plus third-generation cytotoxic drugs has been the gold standard first-line chemotherapy for patients with advanced NSCLC. However, most patients experience disease progression during or after first-line treatment.
The standard second-line therapy for non-squamous NSCLC had been single-agent chemotherapy with docetaxel or pemetrexed. The response rate (RR), median progression-free survival (PFS), and median overall survival (OS) with these drugs range 7.1–9.1%, 2.2–2.9 months, and 6.7–8.3 months, respectively [3, 4].
S-1 is an oral medication that contains tegafur, gimeracil, and oteracil. It is widely used to treat various kinds of cancer. We previously recommended a tailored dose of S-1 adjusted by individual body surface area (BSA) and creatinine clearance (Ccr). Our pharmacokinetic data supported the validity of using Ccr in addition to BSA [5]. Our study and three other phase II trials showed that RR, median PFS, and median OS for S-1 monotherapy as second-line therapy NSCLC range 12.5–26.7%, 2.5–4.2 months and 8.2–16.4 months, respectively [6,7,8,9]. The survival benefit of these non-platinum second-line monotherapies was not satisfactory.
Bevacizumab is a monoclonal antibody that binds to vascular endothelial growth factor. In non-squamous NSCLC, bevacizumab conferred a survival benefit when combined with carboplatin and paclitaxel as first-line treatment [10]. However, the benefit of adding bevacizumab to non-platinum cytotoxic monotherapy is not clear as subsequent treatment. Therefore, we conducted a multi-center, a single-arm phase II study to evaluate the safety and efficacy of combination therapy of tailored-dose S-1 plus bevacizumab in patients with recurrent non-squamous NSCLC.
Patients and methods
Patient eligibility
Patients were enrolled if they met the following eligibility criteria: (1) histologically or cytologically confirmed non-squamous NSCLC; (2) stage III or IV disease, or postoperative recurrence; (3) at least one measurable lesion; (4) more than 4 weeks since the last administration of prior chemotherapy; (5) epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), anaplastic lymphoma kinase (ALK) inhibitors, and mesenchymal epithelial transition factor (MET) inhibitors were not considered chemotherapy; (6) age ≥20 years; (7) Eastern Cooperative Oncology Group performance status of 0–2; (8) ability to tolerate oral intake; (9) adequate organ function, defined as adequate bone marrow function (white blood cell count ≥3500/μL, neutrophil count ≥1500/μL, platelet count ≥100,000/μL, and hemoglobin ≥9.0 g/dL), normal renal function (serum creatinine ≤1.5 mg/dL, urine protein ≤1+ or ≤2 g/24 h in pooled urine), normal liver function (aspartate aminotransferase and alanine aminotransferase ≤100 IU/L, serum total bilirubin ≤1.5 mg/dL), and normal respiratory function (SpO2 ≥90% or PaO2 ≥60 Torr on room air); (10) more than 2 weeks after radiotherapy (except thoracic irradiation); (11) recurrence more than 4 weeks after first-line chemotherapy (anti-cancer therapy given for recurrence of NSCLC within 180 days of previous preoperative or postoperative chemotherapy); (12) estimated life expectancy of more than 3 months; and (13) written informed consent.
Exclusion criteria were: (1) history of S-1 treatment; (2) interstitial lung disease on chest radiography; (3) high risk of bleeding, defined as intrabronchial invasion observed with bronchoscopy, invasion of major vessels on computed tomography (CT), history of hemoptysis (>2.5 mL), or anticoagulant or anti-platelet drug therapy; (4) clinically significant complications or unstable medical condition such as symptomatic brain metastasis, pleural effusion requiring drainage, severe heart disease, uncontrolled diabetes mellitus, active infection, or other active neoplasm; (5) thoracic irradiation within 2 weeks; (6) pregnancy, lactation, and possibility of being pregnant; and (7) current flucytosine therapy. This study followed the ethical principles of the Declaration of Helsinki. The protocol was approved by the institutional review board at each of the three participating institutions.
Study treatment
Patients received S-1 adjusted on the basis of Ccr and BSA. Based on our previous studies [5, 8] and a post-marketing survey [11], we used the S-1 doses, as shown in Table 1. Ccr values were calculated using the Cockcroft–Gault formula. Patients took oral S-1 on days 1–14. Bevacizumab (15 mg/kg) was given intravenously on day 1. This schedule was repeated every 3 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. The starting criteria were as follows: neutrophil count ≥1500/μL, platelet count ≥100,000/μL, aspartate aminotransferase and alanine aminotransferase ≤100 IU/L, total bilirubin ≤1.5 mg/dL, and non-hematologic toxicities of Grade 1 or lower. If a patient did not meet these criteria on day 1, the cycle was postponed. Criteria for discontinuation of protocol treatment included: (1) disease progression; (2) Grade 2 or higher interstitial pulmonary fibrosis or pneumonitis; (3) Grade 4 non-hematologic toxicity; (4) Ccr <30 mL/min; (5) delay of the start of the next course for more than 3 weeks; (6) need for a second dose reduction; and (7) withdrawal of consent. One-level dose reduction of S-1 was permitted if Grade 3 or 4 neutropenia, thrombocytopenia, or non-hematological toxicity occurred in the previous cycle.
Assessment of response and toxicity
Baseline assessments included medical history, physical examination, complete blood count, serum biochemical analyses, chest radiography, electrocardiography, and urinalysis. All patients underwent chest and abdominal CT, brain CT or magnetic resonance imaging, and positron emission tomography or bone scintigraphy at most 4 weeks before enrollment. Response was graded as complete response (CR), partial response (PR), stable disease (PR), progressive disease (PD), or not evaluable (NE) according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1.1. All patients were evaluated with CT every 6 weeks. Toxicity was evaluated every 2 weeks according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical analyses
The primary endpoint was RR. Secondary endpoints consisted of safety, PFS, and OS. In the V15-32 trial for Japanese patients with previously treated NSCLC, the RR for docetaxel as second-line therapy was 12.8% (95% confidence interval (CI): 8.8–17.5) [12]. In a phase II study of bevacizumab in addition to docetaxel or pemetrexed, the RR of combination therapy ranged from 12.5% (95% CI 5.3–25.5%) for docetaxel to 17.9% (95% CI 9.0–32.7%) for pemetrexed [13]. Based on these results, we assumed the threshold for RR, expected RR, two-sided α error, and power to be 9, 25, 5, and 80%, respectively. The required sample size was 27 patients. Taking into account patients who might be withdraw consent or be excluded from analysis, we needed 30 patients. OS and PFS were defined as the time from study enrollment until death or last observation and disease progression or death from any cause, respectively. OS and PFS were calculated using the Kaplan–Meier method. All statistical analyses were performed using JMP® Pro 12.0.0 for Mac (SAS Institute, Cary, NC, USA).
This trial has been registered under the University Medical Hospital Information Network (UMIN) Clinical Trial Registry Identifier UMIN000008158.
Results
Patients
Thirty patients were enrolled from March 2013 to March 2015. We followed them until June 30, 2016. At the cut-off date, one patient was lost to follow-up, 20 had died, and nine were alive. Table 2 shows the patients’ baseline characteristics. Regarding prior regimens, one patient had received pemetrexed monotherapy and 29 patients had received platinum-based chemotherapy, of whom one had received pemetrexed maintenance therapy. Four patients had received EGFR-TKIs.
Treatment
Thirty patients received a total of 203 cycles (median 5 cycles, range 1–24 cycles). Nineteen patients withdrew because of PD, five because of toxicity, and four withdrew consent. Two patients were still on this regimen at the cut-off date. One patient had to quit bevacizumab after 18 cycles because of proteinuria but could continue S-1 alone for another four cycles until PD. Fourteen patients received post-protocol chemotherapy and 16 received palliative radiotherapy or best supportive care.
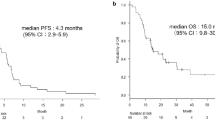
Efficacy
All patients were evaluable for response. One achieved CR, 1 PR, 22 SD, and 6 PD. Thus, the RR and disease control rate (DCR) were 6.7% (95% CI 1.8–21.3%) and 80% (95% CI 62.7–90.5%), respectively. On the other hand, the RR and DCR of 25 patients with wild-type EGFR were 12% (95% CI 4.1–30.0%) and 76% (95% CI 56.7–88.5%), respectively. Median PFS was 4.8 months (95% CI 2.7–6.4 months) (Fig. 1). Median OS was 13.8 months (95% CI 8.4 months–not available) (Fig. 2). Four patients had continued this regimen for more than 1 year with tolerable toxicity. Six patients had received bevacizumab as first-line chemotherapy. The RR of bevacizumab-naïve and bevacizumab-pretreated patients was 8.3% (95% CI 2.3–2.6%) and 0%, respectively. On the other hand, prior use of bevacizumab did not affect PFS (5.4 months for bevacizumab-naïve vs. 3.3 months for bevacizumab-pretreated, p = 0.08). EGFR mutation status did not affect PFS for this regimen (wild type vs. mutant; 4.8 months vs. 5.8 months, p = 0.76). Median PFS of patients with CCr ≥60 mL/min was longer than that of patients with Ccr <60 mL/min (2.6 months vs. 5.4 months, p = 0.02) (Fig. 3).
Safety
All patients were assessed for toxicity (Table 3). No grade 4 severe toxicities or treatment-related deaths were observed. The only grade 3 hematologic toxicity was anemia. Common grade 3 non-hematologic toxicities included diarrhea, anorexia, and infection. One patient withdrew from the protocol treatment due to grade 2 gastric perforation, likely caused by non-steroidal anti-inflammatory drugs. This patient did not require surgery.
Discussion
This is the first study of combination therapy with tailored-dose S-1 and bevacizumab. The RR of 6.7% did not meet the threshold for the primary endpoint of this study. Our study did not demonstrate that bevacizumab has an effect when given in addition to S-1. There were two studies of S-1 plus bevacizumab combination therapy in patients previously treated for non-squamous NSCLC. The results of our study were consistent with those two studies. Nishino et al. compared docetaxel plus bevacizumab with S-1 plus bevacizumab. They reported that median RR and PFS were 2.2% and 3.5 months with S-1 plus bevacizumab and 22.3% and 3.9 months for docetaxel plus bevacizumab. Both regimens were associated with modest PFS, but did not warrant further investigation because of low RR for S-1 plus bevacizumab and severe toxicity with docetaxel plus bevacizumab [14]. Tokito et al. conducted a phase II trial of S-1 plus bevacizumab after platinum-based chemotherapy in patients with advanced non-squamous NSCLC. The RR, DCR, median PFS, and OS were 14.3, 85.7%, 3.2, and 11.4 months, respectively [15].
Our study suggested that S-1 with dose tailored to renal function reduces severe adverse events, even when combined with bevacizumab. We previously reported that S-1 with dose tailored to renal function was reasonable for elderly patients in terms of pharmacokinetics [5]. This study showed that, compared with patients with Ccr ≥60 mL/min, patients with Ccr <60 mL/min had shorter PFS but similar adverse event profiles. Regarding safety, the toxicity of tailored-dose S-1 plus bevacizumab was manageable. Neutropenia of Grade 3 or higher was not observed in our study, although it occurred in 4.4 and 2.1% of patients in the previous two studies [14, 15]. As a result of the latest phase III trial (EAST-LC) demonstrating non-inferiority of S-1 to docetaxel [16], S-1 monotherapy is an alternative second-line regimen. We believe that tailored-dose S-1 is also reasonable in the second-line setting.
In conclusion, the addition of bevacizumab to S-1 was not beneficial in patients with previously treated non-squamous NSCLC. We do not recommend any further study of this regimen.
References
Siegel R, Ma JM, Zou ZH, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S, Grp EGW (2014) Metastatic non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:27–39
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18(10):2095–2103
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TCY, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22(9):1589–1597
Shiroyama T, Kijima T, Komuta K, Yamamoto S, Minami S, Ogata Y, Okafuji K, Imamura F, Hirashima T, Tachibana I, Kawase I, Kumanogoh A (2012) Phase II tailored S-1 regimen study of first-line chemotherapy in elderly patients with advanced and recurrent non-small cell lung cancer. Cancer Chemother Pharmacol 70(6):783–789
Totani Y, Saito Y, Hayashi M, Tada T, Kohashi Y, Mieno Y, Kato A, Imizu H, Yoneda Y, Hoshino T, Uchiyama Y, Takeuchi Y, Okazawa M, Sakakibara H (2009) A phase II study of S-1 monotherapy as second-line treatment for advanced non-small cell lung cancer. Cancer Chemother Pharmacol 64(6):1181–1185
Wada M, Yamamoto M, Ryuge S, Nagashima Y, Hayashi N, Maki S, Otani S, Katono K, Takakura A, Yanaihara T, Igawa S, Yokoba M, Mitsufuji H, Kubota M, Katagiri M, Masuda N (2012) Phase II study of S-1 monotherapy in patients with previously treated, advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 69(4):1005–1011
Shiroyama T, Komuta K, Imamura F, Hirashima T, Kijima T, Tachibana I, Kawase I (2011) Phase II study of S-1 monotherapy in platinum-refractory, advanced non-small cell lung cancer. Lung Cancer 74(1):85–88
Govindan R, Morgensztern D, Kommor MD, Herbst RS, Schaefer P, Gandhi J, Saito K, Zergebel C, Schiller J (2011) Phase II trial of S-1 as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 6(4):790–795
Sandler A, Yi J, Dahlberg S, Kolb MM, Wang LS, Hambleton J, Schiller J, Johnson DH (2010) Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 5(9):1416–1423
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M (2008) Safety evaluation of oral fluoropyrimidine S-1 for short- and long-term delivery in advanced gastric cancer: analysis of 3,758 patients. Cancer Chemother Pharmacol 61(2):335–343
Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F, Eguchi K, Takeda K, Inoue A, Tomii K, Harada M, Masuda N, Jiang H, Itoh Y, Ichinose Y, Saijo N, Fukuoka M (2008) Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 26(26):4244–4252
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A (2007) Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non-small-cell lung cancer. J Clin Oncol 25(30):4743–4750
Nishino K, Imamura F, Kumagai T, Katakami N, Hata A, Okuda C, Urata Y, Hattori Y, Tachihara M, Yokota S, Nishimura T, Kaneda T, Satouchi M, Morita S, Negoro S (2015) A randomized phase II study of bevacizumab in combination with docetaxel or S-1 in patients with non-squamous non-small-cell lung cancer previously treated with platinum based chemotherapy (HANSHIN Oncology Group 0110). Lung Cancer 89(2):146–153
Tokito T, Yamada K, Ichiki M, Takahashi K, Hisamatsu Y, Azuma K, Ishii H, Shukuya T, Takeoka H, Nishikawa K, Hoshino T (2015) A multicenter phase II study of S-1 combined with bevacizumab after platinum-based chemotherapy in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol 33(15):1
Nishino MM, Mok TSK, Nakagawa K, Yamamoto N, Shi Y-K, Zhang L, Lu S, Soo R, Yang J, Morita S, Sugawara S, Nokihara H, Takahashi T, Goto T, Chang J, Maemoto M, Ichinose Y, Cheng Y, Lim W-T, Tamura T (2016) EAST-LC: randomized controlled phase III trial of S-1 versus docetaxel in patients with non-small-cell lung cancer who had received a platinum-based treatment. Ann Oncol 27(Suppl_6):1218PD
Acknowledgements
The authors would like to thank Suguru Yamamoto, Moto Yaga, Kentaro Masuhiro, and Yumi Mitsuyama (Osaka Police Hospital), and Hiroshi Kida, Tomoyuki Otsuka, Osamu Morimura, Yoshiko Takeuchi, Akio Osa, and Mikako Ishijima (Osaka University Hospital) for their roles in patient enrollment and treatment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this work.
Conflict of interest
Tomonori Hirashima and his institution received research grants from Taiho Pharmaceutical Co., Ltd, and Chugai Pharmaceutical Co., Ltd. The other authors have declared no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Nishijima-Futami, Y., Minami, S., Futami, S. et al. Phase II study of S-1 plus bevacizumab combination therapy for patients previously treated for non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol 79, 1215–1220 (2017). https://doi.org/10.1007/s00280-017-3321-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3321-x