Abstract
The aim of this study was to differentiate S-cone responses from other retinal activities using various recording conditions and to optimize these recording conditions for clinical diagnostics. S-cone responses to blue stimuli (449 nm) were studied in 20 healthy subjects and four patients with enhanced S-cone syndrome. The time-integrated luminance of the stimulus varied from 0.008 to 1.0 cd s/m2. Three isoluminant backgrounds were used (100 ph cd/m2 = 40 sc cd/m2): amber (594 nm), green (513 nm), and red (635 nm). With low flash strengths (from 0.008 to 0.032 cd s/m2), the S-cone response appeared as a single positive peak, while with higher strengths (≥0.064 cd s/m2), it appeared as a second peak that followed the L-cone and M-cone components. With a further increase in flash strength (≥0.25 cd s/m2), the S-cone response interfered with the i-wave of the L-cone and M-cone systems. The wavelength and luminance of the background influenced the suppression of the rods, as well as the L-cone- and M-cone-system activities. The S-cone response was measurable in the presence of the amber and green backgrounds, but its amplitude was higher if a strong red background was used. Thus, the function of the retinal S-cone system can be measured if possible interference from other retinal sources can be minimized by the appropriate combination of stimulus and background parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The S-cone response is a slow, positive component that can be seen in the electroretinogram (ERG), and that has been shown to be generated by the S-cone system. This can be elicited with blue stimuli in the presence of a bright photopic, usually amber, background. The unique spectral characteristics of the S-cone response show their highest activity in the blue spectrum of visible light, in agreement with the spectral sensitivity of the S-cones [1–4]. Further suggestion that this response could be generated by the S-cone system was provided from the ERGs of a patient with S-cone monochromatism: the response to the S-cone-specific stimulus was normal, while responses to the L-cone-specific and M-cone-specific stimuli were absent [2, 3]. The importance of the S-cone bipolar cells in generation of the S-cone response has been revealed by studies using a primate model. The S-cone response was completely abolished after exposure of the retina to 2-amino-4-phosphonobutyric acid, a specific agonist of glutamate metabotropic receptors [5]. In general, the S-cone response is delayed in comparison to a component generated by the post-photoreceptor mechanisms of the L-cones and M-cones at the same level of retinal adaptation [2]. This is believed to be a consequence of the slower processing of the S-cone ON-bipolar cells [4]. Furthermore, S-cone-specific stimuli cannot elicit the d-wave [5]. This is consistent with the concept that the S-cones are only connected to the ON-bipolar and not to the OFF-bipolar cells [4].
There is no standardized protocol for the assessment of the S-cone response, which has resulted in differences in recording conditions across studies [6–17]. Furthermore, the response waveform also differs between some of these studies, most probably due to unequal contribution of other retinal sources. One of the reasons for those differences might be the contribution of the L-cone and M-cone systems, which can vary depending on luminance and wavelengths of the stimulus. As was indicated previously, the S-cone response can be considerably contaminated by the rod system activity [13], which could also add to the inconsistency of the results of previous studies. In addition, the S-cone responses have been measured in different manners across these previous studies. Some studies showed the S-cone response as a single prominent component, and the amplitude was measured from the baseline to the positive peak [1, 7, 17]. In other studies that showed a faster component of the L-cone and M-cone systems before the S-cone component, the amplitude of the S-cone response was measured from the trough following the L-cone and M-cone components, to the peak of the S-cone component [3, 4, 9–12, 14–16]. To date, the importance of S-cone response analysis has not been evaluated.
The aim of the present study was to isolate the S-cone response from other retinal activities and to optimize the recording conditions and S-cone response analysis for clinical diagnostics. The characteristics of the S-cone-driven response were also studied in patients with enhanced S-cone syndrome (ESCS), an autosomal recessive hereditary retinal disorder that shows predominant activity of the S-cone mechanisms.
Subjects and methods
This study was performed according to the tenets of the Declaration of Helsinki, and it was approved by the National Ethics Committee. All of the subjects were fully informed about the possible consequences of the study protocol, and they provided their signed approval for participation.
ERGs were recorded in 20 healthy subjects (11 women, 9 men), aged from 18 to 35 years, who had no history of eye disease, a normal ophthalmological examination, normal visual acuity (Snellen 0.8 or better), and clear optic media.
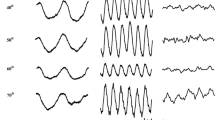
ERGs were also recorded from four patients (1 woman, 3 men) with ESCS, aged from 31 to 49 years. Clinical findings for the patients with ESCS are summarized in Table 1, with their clinical and electrophysiological findings consistent with ESCS. Their fundus examination showed nummular pigment depositions at the level of the retinal pigment epithelium in the mid-periphery of the retina. Their visual acuity varied from counting fingers to 1.0, which correlated well with their degree of macular involvement (evaluated with optical coherence tomography). Mutational screening for the NR2E3 gene, which encodes a photoreceptor-specific nuclear receptor, was also carried out. This confirmed the mutation in three of these patients, while the results of the remaining patient are still under analysis. As shown in Fig. 1, in all four of these patients, their standard full-field ERG responses were pathognomonic for ESCS: the combined rod–cone response (dark-adapted 3.0 ERG) and cone response (light-adapted 3.0 ERG) had similar waveforms, the rod response (dark-adapted 0.01 ERG) was not detectable, and the light-adapted 3.0 flicker response was delayed and had an amplitude lower than that of the cone response a-wave [18].
The ERGs were recorded simultaneously from both eyes. The recording electrode was an HK-loop that was placed in the fornix of the lower eyelid [19]. The silver chloride reference electrode was placed on the ipsilateral temple, and the ground electrode was positioned on the forehead. Photopic ERGs were elicited with a Ganzfeld Espion ColorDome stimulator (Diagnosys LLC, Littleton, MA, USA). The pupils were dilated with 1% tropicamide (Mydriacyl®), and the subjects were light-adapted for 5 min in the presence of each photopic background.
The ERGs were elicited with blue stimuli, which were generated by a xenon bulb, with a blue filter (Wratten 47B; radiance peak, 449 nm) placed between the bulb and the patient. The stimuli were generated by integrated LED diodes (the bandwidth of each light source is indicated in Fig. 2A) and delivered on green (513 nm), amber (594 nm), or red (635 nm) monochromatic backgrounds. The time-integrated luminance of the stimuli were set as increments of 0.3 log units, from 0.008 to 1 cd s/m2, with a stimulus duration of less than 1 ms. The flashes were delivered at 1 Hz, and 30–60 responses were averaged and repeated twice. The signals were amplified with a band-pass from 0.1 to 500 Hz. The maximal possible luminance of the amber background (100 ph cd/m2 = 40 sc cd/m2) was chosen as the reference value, while for the green and red backgrounds, the luminance was calibrated to match with that of the amber background. Due to the differences in the spectral sensitivities of the rod and cone systems for these chromatic backgrounds, the isoluminant values were calibrated separately for the scotopic and photopic spectral efficiencies. The values used for the chromatic backgrounds are summarized in Table 2.
a Relative radiance of the Xenon light and LEDs (green, amber, red) and relative transmittance of the blue Wratten 47B filter. (b, c) Measurement of the S-cone response implicit time (blue horizontal lines) and the amplitude (red vertical lines). b At low flash strength, the amplitude was measured from the baseline to the positive peak. c At high flash strength, the amplitude was measured from the trough following the L-cone and M-cone component, to the peak of the S-cone component
In two healthy subjects, the responses to blue stimuli (0.032 cd s/m2) were elicited after 1 min of illumination with intense white light (5,000 ph cd/m2 = 15,000 sc cd/m2). Norren et al. [1] used the same luminance of bleaching exposure prior to the S-cone recording, to exclude the rod contribution to the ERG of macaque. Thomas and Lamb [21] showed that a similar bleaching exposure results in complete deactivation of the human rod system for at least 5 min. In our study, bleaching exposure was generated by a Grass PS33 plus stimulator (Astro-Med Inc, West Wanwick, Rhode Island, USA), by setting the Grass intensity to a value of 8 and the frequency to 80 Hz; in this manner, it represented an intense sustained source of light. Bleaching was repeated before turning on each of the chromatic backgrounds. This experiment was performed in only two healthy subjects, due to the possible phototoxicity of the intense illumination.
The flash and background luminance were calibrated with a photometer/radiometer (IL-1700, International Light INC, Newburyport, USA), using detectors with integrated photopic and scotopic filters.
The implicit time of the S-cone response was measured from the stimulus onset to the peak of the response, while the S-cone response amplitude was measured and analyzed in two ways. At low stimulus strength, where the S-cone response appeared as a single prominent component, the amplitude was measured from the baseline to the positive peak. At high stimulus strength, the faster component of the L-cone and M-cone systems appeared in front of the S-cone component. Here, the amplitude of the S-cone response was measured from the trough following the L-cone and M-cone components, to the peak of the S-cone component (Fig. 2). The ERG amplitudes, measured in the above-mentioned manners (from baseline to the peak and from the trough after the L-cone and M-cone component to the peak of the S-cone component), were compared by Students’ t tests for each of the backgrounds used. Furthermore, one-way ANOVA with Bonferroni post hoc comparisons was used to evaluate the differences between the amplitudes, elicited with the same strength of the flash at different chromatic backgrounds. Differences were considered significant at P < 0.05. The signal-to-noise ratio was calculated as the ratio between the mean value and the standard deviation. The data were analyzed using the programs Origin 7.0 (OriginLab Corp., Northampton, USA) and SPSS, version 12.0 (SPSS Inc., Chicago, USA).
Results
S-cone responses to chromatic backgrounds
As shown in Fig. 3, the ERG components varied when elicited with a blue stimulus (449 nm) of increasing strength in the presence of the amber, green, and red chromatic backgrounds. According to previous studies, the amber background with the maximum possible luminance produced by the stimulator (100 ph cd/m2 = 40 sc cd/m2) was chosen as the reference background for eliciting the S-cone response. In the presence of this amber background, a blue stimulus of low strength (0.008–0.032 cd s/m2) elicited a response with a positive polarity, a low amplitude (approximately 5 μV), and an implicit time of 40 ms to 45 ms. This was defined as the S-cone response (Fig. 3, indicated as S). With a higher flash strength (0.063–0.125 cd s/m2), an earlier component of the L-cone and M-cone systems appeared before the S-cone response, with an implicit time of 26 ms to 28 ms (Fig. 3, indicated as LM). With a further increase in flash strength (0.25–1.0 cd s/m2), the response was dominated by the L-cone- and M-cone-system components, which were identified as the b-wave of the photopic electroretinogram.
Electroretinograms of a healthy subject (age, 30 years) elicited with increasing luminance (0.008–1.0 cd s/m2) of a blue stimulus (449 nm) in the presence of three chromatic backgrounds (green 513 nm, amber 594 nm, red 635 nm). The luminance of the green and red backgrounds was calibrated to the same (isoluminant) value as that used for the amber reference background (center, shaded). The strength of the stimulus is given on the left, and the background wavelength and luminance are given above the traces. “S” indicates the component of the S-cone system and “S?” indicates a component with some of the features, unusual for the S-cone system. “LM” indicates the positive component of the L-cone and M-cone systems, which with the highest strengths could be identified as the photopic ERG with the a-, b-, and i-waves
Other chromatic backgrounds were calibrated to the same luminance as the amber background (Fig. 3). The bright green (100 ph cd/m2 = 300 sc cd/m2; photopic isoluminance) and bright red (515 ph cd/m2 = 40 sc cd/m2; scotopic isoluminance) backgrounds elicited the S-cone response in a similar manner to the amber reference background; this emerged with the strengths of the stimulus, while with higher strengths, the predominance of the L-cone and M-cone components was also observed here. With a dim green background (14 ph cd/m2 = 40 sc cd/m2; scotopic isoluminance), the S-cone response could not be identified, because the L-cone- and M-cone-system components already emerged at the lowest flash strengths (0.008–0.016 cd s/m2). In the presence of a dim red background (100 ph cd/m2 = 7 sc cd/m2; photopic isoluminance), a component of a higher amplitude was elicited, although with an implicit time similar to the S-cone response (40–45 ms). This component showed markedly higher amplitudes in some of the subjects investigated, and therefore there appeared to be a possible contribution from the rod system.
Elimination of rod system activity
While trying to exclude potential contributions from the rod system, an experiment with an additional 1 min illumination with intense bleaching light was performed. Figure 4 shows the responses that were elicited before and after this bleaching exposure. When the amber, bright green, or bright red backgrounds were used, the amplitude of the S-cone response did not show any significant changes after this intense illumination. Small amplitude variations that were observed in the presence of these backgrounds might have been caused by muscle artifacts or other noise activities. In contrast, when the dim red background was used, the S-cone response was considerably lower after the bleaching exposure, by 35% in first subject and 44% in the second (Fig. 4). When comparing the responses before and after the intense illumination, this amplitude difference most probably indicated that prior to the bleaching exposure, the dim red background did not provide sufficient suppression of the rod system.
Electroretinograms of two healthy subjects elicited before and after intense illumination with a bleaching light (1 min illumination with 5000 ph cd/m2 light). The responses were elicited with 0.032 cd s/m2 blue (449 nm) stimulus, which was delivered on three chromatic backgrounds, the values of which are shown at the top of the traces
S-cone response in relation to stimulus strength
The responses of the S-cone system were further explored in relation to the strength of the stimulus. This part of the experiment was focused only on those backgrounds that successfully elicited the S-cone response (amber, bright green, and bright red backgrounds). Figure 5 shows the averaged traces of the 20 healthy subjects. At low strengths of the stimuli (0.008–0.016 cd s/m2), the S-cone response appeared as a single component. Its amplitude was highest with the red background, lower with the amber, and lowest with the green background. At higher flash strengths (0.032 and 0.064 cd s/m2), the simple waveform of the S-cone response was apparent only with the red background. With the green and amber backgrounds, a component of the L-cone and M-cone systems appeared before the S-cone response, thereby producing a compound waveform. With the flashes of 0.125 cd s/m2, the S-cone response was evident only in the presence of the red background. With the amber and green backgrounds, as well as with a further increase in flash strength (0.25–1.00 cd s/m2), the S-cone response cannot be discriminated from the i-wave, which appeared with the same implicit time (40–45 ms).
Comparisons of the averaged ERG responses (20 normal subjects; 40 eyes) elicited with a progressively brighter blue stimulus (449 nm; from 0.008 to 1.0 cd s/m2; upper left) in the presence of three chromatic backgrounds (green: 513 nm, 100 ph cd/m2 = 300 sc cd/m2; amber: 594 nm, 100 ph cd/m2 = 40 sc cd/m2; red: 635 nm, 515 ph cd/m2 = 40 sc cd/m2)
The response amplitudes were plotted against the time-integrated luminance of the stimulus, providing the stimulus/response (S/R) curves (Fig. 6). The S/R curves of the S-cone response showed slow, linear increases with low stimulus strengths of ≤0.125 cd s/m2. A slow increase in the S/R function was previously shown to be characteristic for the S-cone system [1, 26]. With higher strengths of the stimuli (>0.125 cd s/m2), the S/R curves showed a rapid increase, particularly in the presence of the green and amber backgrounds. Therefore, a range of stimulus luminance >0.125 cd s/m2 is not suitable for eliciting the S-cone response, as it appears that the contribution of the i-wave and the L-cone and M-cone systems predominate over the response (Fig. 6).
Averaged stimulus/response curves of the S-cone responses (20 normal subjects; 40 eyes), elicited with a progressively brighter blue stimulus (449 nm; from 0.008 to 1.0 cd s/m2; upper left) in the presence of the three chromatic backgrounds (green: 513 nm, 100 ph cd/m2 = 300 sc cd/m2; amber: 594 nm, 100 ph cd/m2 = 40 sc cd/m2; red: 635 nm, 515 ph cd/m2 = 40 sc cd/m2). At low flash strength, the amplitude was measured from the baseline to the positive peak (a simple waveform), while at higher flash strengths, the amplitude was measured from the trough following the L-cone and M-cone components, to the peak of the S-cone response (a combined waveform), as indicated
Evaluation of S-cone response measurements and analysis
Table 3 summarizes the comparisons between the two S-cone response analyses (a simple waveform at low strengths of the stimulus and a combined waveform at higher strengths). In the presence of all three backgrounds (amber, green, and red), the amplitudes were significantly higher when the simple waveform of the S-cone response was analyzed. Measurement of the combined waveform gave lower amplitudes of the S-cone response (Fig. 6, Table 3). Furthermore, measurement of the simple waveform showed a higher signal-to-noise ratio, indicating that the S-cone response was better isolated from the underlying noise activity. The implicit time did not differ between these analyses. The amplitude of the S-cone response was higher in the presence of the red background, when compared to the response of the amber and green backgrounds at the same strength of flash (a part of the multiple comparison is summarized in Table 3).
Responses in patients with enhanced S-cone syndrome
The characteristics of the ERGs elicited with the blue stimuli in the presence of the amber, bright green, and bright red backgrounds were also examined in the four patients with ESCS. The response waveform in these patients was different from the S-cone response of the healthy subjects, as it showed broader waves and significantly longer implicit times (P < 0.001). In these patients, a simple waveform was maintained across the whole spectrum of the stimulus luminance. This was in contrast to the normal subjects, where there was a combined contribution from the L-cone and M-cone systems at higher flash strengths. In two of the patients (the first and the fourth), the amplitudes of the S-cone responses were lowest with the green background, higher with the amber background, and highest with the red background. In the other two patients (second and third), the S-cone responses showed the lowest amplitude in the presence of the green background as well, while both the amber and red backgrounds had similar effects on the amplitudes and waveforms of the responses (Fig. 7).
ERG responses in the four patients with ESCS, elicited with progressively brighter blue (449 nm) stimuli (from 0.016 to 0.25 cd s/m2; upper left) in the presence of the three chromatic backgrounds (green: 513 nm, 100 ph cd/m2 = 300 sc cd/m2; amber: 594 nm, 100 ph cd/m2 = 40 sc cd/m2; red: 635 nm, 515 ph cd/m2 = 40 sc cd/m2)
Discussion
S-cone responses can be recorded with specialized commercially available equipment, although there still remains the need to optimize their recording. Our data show that the S-cone response can be elicited under various recording conditions without significant interference from other retinal sources and with a satisfactory signal-to-noise ratio.
ERG function of the S-cone system has been elicited in most studies with blue stimuli in the presence of either an amber background [1, 11, 13, 22] or a broad-band white background [2–4, 9]. An amber background has been described as more appropriate, as it provides a more obvious separation of the S-cone response from the L-cone and M-cone components [4]. The present study also confirms that the activity of the S-cone system can be recorded using a blue flash (449 nm) on an intense amber background (594 nm). To our knowledge, the S-cone response recording has not been previously studied with other wavelengths of monochromatic background. In the present study, we have shown that the S-cone response can be elicited in the presence of green and red monochromatic backgrounds as well. However, the luminance of these chromatic backgrounds needs to be bright enough, to efficiently suppress the activities of the rod system, as well as the L-cone and M-cone systems.
The S-cone response has been described to date as a low-amplitude ERG component in the range of 1–10 μV, with a time to peak in the range of 40 ms to 50 ms [1–4, 7, 9–12, 14–17]. Accordingly, the data from the present study show the S-cone response as a component with mean amplitude of 5–8 μV and a time to peak of around 43 ms, when evoked in the presence of bright amber, green, or red backgrounds. In a review article, Gouras [4] described that the low amplitude of the S-cone response is a consequence of the small population of the S-cones and S-cone bipolar cells in the healthy human retina. Therefore, these retinal neurons represent a much smaller proportion of the ERG generators, as is the case for the L-cones and M-cones.
Some studies have shown responses of the S-cone system that have significantly greater amplitudes (20–100 μV) and longer times to peak (50–100 ms) [8, 13, 23, 24]. It might be assumed that such an outcome might be partially caused by insufficient inhibition of the rod system. The rod and S-cone mechanisms are technically more difficult to separate because their spectral sensitivities overlap to a large extent. Furthermore, the signals from the rods might feed into the cone system through the rod–cone gap junctions even at higher light levels, representing the so-called fast rod pathway [25]. Arden and co-authors [13] demonstrated that the S-cone response can include a significant contribution from the rod system, despite very high luminance of the amber background (300 cd/m2). In the present study, a contribution of the rod system was designed to be excluded with prior extremely bright illumination with white light. According to data from the literature, this would result in complete deactivation of the rods for at least 5 min [21]. This principle for the exclusion of the rod contribution in the response of the S-cones was previously used by Norren et al. [1]. Furthermore, similar bleaching exposure was shown to sufficiently suppress the activation of the fast rod pathway [26]. In the present study, the bleaching exposure was performed in only two subjects, due to the potential phototoxicity of such intense illumination [27]. If we assume that this methodology of rod suppression was sufficient, the contribution of the rod system was recognizable only in the presence of the dim red background that matched the photopic isoluminance of the amber background. Under these conditions, a high-amplitude component was seen, and due to the similar times to peak and the similar waveforms, this might be mistakenly equated with the response of the S-cone system. However, one cannot exclude the possibility that a proportion of rods was not completely bleached and contributed to the measured signal through the less-sensitive fast rod pathway.
Among the studies to date, there have been major differences in the morphology and the analysis of the S-cone responses. Some studies have shown these as late-positive waves with a simple morphology [1, 7, 17]. In such cases, the amplitude was measured from the baseline to the top of the wave. Other studies have shown these as the waves that follow the L-cone and M-cone components, and their amplitudes were measured from the negative wave following this component to the peak of the S-cone component [3, 4, 9–12, 14–16]. In agreement with the description of Marmor et al. [17], we also measured the S-cone response as a single simple component with low flash strengths and with slightly higher strengths as a component following the L-cone- and M-cone-system activities. To date, it has not been shown which stimulus strengths are more appropriate to isolate the S-cone response from the activity of the L-cone and M-cone mechanisms. For this differentiation, the S/R curves were analyzed. Two previous studies [1, 28] have shown that the L-cone and M-cone S/R curves increase at a much greater rate than that originating from the S-cone system. The present study shows a slow increase in the S/R curves in the range up to 0.125 cd s/m2, thus characterizing the activity of the S-cone mechanisms. However, within this luminance range, lower flash strengths (0.008–0.032 cd s/m2) elicited the S-cone response as a single component. This might be a more appropriate technique for the S-cone response, due to the higher amplitude as well as the higher signal-to-noise ratio. At much higher flash strengths (>0.125 cd s/m2), there was a steep increase in the S/R curves, which was most likely a consequence of the i-wave intrusion. We propose to avoid the i-wave intrusion in the S-cone response recordings, as the i-wave most probably originates from the OFF-pathway activity of the L-cone and M-cone systems [29–31].
The S-cone response recording is important for clinical use, but large variabilities for these responses have been reported [17]. The present study also shows variation among the healthy subjects, and sometimes, the signal from the S-cone response could not be separated easily from the underlying noise activity. However, with the repetition of a greater number of responses, the S-cone response was detected in all of the subjects studied here. According to results of present study, a higher amplitude of the S-cone response can be achieved by the use of a flash strength that elicits a single, simple configuration of the S-cone component. Alternatively, a higher amplitude could also be obtained by using an intense red background, which suppressed the activity of the L-cone and M-cone systems more effectively. Therefore, a promising method for eliciting the S-cone response might be the use of a blue flash on a strong red L-cone- and M-cone-suppressing background that is triggered immediately after a strong, rod-suppressing flash.
ESCS is a hereditary autosomal recessive retinal disease that is characterized by an increased number of S-cones, a lack of rod function, and L-cone and M-cone dysfunction [18, 32–34]. To some extent, this pathology allows the examination of the spectral characteristics of the S-cone system without substantial contribution from other retinal systems. In our study, this issue was investigated to determine the effects of the stimulus backgrounds on the S-cone mechanism. According to our hypothesis, similar changes to those seen in normal S-cone responses after modifying the wavelength of the chromatic background would be expected to be seen also in ESCS. Such changes would be seen as the highest amplitude of the response in the presence of the red background and the lowest amplitude in the presence of the green background. These would be explained by an adapting effect of the chosen background on the S-cone system. This effect is greatest with the green background and lowest with the red background. However, our hypothesis was confirmed in only two of the four patients with ESCS. In the other two patients, the red background affected the response amplitude in the same way as the amber background. A previous study proposed that the S-cones might replace the rod post-photoreceptor connections [35], while another study showed that there can be abnormal connections of the S-cones with the L-cone and M-cone OFF-pathway [18]. It is still not known to what degree the L-cone and M-cone functions might be preserved in ESCS pathology. Therefore, the variability of the abnormal connections, as well as the preserved but abnormal L-cone and M-cone activities, might be the reasons for these findings in patients with ESCS. ESCS, thus, appears not to be an ideal pathology for examination of the function of the S-cone mechanism.
To conclude, the S-cone response can be isolated from the activity of the other retinal systems when a blue stimulus of low strength is used with a strong amber background. The S-cone response amplitude can be improved by the use of a strong red background, although care needs to be taken to establish sufficient rod suppression. For our recording equipment, this study indicates the use of a 0.016–0.032 cd s/m2 blue stimuli in the presence of a 100 cd/m2 amber background. However, further studies are needed to standardize the recording procedures for clinical use and to define a single protocol for S-cone response recording.
References
Norren DV, Padmos P (1973) Human and macaque blue cones studied with electroretinography. Vis Res 13(7):1241–1254
Gouras P, MacKay CJ (1990) Electroretinographic responses of the short-wavelength-sensitive cones. Invest Ophthalmol Vis Sci 31(7):1203–1209
Gouras P, MacKay CJ, Yamamoto S (1993) The human S-cone electroretinogram and its variation among subjects with and without L and M-cone function. Invest Ophthalmol Vis Sci 34(8):2437–2442
Gouras P (2003) The role of S-cones in human vision. Doc Ophthalmol 106(1):5–11
Evers HU, Gouras P (1986) Three cone mechanisms in the primate electroretinogram: two with, one without off-center bipolar responses. Vis Res 26(2):245–254
Padmos P, van Norren D, Faijer JW (1978) Blue cone function in a family with an inherited tritan defect, tested with electroretinography and psychophysics. Invest Ophthalmol Vis Sci 17(5):436–441
Román AJ, Jacobson SG (1991) S cone-driven but not S cone-type electroretinograms in the enhanced S cone syndrome. Exp Eye Res 53(5):685–690
Swanson WH, Birch DG, Anderson JL (1993) S-cone function in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 34(11):3045–3055
Yamamoto S, Kataoka Y, Kamiyama M, Hayasaka S (1995) Nondetectable S-cone electroretinogram in a patient with crystalline retinopathy. Doc Ophthalmol 90(3):221–227
Yamamoto S, Kamiyama M, Nitta K, Yamada T, Hayasaka S (1996) Selective reduction of the S cone electroretinogram in diabetes. Br J Ophthalmol 80(11):973–975
Simonsen SE, Rosenberg T (1995–1996) Reappraisal of a short-wavelength-sensitive (S-cone) recording technique in routine clinical electroretinography. Doc Ophthalmol 91(4):323–332
Yamamoto S, Hayashi M, Takeuchi S, Shirao Y, Kita K, Kawasaki K (1997) Normal S cone electroretinogram b-wave in Oguchi’s disease. Br J Ophthalmol 81(12):1043–1045
Arden G, Wolf J, Berninger T, Hogg CR, Tzekov R, Holder GE (1999) S-cone ERGs elicited by a simple technique in normals and in tritanopes. Vis Res 39(3):641–650
Yamamoto S, Hayashi M, Takeuchi S (1999) S-cone electroretinogram to Ganzfeld stimuli in patients with retinitis pigmentosa. Doc Ophthalmol 99(2):183–189
Yamamoto S, Hayashi M, Tsuruoka M, Ogata K, Tsukahara I, Yamamoto T, Takeuchi S (2002) Selective reduction of S-cone response and on-response in the cone electroretinograms of patients with X-linked retinoschisis. Graefes Arch Clin Exp Ophthalmol 240(6):457–460
Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM (2003) Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 424(6950):805–808
Marmor M, Cabael L, Shukla S, Hwang JC, Marcus M (2004) Clinical S-cone ERG recording with a commercial hand-held full-field stimulator. Doc Ophthalmol 109(1):101–107
Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, Neveu MM, Hogg CR, Hunt DM, Moore AT, Bird AC, Webster AR, Holder GE (2008) Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci 49(5):2082–2093
Hawlina M, Konec B (1992) New noncorneal HK-loop electrode for clinical electroretinography. Doc Ophthalmol 81(2):253–259
Stockman A, Sharpe LT (2000) Spectral sensitivities of the middle- and long-wavelength sensitive cones derived from measurements in observers of known genotype. Vis Res 40:1711–1737
Thomas MM, Lamb TD (1999) Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J Physiol 518:479–496
Horiguchi M, Miyake Y, Kondo M, Suzuki S, Tanikawa A, Koo HM (1995) Blue light-emitting diode built-in contact lens electrode can record human S-cone electroretinogram. Invest Ophthalmol Vis Sci 36(8):1730–1732
Sawusch M, Pokorny J, Smith VC (1987) Clinical electroretinography for short wavelength sensitive cones. Invest Ophthalmol Vis Sci 28(6):966–974
Nilsson A, Wright T, Westall CA (2008) A promising S-cone isolating protocol. Doc Ophthalmol 117(supl):40–41
Sharpe LT, Stockman A (1999) Rod pathways: the importance of seeing nothing. Trends Neurosci 22(11):497–504
Stockman A, Sharpe LT, Zrenner E, Nordby K (1991) Slow and fast pathways in the human rod visual system: electrophysiology and psychophysics. J Opt Soc Am A 8(10):1657–1665
Glickman RD (2002) Phototoxicity to the retina: mechanisms of damage. Int J Toxicol 21(6):473–490
Zrenner E, Gouras P (1979) Blue-sensitive cones of the cat produce a rodlike electroretinogram. Invest Ophthalmol Vis Sci 18(10):1076–1081
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45(10):3827–3837
Rufiange M, Dumont M, Lachapelle P (2005) Modulation of the human photopic ERG luminance-response function with the use of chromatic stimuli. Vis Res 45(17):2321–2330
Ueno S, Kondo M, Ueno M, Miyata K, Terasaki H, Miyake Y (2006) Contribution of retinal neurons to d-wave of primate photopic electroretinograms. Vis Res 46(5):658–664
Jacobson SG, Marmor MF, Kemp CM, Knighton RW (1990) SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci 31(5):827–838
Greenstein VC, Zaidi Q, Hood DC, Spehar B, Cideciyan AV, Jacobson SG (1996) The enhanced S cone syndrome: an analysis of receptoral and post-receptoral changes. Vis Res 36(22):3711–3722
Lam BL, Goldberg JL, Hartley KL, Stone EM, Liu M (2007) Atypical mild enhanced S-cone syndrome with novel compound heterozygosity of the NR2E3 gene. Am J Ophthalmol 144(1):157–159
Marmor MF, Tan F, Sutter EE, Bearse MA Jr (1999) Topography of cone electrophysiology in the enhanced S cone syndrome. Invest Ophthalmol Vis Sci 40(8):1866–1873
Acknowledgments
The authors thank Mrs. Marija Jesenšek and Mrs. Ana Jeršin, who were involved in the clinical ERG recording. This study was supported by Slovenian Research Agency, Grant No. J3-6167 and P3-0333.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sustar, M., Hawlina, M. & Brecelj, J. Electroretinographic evaluation of the retinal S-cone system. Doc Ophthalmol 123, 199–210 (2011). https://doi.org/10.1007/s10633-011-9299-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-011-9299-5