Abstract
Background
Circulating tumors cells (CTCs) may be a promising prognostic marker for patients with malignant tumors. However, there are few reports regarding its value for hepatocellular carcinoma (HCC) patients.
Aims
To investigate CTCs with epithelial and mesenchymal phenotypes as a potential prognostic biomarker for HCC patients.
Methods
Peripheral blood samples were obtained from 165 HCC patients before radical surgery. CTCs were isolated via the CanPatrol CTC enrichment technique and classified using epithelial–mesenchymal transition (EMT) markers. The relationship of CTC phenotype with clinicopathological factors and HCC recurrence in patients was analyzed.
Results
CTC-positive status (count ≥ 2/5 mL) was found in 70.9% of the 165 HCC patients. Increased CTC number was more common in patients with higher AFP levels, multiple tumors, advanced TNM and BCLC staging, and presence of embolus or microembolus (P < 0.05). CTCs heterogeneity was noted using EMT markers. Mesenchymal CTCs were significantly correlated with high AFP levels, multiple tumors, advanced TNM and BCLC stage, presence of embolus or microembolus, and earlier recurrence (P < 0.05). The presence of mesenchymal CTCs predicted the shortest relapse-free survival, followed by mixed phenotypic CTCs, and then epithelial CTCs (P < 0.001).
Conclusion
CTC phenotype may serve as a prognostic indicator for HCC patients. CTCs assessment should include phenotypic identification tailored to characterize cells based on epithelial and mesenchymal markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies in China, with a high incidence of cancer-related death in this country [1]. The vast majority of HCC cases in China develop as a result of chronic infection with hepatitis B virus (HBV) in this country [2]. Accordingly, previous studies from our research group showed that greater than 85% of HCC patients were diagnosed with HBV infection [3, 4]. Although great improvements have been made in HCC diagnosis and treatment during the past few decades, the long-term survival of these patients remains unfavorable, due to a high rate of recurrence and mortality. Conventional prognostic factors for HCC consist of alpha-fetal protein (AFP) and tumor, node, metastases (TNM) and Barcelona Clinic Liver Cancer (BCLC) staging system, but their value varies between patients with this disease [5]. Therefore, new methods are urgently needed to provide predictive information regarding existing metastasis as well as the probability of early recurrence.
Circulating tumor cells (CTCs), which are defined as a small population of cancer cells that have escaped from the primary tumor into the body’s circulatory system, have great promise as a noninvasive method of assessing tumor progression in real time [6,7,8,9]. Indeed, the significance of CTCs in the peripheral blood (PB) of patients has been studied extensively for various malignancies. Many studies have demonstrated that CTCs are closely related to tumor metastasis, recurrence, and patient prognosis [7, 9, 10].
Cancer cells often lose some of their epithelial characteristics and gain features of a more mesenchymal phenotype, deem an “epithelial-to-mesenchymal transition (EMT)” [11]. EMT allows for increased mobility and invasion and is thought to facilitate metastasis [12,13,14]. Aberrant activation of the EMT progress has been implicated not only in the generation of CTCs, but also in their survival in the bloodstream. The current FDA standard for capturing CTCs (CellSearch, Menarini Silicon Biosystems Inc., San Diego, CA, USA) relies on an epithelial marker, but this system fails to capture cells that have undergone an EMT. Specifically, the CellSearch technology depends on the EpCAM marker for the isolation of CTCs, which may not detect the critical mesenchymal subset of CTCs [15]. Moreover, a study comparing patients with early-stage and metastatic breast cancer found a statistically higher detection of vimentin (+) and cytokeratin (CK) 19 (+) CTCs in those with metastasis, suggesting a strong association between the presence of mesenchymal CTCs and tumor progression [16]. There were also several studies focused on the importance of mesenchymal CTCs in HCC. For instance, a recent study showed snail expression in CTCs might be associated with extrahepatic metastasis of HCC [17]. Another study attempted to predict HCC metastasis more accurately by the detection of both TWIST and vimentin in CTCs and found that the co-expression of these proteins was significantly associated with the presence of embolus in the portal vein [18]. However, these results were not conclusive, due to a limited sample size and the incomplete use of EMT phenotypes. However, using a combination of multiple epithelial and mesenchymal markers could provide more convincing evidence of the clinical significance of mesenchymal CTC phenotypes, and therefore expand our understanding of their contribution to the development and aggressiveness of malignances.
In this study, we evaluated CTCs in the peripheral blood of 165 patients with HCC prior to surgery, beginning in 2013. First, we employed the CanPatrol™ CTC enrichment technique [19] using EMT markers to stratify the CTCs into 3 subpopulations. We then evaluated the relationship between CTC positivity and the clinicopathologic features of HCC patients. The main purpose of this prospective study was to determine the impact of CTC on recurrence risk, in an effort to determine if CTCs phenotype is a potential biomarker for tumor recurrence.
Materials and Methods
Patients and Blood Samples
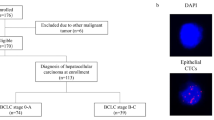
A total of 165 HCC patients, who underwent radical surgical resection in the Department of Hepatobiliary Surgery of Nanfang Hospital of Southern Medical University between 2013 and 2016, were recruited into this prospective study, based on the following criteria: (a) curative resection of HCC was performed; (b) without history of any other anticancer therapy; and (c) complete clinicopathological and follow-up data were available. Patient characteristics of this study cohort are listed in Table 1. The Southern Medical University Ethics Committee approved the protocols according to the Declaration of Helsinki (6th revision, 2008), and informed consent was signed by each patient enrolled in the study.
Samples consisting of 5 mL of peripheral blood (PB) were collected and subjected to the optimized CanPatrol™ CTC enrichment technique. CTCs were quantified in each 5 mL PB sample and classified using EMT markers into 3 subpopulations including epithelial CTCs, mesenchymal CTCs, and mixed phenotypic CTCs.
Patient Follow-Up
Patients were followed up every 3 months during the first postoperative year and 6 months thereafter until April 2017. All patients were followed in a manner previously reported [20]. Briefly, patients were monitored by abdomen ultrasonography and serum alpha-fetoprotein (AFP). Computed tomography (CT) and/or magnetic resonance imaging (MRI) were performed for patients who were suspected of recurrence. A diagnosis of recurrence was based on typical imaging appearances on CT and/or MRI scans and elevated AFP levels. Relapse-free survival (RFS) was defined as the interval from the date of resection to the date of recurrence diagnosis or the date of the last follow-up.
CTCs Filtration, Quantification, and Characterization
Peripheral blood samples were collected in EDTA tubes by venipuncture. After erythrocytes were removed using a red blood cell lysis buffer, the remaining cells were fixed in 4% paraformaldehyde and isolated by the filtration system. This system consisted of a filtration tube containing an 8-µm-diameter multi-pore calibrated membrane (SurExam, Guangzhou, China), a manifold vacuum plate with adjustable valve settings (SurExam, Guangzhou, China), an E-Z 96 vacuum manifold (Omega Bio-Tek, Norcross, GA, USA), and a vacuum pump (Auto Science, Tianjin, China). Then, an RNA in situ hybridization (RNA-ISH) assay, as described previously [19], was performed to conjugate the cells with red fluorescent dyes Alexa Fluor 594 (for the epithelial biomarkers EpCAM and CK8/18/19), using Alexa Fluor 488 (for the mesenchymal biomarkers vimentin and TWIST), and Alexa Fluor 647 (for the leukocyte biomarker CD45). The labeled cells were counted and classified with a fluorescence microscope using a 100× oil objective (Olympus BX53, Tokyo, Japan). In our investigation, we used a threshold of 2 CTCs/5 mL of blood to determine whether the patient was CTCs positive. Epithelial CTCs were defined as cells greater than 15 μm in diameter with cytoplasmic labeling for epithelial biomarkers and no expression of CD45. Mesenchymal-like CTCs were defined as cells greater than 15 µm in diameter, with cytoplasmic labeling for mesenchymal biomarkers and no expression of CD45. The leukocytes were defined as cells with CD45 and should not be counted as CTCs.
Statistical Analysis
Statistical analysis was performed with the SPSS version 19.0 software package (IBM Corp., Armonk, NY, USA). The comparison between CTC count and clinicopathological parameters was tested by the Student’s t test or the one-way ANOVA test. The correlation between CTCs and clinicopathological parameters was examined by the Pearson Chi-square test. Relapse-free survival plots were made using the Kaplan–Meier method, and the significance was estimated by the log-rank test. P < 0.05 was considered statistically significant.
Results
CTCs ≥ 2/5 mL were detected in 117 out of 165 (70.9%) blood samples. The number of CTCs per 5 mL of blood in the CTC-positive patients ranged from 2 to 76 (median 9) (Fig. 1).
First, we evaluated the relationship between clinicopathological factors and patients with a high CTC count. As shown in Table 1, an increased number of CTCs was observed in patients with high levels of AFP, multiple tumors, advanced TNM and BCLC stage, and the presence of embolus or microembolus (P < 0.05). There was no correlation between CTC count and patient sex, age, diagnosis of cirrhosis, expression of HBsAg, tumor size, or tumor differentiation (P > 0.05).
Then, we classified patient CTC phenotypes into 3 subpopulations according to the EMT markers, including epithelial CTCs, mesenchymal CTCs, and mixed phenotypic CTCs. Figures 2 and 3 describe the distribution of CTC phenotypes. Sixty-four patients (38.8%) had CTCs that showed staining for EpCAM or CK8/18/19 expression, but without CD45 expression, constituting an epithelial phenotype. Forty-two patients (25.4%) had CTCs that were vimentin (+) or TWIST (+) and CD45 (−), constituting a mesenchymal phenotype. Eleven patients (0.07%) had CTCs that showed both epithelial and mesenchymal markers, constituting a mixed epithelial/mesenchymal phenotype.
Circulating tumor cell (CTC) labeled by epithelial–mesenchymal transition (EMT) markers in peripheral blood from HCC patients. Representative images of the 3 CTC phenotypes. Red fluorescence indicates epithelial biomarkers; green fluorescence indicates mesenchymal biomarkers. Red fluorescence represents epithelial CTCs, green fluorescence represents mesenchymal CTCs, and mixed red and green fluorescence represents mixed phenotypic CTCs. The cells were analyzed using a ×100 oil objective
We analyzed the relationship between the CTC phenotype and the clinicopathological features, which are summarized in Table 2. In this analysis, no significant association was observed between CTC phenotype and gender, age, tumor size, diagnosis of cirrhosis, expression of HBsAg, tumor size, or tumor differentiation. However, the analysis did show that mesenchymal CTCs were more common in patients with high levels of AFP, multiple tumors, advanced TNM and BCLC stage, and the presence of embolus or microembolus.
We then analyzed tumor recurrence for any correlation with patient variables, including CTC phenotype, sex, age, cirrhosis, HBsAg, AFP levels, tumor number, tumor size, tumor differentiation, TNM and BCLC stage, and the presence of embolus or microembolus. In this study cohort, the median patient follow-up time was 14.0 months. CTC phenotype, AFP levels, tumor number, tumor differentiation, TNM and BCLC stage, and the presence of embolus or microembolus were significantly associated with tumor recurrence. As shown in Fig. 3, among patients with positive CTC, the presence of mesenchymal CTCs predicts a decreased median time to those with mixed phenotypic CTCs or epithelial CTCs (Table 3).
Discussion
Given the poor outcome for HCC patients, current research has focused on new methods of early disease detection, stratification based upon prognosis, and the prediction of local or distant tumor recurrence. CTCs are of particular interest given their presence in patients with various forms of malignancy, as well as their location in the vasculature, which allows for easy sampling and analysis. Recent studies have demonstrated that the presence of CTCs in the blood of patients with metastatic disease, suggesting that CTCs in addition to be a result, could also as a possibly be a source of metastatic disease [8,9,10, 21,22,23,24,25]. In addition, several studies have found an association between the presence of CTCs and poor survival [26,27,28,29]. To the best of our knowledge, this study is the first to prospectively evaluate the clinical relevance of the phenotypic subtypes of CTCs for characterizing HCC.
We identified epithelial and mesenchymal CTCs in the blood of the majority (70.9%) of HCC patients. This indicates that CTCs are a heterogeneous population. The CanPatrol™ CTC enrichment technique detects CTCs not only by size, but also by the expression of EMT markers, allowing for the identification of both epithelial and mesenchymal characteristics. Unlike other studies of other tumor types [16, 28], we not only confirmed the presence of CTCs, but also conducted a comprehensive study involving epithelial and mesenchymal markers for HCC.
The fraction of each CTC phenotypes varied from the patients with different clinicopathological factors. In our cohort, 3 subgroups of CTCs were detected in all patients at all disease stages. However, the presence of mesenchymal CTCs has been linked to aggressive tumors. Patients with malignant features, including high levels of AFP, multiple tumors, advanced TNM and BCLC stage, and the presence of embolus or microembolus were shown to have a much higher positivity for mesenchymal CTCs. These results demonstrate that CTCs might be indicative of a separate feature of tumor biology that influences disease progression. We also found a strong association of CTC phenotypic subtypes with tumor recurrence: Firstly, the positivity of mesenchymal CTCs was higher in patients with early tumor recurrence, and secondly, the survival analysis indicated that patients with mesenchymal CTCs had shorter RFS, when compared with those patients in either of the other 2 CTC subgroups. This finding is consistent with other studies that have shown markers of EMT in CTCs have the potential to provide both prognostic and predictive information [3, 30, 31].
This study, although a prospective analysis, had several limitations. The relationship between the CTCs phenotype and overall survival has not been discussed in our study due to the limitation of follow-up time and the number of patients included in this study, and further analysis in a larger group of HCC patients is required to reevaluate these findings. As our understanding of the heterogeneity of CTCs is enhanced, additional work regarding the genetics and gene expression patterns in individual cells will be needed to characterize epithelial and mesenchymal CTCs and determine their relationship to primary and metastatic tumors. These findings would clarify which CTCs are responsible for tumor recurrence and identify genetic factors that contribute to decreased survival in HCC.
In addition, our study design did not include the collection of samples at multiple time points to observe how CTC characteristics predict outcomes over time and the course of treatment. Although such studies may help to reflecting an exacting CTC phenotypes status and guiding the use of CTCs in evaluating response to surgical treatment, our study does provide data on the utility of CTCs as a biomarker of surgical treatment decision-making.
In conclusion, our results indicated that the evaluation of CTC phenotypes in HCC patients could be used to characterize disease progression and predict recurrence. This study confirms that CTC populations are heterogeneous and that their assessment in HCC patients should include methods tailored for the identification of CTCs for both epithelial and mesenchymal markers. The presence of mesenchymal CTCs tended to occur in advanced stage patients and was associated with an earlier recurrence.
References
Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. https://doi.org/10.3978/j.issn.1000-9604.2015.01.06.
Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538.
Liu H, Zhang X, Li J, et al. The biological and clinical importance of epithelial–mesenchymal transition in circulating tumor cells. J Cancer Res Clin Oncol. 2015;141:189.
Liang B, Jia C, Huang Y, et al. TPX2 level correlates with hepatocellular carcinoma cell proliferation, apoptosis, and EMT. Dig Dis Sci. 2015;60:2360–2372.
Qin LX. Tang ZY Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513.
Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. https://doi.org/10.1158/1078-0432.CCR-06-2701.
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. https://doi.org/10.1056/NEJMoa040766.
Chausovsky G, Luchansky M, Figer A, et al. Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer Am Cancer Soc. 1999;86:2398–2405.
Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. https://doi.org/10.1038/nature06385.
Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–835. https://doi.org/10.1158/1078-0432.CCR-10-0445.
Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cell. 2016;166:21–45. https://doi.org/10.1016/j.cell.2016.06.028.
Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. https://doi.org/10.1016/j.devcel.2008.05.009.
Thiery JP, Acloque H, Huang RY, et al. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–890. https://doi.org/10.1016/j.cell.2009.11.007.
Lee JM, Dedhar S, Kalluri R, et al. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. https://doi.org/10.1083/jcb.200601018.
Grover PK, Cummins AG, Price TJ, et al. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25:1506.
Kallergi G, Papadaki MA, Politaki E, et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13:R59. https://doi.org/10.1186/bcr2896.
Min AL, Choi JY, Woo HY, et al. High expression of Snail mRNA in blood from hepatocellular carcinoma patients with extra-hepatic metastasis. Clin Exp Metastas. 2009;26:759–767.
Li YM, Xu SC, Li J, et al. Epithelial–mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013;4:e831.
Wu S, Liu S, Liu Z, et al. Classification of circulating tumor cells by epithelial–mesenchymal transition markers. PLoS ONE. 2015;10:e123976.
Liu X, Ou H, Xiang L, et al. Elevated UHRF1 expression contributes to poor prognosis by promoting cell proliferation and metastasis in hepatocellular carcinoma. Oncotarget. 2017;8:10510.
Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepat Pancreat Surg. 2008;15:189–195. https://doi.org/10.1007/s00534-007-1250-5.
de Albuquerque A, Kubisch I, Breier G, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncol Basel. 2012;82:3–10. https://doi.org/10.1159/000335479.
Tjensvoll K, Nordgard O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer. 2014;134:1–8. https://doi.org/10.1002/ijc.28134.
Z’Graggen K, Centeno BA, Fernandez-del CC, et al. Biological implications of tumor cells in blood and bone marrow of pancreatic cancer patients. Surgery. 2001;129:537–546. https://doi.org/10.1067/msy.2001.113819.
Soeth E, Grigoleit U, Moellmann B, et al. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol. 2005;131:669–676. https://doi.org/10.1007/s00432-005-0008-1.
Okabe H, Tsunoda S, Hosogi H, et al. Circulating tumor cells as an independent predictor of survival in advanced gastric cancer. Ann Surg Oncol. 2015;22:1–8.
Abrahamsson J, Aaltonen K, Engilbertsson H, et al. Circulating tumor cells in patients with advanced urothelial carcinoma of the bladder: Association with tumor stage, lymph node metastases, FDG-PET findings, and survival. Urol Oncol. 2017;35:606.e9–606.e16.
Li Y, Cheng X, Chen Z, et al. Circulating tumor cells in peripheral and pulmonary venous blood predict poor long-term survival in resected non-small cell lung cancer patients. Int J Radiat Oncol Biol Phys. 2014;90:S49.
Qiao Y, Li J, Shi C, et al. Prognostic value of circulating tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Oncotargets Therapy. 2017;10:1363–1373.
Zhao R, Cai Z, Li S, et al. Expression and clinical relevance of epithelial and mesenchymal markers in circulating tumor cells from colorectal cancer. Oncotarget. 2016;8:9293.
Polioudaki H, Agelaki S, Chiotaki R, et al. Variable expression levels of keratin and vimentin reveal differential EMT status of circulating tumor cells and correlation with clinical characteristics and outcome of patients with metastatic breast cancer. BMC Cancer. 2015;15:1–10.
Funding
Funding for this study was provided by Guangdong Provincial Science and Technology Projects of China (2013B02200069, 2017A020215132).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Additional information
Dinghua Yang is the first corresponding author of manuscript.
Rights and permissions
About this article
Cite this article
Ou, H., Huang, Y., Xiang, L. et al. Circulating Tumor Cell Phenotype Indicates Poor Survival and Recurrence After Surgery for Hepatocellular Carcinoma. Dig Dis Sci 63, 2373–2380 (2018). https://doi.org/10.1007/s10620-018-5124-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5124-2