Abstract
Goals and Background
Ustekinumab (UST) is a monoclonal antibody inhibitor of IL-12/IL-23 approved for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). We conducted a meta-analysis to compare rates of adverse events (AEs) in randomized controlled trials (RCTs) of UST for all indications.
Study
A systematic search was performed of MEDLINE, Embase, and PubMed databases through November 2019. Study inclusion included RCTs comparing UST to placebo or other biologics in patients aged 18 years or older with a diagnosis of an autoimmune condition.
Results
Thirty RCTs with 16,068 patients were included in our analysis. Nine thousand six hundred and twenty-six subjects were included in the UST vs placebo analysis. There was no significant difference in serious or mild/moderate AEs between UST and placebo with an OR of 0.83 (95% CI 0.66, 1.05) and 1.08 (95% CI 0.99, 1.18), respectively, over a median follow-up time of 16 weeks. In a sub-analysis of CD and UC trials, no difference in serious or mild/moderate AEs in UST versus placebo was seen.
Conclusions
UST was not associated with an increase in short-term risk of AEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interleukin (IL)-12 and IL-23 are cytokines that play a role in the maturation and proliferation of T-helper (TH) 1 and TH17 cells and thereby influence cell-mediated immunity and inflammation. Ustekinumab (UST), a monoclonal antibody that inhibits the p40 subunit of IL-12 and IL-23, has demonstrated efficacy in a variety of immunologic disorders including psoriasis, psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease (IBD), which encompasses Crohn’s disease (CD) and ulcerative colitis (UC) [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
The pro-inflammatory cytokines IL-12 and IL-23 have several other important biologic roles, including driving the body’s response to viral and bacterial infection and shaping the balance between anti-tumor and pro-tumor immunity. Mutations in IL-12 and IL-23 have been reported to play a role in mycobacterial disease and non-typhi Salmonella infections [26, 27]. In a murine model, blockade of IL-12 enabled tumor progression, whereas in vitro IL-12 stimulation of T cells showed greater effect in controlling tumors [28,29,30]. Thus, it is important to monitor the safety of anti-IL-12 and anti-IL-23 as the number of disease indications for UST increases.
The IL-12 and IL-23 pathway is a promising therapeutic target. To date, there are no major trends in adverse effects (AEs) documented from multiple individual clinical trials of UST, although it is acknowledged that many adverse effects are rare. The primary aim of this study is to conduct a meta-analysis to compare rates of adverse events in randomized controlled trials (RCTs) of UST compared to placebo for the treatment of any autoimmune condition. As secondary analyses, we will compare the safety of UST in IBD (UC and CD), high- versus low-doses of UST in IBD, and UST versus other biologics in any autoimmune condition.
Methods
Study Selection
This study was conducted according to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines (Supplementary Table 7). A systematic electronic literature search was performed through November 2019 from MEDLINE, Embase, and PubMed databases using the keywords and MeSH terms for “ustekinumab” and “randomized control trials”. The PubMed search strategy was as follows: (((ustekinumab) OR (“ustekinumab”[MeSH Terms] OR “ustekinumab”[All Fields]))) AND ((((randomized controlled trial) OR (“randomized controlled trial”[Publication Type] OR “randomized controlled trials as topic”[MeSH Terms] OR “randomized controlled trial”[All Fields] OR “randomized controlled trial”[All Fields])) OR randomized study) OR ((“random allocation”[MeSH Terms] OR (“random”[All Fields] AND “allocation”[All Fields]) OR “random allocation”[All Fields] OR “randomized”[All Fields]) AND study[All Fields])). A review of the selected titles and abstracts and a full review of potentially relevant studies was independently performed by all reviewers (JK, VR, and SH). Manuscripts that met inclusion criteria were evaluated by both reviewers and any discrepancies were resolved by discussion and consensus with senior authors (SC and VP).
Inclusion/Exclusion Criteria
We included all RCTs comparing UST to placebo and UST to other biologic agents in patients aged 18 years or older with a diagnosis of any autoimmune condition. Studies included in the analysis required documentation of mild/moderate and severe AEs of UST compared to placebo, or UST compared to another biologic (golimumab, brodalumab, etanercept, ixekizumab, risankizumab, and secukinumab). Studies including crossover between placebo or other biologic to UST or vice versa without specifically delineating AEs during these separate study periods were excluded.
Data Extraction
Data were extracted by three authors (JK, VR, and SH) independently with cross-comparison. Any disagreements were resolved by discussion with senior authors (SC and VP). The following information was extracted: author and trial names, country of origin, type of study, study design, primary diagnoses of subjects, number of subjects, primary outcome, inclusion age of the study participants, percent female, duration of time during which AEs were documented, number of patients treated with each regimen (UST versus placebo versus other biologic), drug dosing and intervals, number of serious AEs, number of mild/moderate AEs, and common AEs listed within the included trials (including infections, cough, headache, upper respiratory infection, nausea, nasopharyngitis, injection site/infusion reaction, cardiovascular event, malignancy, and death). The designation of mild/moderate or serious AEs was defined by the individual studies. The senior authors were contacted for more information when the published data were unclear.
Assessment of Outcomes
The primary outcome was the risk of adverse events based on calculating a weighted pooled odds ratio (OR) of serious and mild/moderate AEs in patients treated with UST compared to placebo. Secondary outcomes included ORs of mild/moderate and serious AEs in UST compared to other biologics. Additional analyses were performed for ORs of mild/moderate and serious AEs comparing low-dose to high-dose UST in IBD trials. Dosing varied among the IBD trials. The CERTIFI trial used doses of 1 mg/kg, 3 mg/kg, and 6 mg/kg; the Sandborn 2008 trial used doses of 90 mg or 4.5 mg/kg; and the UNITI and UNIFI trials used doses of 130 mg or 6 mg/kg [11,12,13, 18]. Based on these trials, we dichotomized trials into low-dose (90 mg, 130 mg, or less than 4.5 mg/kg) and high-dose (4.5 mg/kg or higher).
Statistical Analysis
For all included studies, we calculated the rate of serious AEs expressed as a pooled event rate and 95% confidence interval (95% CI). The primary and secondary outcomes are expressed as ORs to estimate risks of adverse event rates among UST-treated and comparator groups. Mantel-Haenzel fixed effects analysis was performed for all of our outcomes. Heterogeneity was assessed by the I2 statistic. All the analyses were performed using Review Manager (RevMan) version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Publication bias was assessed using Comprehensive Meta-Analysis version 3.0 (Biostat, Englewood, NJ). Risk-of-bias assessment of trials was done using the Cochrane Collaboration risk of bias tool (Supplementary Table 6) [31].
Results
Characteristics of Included Studies
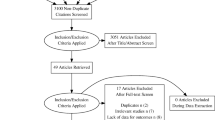
We identified 813 citations that matched our initial search criteria. These citations were reviewed, and ultimately 30 studies met criteria for inclusion in our analysis (Fig. 1). A total of 16,068 subjects were included in our analysis. The UST versus placebo analysis included 9626 subjects, of whom 6163 were in the UST group. The total number of subjects in the UST versus other biologics comparison was 7418, of whom 976 were patients also included in the UST versus placebo comparison. The IBD analysis included 2960 subjects, of whom 2000 were included in the low- versus high-dose analysis.
All included trials were RCTs. Twelve trials involved subjects with psoriasis, four with psoriatic arthritis, four with CD, three with axial spondyloarthritis, two with atopic dermatitis, and one each with UC, systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and multiple sclerosis (Supplementary Table 5). The duration of follow-up ranged from 8 to 56 weeks. Sixteen weeks was the median follow-up period. The median age within trials ranged from 34 to 51 years of age. The range of female participants was 15–91% and the majority of studies had less than 50% female participants.
Primary Outcomes
Serious Adverse Events (SAE)
Definition of AE severity was identified in 53% (16/30) of the studies. Ten of these definitions were based on International Conference on Harmonisation (ICH) and European Union (EU) Guidelines for Pharmacovigilance for Medicinal Products for Human Use, two were based on the Medical Dictionary for Regulatory Activities (MedDRA) and three were obtained directly by contacting authors. An additional 37% (11/30) of studies listed each individual SAE occurrence (e.g., myocardial infarction, gastrointestinal hemorrhage, pancreatitis, etc.) but did not state the definition of severity. 10% (3/30) of studies did not describe SAE definition or occurrences; the authors of these studies were contacted without response. There was no significant difference in the incidence of serious AEs between UST and placebo, with an OR of 0.83 (95% CI 0.66, 1.05, I2 = 0%, p = 0.13) (Table 1).
Mild/Moderate Adverse Events
There was no significant increase in overall incidence of mild/moderate AE when comparing UST to placebo, with an OR of 1.08 (95% CI 0.99, 1.18, I2 = 0%, p = 0.07) (Table 2). When individual adverse events were compared, UST was associated with a small, but statistically significant, increase in rates of infections and infusion/injection site reactions (Table 3).
Secondary Outcomes
UST Versus Placebo, in Patients with IBD
A sub-analysis of CD and UC trials was performed to compare rates of AEs in UST versus placebo in patients with IBD. Five studies were included with a total of 2960 patients [11,12,13]. We found no increased risk in incidence of serious AEs, with an OR of 0.77 (95% CI 0.55, 1.06, I2= 0%, p = 0.11) (Table 4). Similarly, there was no increased risk of mild/moderate AEs in UST versus placebo, with an OR of 1.01 (95% CI 0.86, 1.18, I2 = 0%, p = 0.95) (Table 5).
UST Versus Other Biologics
UST was compared with brodalumab in two studies, secukinumab in two studies, guselkumab in two studies, risankizumab in two studies, golimumab in one study, etanercept in one study, and ixekizumab in one study. A total of 7418 subjects were included in this analysis across 11 RCTs [1,2,3,4,5,6]. The incidence of serious AEs between UST and other biologics was not significantly different, with an OR of 0.88 (95% CI 0.65, 1.18, I2 = 0%, p = 0.38) (Supplementary Table 1). There was also no increased incidence of mild/moderate AEs, with an OR of 1.07 (95% CI 0.96, 1.19, I2= 35%, p = 0.25) (Supplementary Table 2).
Low- Versus High-Dose UST in IBD Trials
An analysis was performed comparing high- versus low-dose UST in IBD trials. A total of 2000 subjects were included [11, 12]. Dosing in IBD trials ranged from 1–6 mg/kg or 90 to 130 mg. The rates of both serious and mild/moderate AEs between low- and high-doses were similar, with OR of 0.89 (95% CI 0.59, 1.35, I2= 0, p = 0.57) and 0.90 (95% CI 0.75, 1.08, I2= 50%, p = 0.26), respectively (Supplementary Tables 3 and 4).
Assessment of Heterogeneity
In the majority of studies included, there was low heterogeneity. Outcomes and heterogeneity are summarized in Table 6.
Publication Bias
Publication bias was assessed for primary outcomes. Funnel plots were examined visually and revealed no obvious asymmetry to suggest bias. Egger’s regression tests were not significant, with 2-tailed p value of 0.1 for serious AEs and 0.32 for mild/moderate AEs (Supplementary Figure 1).
Discussion
This meta-analysis is an evaluation of documented AEs associated with UST use among a spectrum of autoimmune diseases, with a total of 30 RCTs comprising 16,068 subjects. We found that there was no increased risk of serious AEs or mild/moderate AEs with UST when compared with either placebo or other biologics. Additionally, there was no difference in AEs when comparing low-dose versus high-dose UST therapy in patients with IBD. It is noted that UST was associated with increased rates of infection and infusion/injection site reactions. However, these differences were small and similar to reported AEs associated with other biologics. Prior reviews have evaluated available trials and demonstrated similar favorable safety profiles [32].
Five studies included in our analysis were conducted in patients with CD or UC. There were no differences in rates of serious or mild/moderate AEs for UST versus placebo or rates of AEs for low- versus high-dose UST. Of note, three of these studies (UNITI-1, UNITI-2 and UNIFI) defined severity using ICH and EU guidelines and two (Sandborn 2008 and CERTIFI) listed each severe AE individually [11,12,13, 18].
The results of this meta-analysis indicate that UST has a low risk of both serious and mild/moderate adverse events during induction and short-term maintenance in RCTs, adding further support to the overall favorable safety profile of UST in IBD. These findings are supported by a recent meta-analysis of observational real-world studies of UST in CD patients which reported low pooled incidence rates for AEs and infections [33]. However, safety data from long-term extension studies are needed to further characterize the long-term safety profile of UST in IBD.
Our meta-analysis is limited by variability across trials in UST doses and dosing intervals, which differed by disease state. Another limitation is the varied length of follow-up for reporting of adverse events, with several studies reporting a study duration of 12 weeks or less prior to crossover. Longer follow-up durations in IBD patients specifically are needed to evaluate for AEs.
To our knowledge, this is the first meta-analysis to evaluate the aggregate risk of AEs in UST use across multiple autoimmune diseases. Though AEs studied in other autoimmune diseases may not be directly applicable to the IBD population, the available trial data does help provide supplementary data for patients or providers about potential risks. Given the increasing use of UST in CD and UC, this study offers further insight into the safety of UST as it becomes more integrated into the IBD armamentarium.
Conclusion
This is the first meta-analysis of adverse events associated with UST use in RCTs across multiple autoimmune disease states. There was no increased risk of AEs with the use of UST compared to placebo in any of the disease states studied. There was no difference in risk of AEs with UST in IBD, which was maintained when comparing low- versus high-dose UST. As the use of UST in the treatment of IBD becomes more common, these findings will be beneficial to practitioners when assessing the risks and benefits of treatment with UST. Further trials with long-term follow-up studies are needed to assess late-onset AEs and the durability of these results.
References
Judson MA, Baughman RP, Costabel U, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–1307. https://doi.org/10.1183/09031936.00000914.
Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242–252. https://doi.org/10.1111/j.1346-8138.2011.01347.x.
Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet Lond Engl. 2009;373:633–640. https://doi.org/10.1016/S0140-6736(09)60140-9.
Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet Lond Engl. 2008;371:1665–1674. https://doi.org/10.1016/S0140-6736(08)60725-4.
Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet Lond Engl. 2008;371:1675–1684. https://doi.org/10.1016/S0140-6736(08)60726-6.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318–1328. https://doi.org/10.1056/NEJMoa1503824.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet Lond Engl. 2013;382:780–789. https://doi.org/10.1016/S0140-6736(13)60594-2.
Segal BM, Constantinescu CS, Raychaudhuri A, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. https://doi.org/10.1016/S1474-4422(08)70173-X.
Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999. https://doi.org/10.1136/annrheumdis-2013-204655.
Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26:28–35. https://doi.org/10.1111/exd.13112.
Sandborn WJ, Gasink C, Gao L-L, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. https://doi.org/10.1056/NEJMoa1203572.
Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. https://doi.org/10.1056/NEJMoa1602773.
Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. https://doi.org/10.1053/j.gastro.2008.07.014.
Blauvelt A, Reich K, Tsai T-F, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60–69.e9. https://doi.org/10.1016/j.jaad.2016.08.008.
Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. https://doi.org/10.1056/NEJMoa0810652.
Gelfand JM, Shin DB, Alavi A, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U Trial). J Invest Dermatol. 2020;140:85–93.e2. https://doi.org/10.1016/j.jid.2019.07.679.
van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet Lond Engl. 2018;392:1330–1339. https://doi.org/10.1016/S0140-6736(18)32167-6.
Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. https://doi.org/10.1056/NEJMoa1900750.
Saeki H, Kabashima K, Tokura Y, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol. 2017;177:419–427. https://doi.org/10.1111/bjd.15493.
Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet Lond Engl. 2018;392:650–661. https://doi.org/10.1016/S0140-6736(18)31713-6.
Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of Ustekinumab in axial spondyloarthritis. Arthritis Rheumatol Hoboken NJ. 2019;71:258–270. https://doi.org/10.1002/art.40728.
Smolen JS, Agarwal SK, Ilivanova E, et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis. 2017;76:831–839. https://doi.org/10.1136/annrheumdis-2016-209831.
Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80:70–79.e3. https://doi.org/10.1016/j.jaad.2018.06.039.
Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week clarity results). Dermatol Ther. 2018;8:571–579. https://doi.org/10.1007/s13555-018-0265-y.
Deodhar A, Gottlieb AB, Boehncke W-H, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Lond Engl. 2018;391:2213–2224. https://doi.org/10.1016/S0140-6736(18)30952-8.
Frucht DM, Holland SM. Defective monocyte costimulation for IFN-gamma production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J Immunol Baltim Md 1950. 1996;157:411–416.
Godinez I, Keestra AM, Spees A, Bäumler AJ. The IL-23 axis in Salmonella gastroenteritis. Cell Microbiol. 2011;13:1639–1647. https://doi.org/10.1111/j.1462-5822.2011.01637.x.
Awoniyi M, Miller SI, Wilson CB, Hajjar AM, Smith KD. Homeostatic regulation of Salmonella-induced mucosal inflammation and injury by IL-23. PLoS ONE. 2012;7:e37311. https://doi.org/10.1371/journal.pone.0037311.
Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. 2017;. https://doi.org/10.1101/cshperspect.a028530.
Schurich A, Raine C, Morris V, Ciurtin C. The role of IL-12/23 in T cell-related chronic inflammation: implications of immunodeficiency and therapeutic blockade. Rheumatol Oxf Engl. 2017. https://doi.org/10.1093/rheumatology/kex186.
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (Updated on March 2011) 2011.
Deepak P, Sandborn WJ. Ustekinumab and anti-interleukin-23 agents in Crohn’s disease. Gastroenterol Clin N Am. 2017;46:603–626. https://doi.org/10.1016/j.gtc.2017.05.013.
Macaluso FS, Maida M, Ventimiglia M, Cottone M, Orlando A. Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: a meta-analysis of observational studies. Expert Opin Biol Ther. 2020;20:193–203. https://doi.org/10.1080/14712598.2020.1707800.
Funding
There was no funding source for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Hudesman was the consultant for Pfizer, Takeda, Janssen Biotech, Abbvie, and Salix. Research support was from Pfizer. Shannon Chang was the consultant for Takeda, Pfizer, and Oshi Health. Lisa Malter was the consultant for Takeda, Pfizer, Abbvie, Prometheus, Janssen, Merck, UCB, and Gilead. The remaining authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rolston, V.S., Kimmel, J., Popov, V. et al. Ustekinumab Does Not Increase Risk of Adverse Events: A Meta-Analysis of Randomized Controlled Trials. Dig Dis Sci 66, 1631–1638 (2021). https://doi.org/10.1007/s10620-020-06344-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06344-w