Abstract
Background and Aim
Many patients with quiescent Crohn’s disease are maintained on long-term treatment with azathioprine (AZA), but controlled data are limited. We aimed to evaluate the efficacy of AZA therapy for more than 4 years to maintain clinical remission.
Methods
We performed a randomized double-blind placebo-controlled AZA withdrawal trial with a follow-up period of 24 months. Patients had to have continuous AZA therapy ≥4 years without exacerbation of disease during the 12 months before enrollment, and a Crohn’s disease activity index <150 at baseline. Patients were randomized to continue on AZA or switch to placebo. The primary endpoint was time to clinical relapse during follow-up.
Results
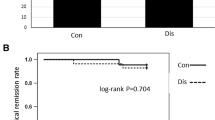
After inclusion of 52 patients, the trial was stopped prematurely due to slow recruitment. During the 2-year follow-up, clinical relapse occurred in 4 of 26 (15 %) patients on continued AZA and in 8 of 26 (31 %) patients on placebo. Time to clinical relapse averaged 22.3 months (95 % CI 20.6–24.0) on AZA and 19.2 months (95 % CI 16.4–22.1) on placebo (p = 0.20). According to life-table analysis, the proportion of patients in remission after 12 and 24 months was 96 ± 4 and 86 ± 7 % in patients receiving AZA versus 76 ± 8 and 68 ± 9 % in patients receiving placebo (month 12, p = 0.035; month 24, p = 0.30). A higher AZA dose at enrollment was an independent predictor for relapse (p < 0.05).
Conclusions
AZA withdrawal resulted in a significantly increased relapse risk after 1 year and a nonstatistically significant trend for relapse after 2 years. Our results are in line with previous observations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract that increases in incidence and preferentially affects young adults [1, 2]. In spite of ongoing efforts and progress in the field [3], there is currently no cure, and therapy is primarily directed toward unspecific abatement of inflammation by the use of medications that modify or suppress the function of the immune system. Azathioprine (AZA), with its metabolite 6-mercaptopurine (6-MP), is among the agents most widely used for treatment of patients with CD [4]. These thiopurine drugs have steroid-sparing properties and proven efficacy for maintaining remission in chronic active disease [5–7] and for reducing the risk of postoperative recurrence after resective surgery for luminal CD [8]. Treatment with immunomodulators, such as AZA, has also been advocated for patients with extensive or early relapsing CD [9]. Finally, thiopurines have a role in closing and maintaining closure of perianal fistulas [6, 10, 11] and are recommended for this indication by current guidelines [12].
Despite the widespread use and available evidence on the consequences of chronic intake and withdrawal of thiopurine drugs [13–22], the optimum duration of treatment is still undefined. In randomized controlled trials, efficacy of newly initiated AZA therapy to preserve remission is limited to 15 months [5, 7], whereas results from withdrawal studies as well as clinical experience suggest persistence of the effect for up to 5 years, or even longer [14–16, 18–20]. Long-term exposure to AZA, however, has been associated with the occurrence of uncommon, but serious side effects, such as lymphoma [23, 24], skin cancer [25, 26], severe late myeloid depression [27], and opportunistic infection [19, 28, 29]. Many patients and doctors are concerned about these risks and feel uncomfortable with indefinite AZA treatment [30].
In favor of chronic therapy, decision analysis using a Markov model led to the conclusion that treatment with AZA to maintain remission in patients with CD results in increased quality-adjusted life expectancy, especially in young patients [31]. This view has been challenged by the results of a more recent study involving 660 CD patients, indicating that the benefits of responders to long-term AZA could be offset by an increased risk of malignancies [32]. Further support for a restricted duration of AZA therapy comes from a retrospective multicenter longitudinal study including 818 patients with CD that did not show an increased risk of disease reactivation when the drug had been discontinued after three to 4 years [33]. Similarly, a retrospective analysis of the clinical course of CD patients, part of which had voluntarily stopped AZA [13], did not detect an increased relapse rate when AZA had been taken for more than 4 years before discontinuation of the drug. A prospective trial by same group [18] indicated, however, continued AZA therapy to be effective in patients with clinically inactive CD on AZA for ≥3.5 years. Long-term follow-up of these patients [19], as well as clinical experience from others [20], substantiates the notion that the relapse risk is enhanced after stopping AZA, irrespective of the duration of remission under this treatment. Nevertheless, prospective data on the issue are limited, and a recent European consensus guides clinicians to consider cessation of AZA therapy for patients after 4 years of remission on AZA maintenance therapy [9].
In an effort to determine the efficacy of continued AZA therapy beyond 4 years, we performed a randomized, placebo-controlled, double-blind AZA withdrawal study over 2 years including patients with clinically inactive CD who had been treated with AZA for four or more years.
Materials and Methods
Patients were recruited from the IBD outpatient clinics of two tertiary academic centers in Austria, the medical university of Graz, and the medical university of Vienna. A total of 52 outpatients (23 Graz, 29 Vienna) between 19 and 70 years of age and an established diagnosis of CD were included. They were required to be on continuous therapy with AZA for 4 or more years, and to be in stable clinical remission without the need of oral prednisone, budesonide, or anti-TNF drugs during the last 12 months. Patients treated with 6-MP were not included. The Crohn’s Disease Activity Index (CDAI) [34] determined within 2 weeks before inclusion had to be less than 150. Indications for institution of AZA therapy included chronic active disease and postoperative prevention of relapse.
Patients were excluded if they had one or more clinical relapses defined as increased disease activity with the need of oral corticosteroid therapy during the last 12 months. They were also excluded in case of concurrent treatment (within the following time-periods before enrollment) with systemic corticosteroids or budesonide, anti-TNF drugs, cyclosporine or methotrexate (12 months); allopurinol or cholestyramine (4 weeks); rectal 5-aminosalicylates or rectal steroids, metronidazole or chinolones, NSAIDs including aspirin (>3 courses up to 7 days within the last 12 months); changed dose of oral 5-aminosalicylates (within the last 4 weeks). Further exclusion criteria were malignant disease; intestinal stoma; leukopenia (<3.0 G/L) or neutropenia (<2.0 G/L); participation in an investigational drug trial within 6 months before enrollment; and pregnant or nursing females.
Study Design and Procedures
This was a prospective, randomized, placebo-controlled, double-blind, two-center trial. Patients meeting the selection criteria were randomized to treatment in a 1:1 ratio with the help of randomization tables; the randomization process was performed centrally and stratified by center. A third center initially consented to participate but recruited only one patient; we did not obtain further information on this patient, and the patient was not considered for analysis.
Study medication (azathioprine and placebo) was formulated as 50-mg tablets of identical form, shape, and color, and provided by GlaxoSmithKline, Vienna, Austria. The number and diurnal distribution of tablets were the same as during AZA therapy before study entry. Study medication—delivered in bottles each containing 50 tablets—was provided for 3 months; thereafter, bottles were returned to the study center, and patients were supplied with new medication.
Adherence (compliance) was assessed by pill counts at each study visit; patients taking less than 80 % of the prescribed dosage were withdrawn from per-protocol analysis (this was not necessary owing to excellent compliance).
Patients were seen by experienced gastroenterologists in 3-monthly intervals, or in case of suspected exacerbation of disease. At baseline and at each visit, the CDAI and, in case of perianal disease, also the Perianal Disease Activity Index (PDAI) [35] were recorded. In addition, patients completed the Inflammatory Bowel Disease Questionnaire (IBDQ) [36] for estimation of quality of life. Blood specimens were taken for assessment of efficacy and safety, including blood counts, liver enzymes, C-reactive protein (CRP), and serum albumin. Patients were given diary cards for daily documentation of features necessary for calculation of the CDAI during the last 7 days before the next scheduled visit, or in case of suspected disease exacerbation. In case of leukopenia, dose reduction by 50 % was allowed.
Endoscopy
Colonoscopy was not part of the study protocol. Available endoscopy results from patients who had undergone colonoscopy during the 12 months before inclusion and who had been on a stable AZA dose for at least 12 months before endoscopy were analyzed retrospectively. “Mucosal healing” was defined as complete absence of mucosal ulcerations [37].
End Points
The primary efficacy parameter was the time interval between first intake of the study drug and disease relapse during the follow-up of 24 months.
Relapse was defined as either of the following: (1) A CDAI score >150 with an increase of at least 60 points above the baseline CDAI in the absence of infectious diarrhea. (2) Development of one or more new fistulas in a patient without fistula at enrollment. (3) Increase in the PDAI by >4 points. (4) Any increase in disease activity that leads to institution of therapy with oral steroids or anti-TNF alpha drugs. (5) Surgery for CD (abdominal or perianal).
Additional outcome parameters were disease activity as measured by CDAI, quality of life as measured by IBDQ, and laboratory parameters associated with active disease (CRP, serum hemoglobin, serum albumin, and platelet count).
The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP), and was approved by the local research ethics board of participating centers. All patients provided written informed consent prior to study entry.
Statistics
Estimation of required patients was based on the assumption that at 1 year, clinical relapse of CD would occur in 10 percent of patients in the AZA group and in 40 percent in the placebo group [14]. It was estimated that we required a minimum of 50 patients in each treatment group to demonstrate an absolute difference of 30 percentage points with a type I error of 0.05 and a type II error of 0.2 (in two-sided tests).
The homogeneity of the treatment groups at baseline was evaluated by the Chi-square test for categorical data; Student’s t test was used for continuous variables and a nonparametric test (Mann–Whitney U test) if their distribution were abnormal. Relapse rates were compared by Fisher’s exact test. The probability of clinical relapse during follow-up was analyzed with the use of Kaplan–Meier estimated survival functions. Patients who discontinued the study for another reason than relapse had their follow-up censored at that time. The variance of the Kaplan–Meier estimator was computed by the Greenwood formula. The survival rates at 12, 18, and 24 months were estimated and compared by the asymptotic z test between the two treatment groups. The log-rank test was applied to compare patients’ time to clinical relapse. To examine the influence of co-variates on time to relapse, we used the Cox proportional hazards model adjusted on study treatment group. Factors entered consecutively as co-variates together with treatment group included age, sex, smoking history, disease location, disease behavior, presence of perianal lesions, history of previous respective surgery, indication of AZA therapy, AZA dose (mg/day), AZA dose (mg/kg/day), AZA dose <2 mg/kg, duration of AZA therapy, relapse on AZA 1–4 years before entry, CRP level, leukocyte count, lymphocyte count, mean cellular volume (MCV), hemoglobin, hematocrit, thrombocytes, serum albumin, body mass index, CDAI, and IBDQ. Continuous variables were categorized into two or three groups, as described previously [18, 38]. Each variable was first divided into three categories at approximately the 33rd and 67th percentiles. If the relative relapse rates were not substantially different in the adjacent categories, then these two categories were grouped together. If no clear pattern was observed, then the median was taken as a cut point. Classic cutoff values, when available, were also used [18]. Variables with a p value of <0.10 in the univariate analysis were included in a multivariate analysis by using a stepwise approach. P values <0.05 were considered as statistically significant. Results are presented as hazard ratios (HRs) with 95 % CIs. Statistical analysis of clinical events included the intention-to-treat population consisting of all patients who were enrolled in the study and underwent randomization. A per-protocol analysis was also performed. The SPSS 15.0 software (SPSS, Chicago, IL, USA) was used to perform statistical analyses.
Results
The trial was stopped prematurely due to slow enrollment. From the 52 randomized patients, 26 were continued on AZA, and 26 were switched to placebo (Fig. 1). At baseline, patient characteristics did not differ significantly between treatment groups (Table 1). Patient age averaged 39 years, and the median duration of disease was 9 years. Patients had been on AZA therapy for a median of 5.2 years, and the median AZA dose was 125 mg per day. The median duration of stable AZA dosing before study entry was 37 months (range 1–73), and with the exception of one patient, all had been on a stable AZA dose during the 3 months before inclusion. At baseline, 42 patients (81 %) had been in remission without need of steroids for more than 4 years. None of the patients had received previous treatment with anti-TNF drugs.
Time to Relapse
During the 24-month study period, clinical relapse occurred in four patients (15 %) in the AZA group and in eight patients (31 %) in the placebo group. Relapse rates in various patient subgroups are compared in Table 2. Figure 2 shows the Kaplan–Meier estimates for clinical relapse with a trend for continuation of AZA treatment (HR 0.46, 95 % CI 0.14–1.5; p = 0.20). According to life-table analysis, the fraction of patients maintaining remission (mean ± SD, AZA vs. placebo) as a function of time was as follows: month 12, 96 ± 4 versus 76 ± 8 % (p = 0.035); month 18, 86 ± 7 versus 68 ± 9 % (p = 0.12); and month 24, 81 ± 9 versus 68 ± 9 % (p = 0.30). Time to clinical relapse averaged 22.3 months (95 % CI 20.6–24.0) in patients receiving AZA and 19.2 months (95 % CI 16.4–22.1) in patients receiving placebo (p = 0.20).
Secondary Study Objectives
There was no important difference and no consistent trend in average CDAI index and in IBDQ (Fig. 3) during the study period; similarly, mean values of various laboratory parameters (CRP, hemoglobin, platelet count, serum albumin) did not differ significantly between study groups (data not shown).
Risk Factors for Relapse
Several baseline variables were analyzed to assess their potential effect on outcome. When data were controlled for the effect of the study drug, a higher daily AZA dose and involvement of both colon and ileum were significantly associated with an increased risk of relapse (Table 3). According to multivariate analysis, only a higher AZA dose maintained statistical significance as a predictor of clinical recurrence independent of treatment modality (HR 2.2, 95 % CI 1.06–4.42; p = 0.034).
Endoscopy
Colonoscopy results were available in 28 patients. The median time between endoscopy and study entry was 10 weeks. Mucosal ulcerations were present in 10/28 (36 %) patients without predicting outcome (Table 2).
Adverse Events
During the study period, 108 adverse events were recorded, most of which were mild und unrelated to the study drug (Table 4). Mild asymptomatic leukopenia/lymphocytopenia occurred in 6 patients in the AZA group and in 2 patients in the placebo group. White blood cell count increased again spontaneously (n = 6) or after dose adaptation of the study drug (2 patients on AZA); all of these patients could continue the study. Three patients (1 AZA, 2 placebo) developed active perianal disease (abscess/fistulas), two of which required surgery. Three patients in the AZA group terminated the study because of adverse events: One patient was diagnosed with Clostridium difficile infection (month 2); another patient had symptoms of viral infection and was displeased with the study medication (month 9)—symptoms spontaneously disappeared a few days after study drug termination; and the third patient, a 58-year-old man who had been on AZA for 5 years, was diagnosed with early-stage prostate cancer (month 15) requiring surgery. No adverse event in the placebo group led to discontinuation of the study.
Per-protocol Analysis
Per-protocol analysis including the 42 patients who completed the study showed similar results as the intention-to-treat analysis. Relapse rate was 4/19 (21 %) in the AZA group and 8/23 (35 %) in the placebo group. Kaplan–Meier estimates for clinical relapse showed a weak trend for continuation of AZA treatment (HR 0.51, 95 % CI 0.15–1.69; p = 0.26). According to life-table analysis, the fraction of patients maintaining remission (mean ± SD, AZA vs. placebo) as a function of time was as follows: month 12, 95 ± 5 versus 74 ± 9 % (p = 0.048); month 18, 84 ± 8 versus 70 ± 10 % (p = 0.25); and month 24, 79 ± 9 versus 65 ± 10 % (p = 0.32). Time to clinical relapse averaged 22.0 months (95 % CI 20.0–23.0) in patients receiving AZA and 18.8 months (95 % CI 15.8–21.9) in patients receiving placebo (p = 0.26).
Discussion
In this investigator-driven placebo-controlled trial, long-term AZA therapy was withdrawn in patients with quiescent CD. During the follow-up period of 2 years, relapse occurred in 4 of 26 patients in the AZA group and 8 of 26 patients in the placebo group. Kaplan–Meier analysis (Fig. 2) showed a decreased risk of relapse in patients on continued AZA treatment (HR 0.46, 95 % CI 0.14–1.5) without reaching statistical significance (p = 0.20).
One of the most difficult tasks in the treatment of patients with CD is discontinuation of effective maintenance therapy. A large variety of factors, such as severity and extent of disease before institution of therapy, remaining absorptive capacity after intestinal resection, issues related to toxicity, and the patient wish, will have influence on the decision. In this situation, knowledge of risk factors for future relapse can be helpful. In previous studies, several different predictors of recurrence after cessation of AZA have been reported including young age [15, 18], male sex [14], short time without steroids [18], duration of remission less than 4 years [14], low hemoglobin [18, 19], a high neutrophil count [18, 19], and an elevated level of C-reactive protein [18, 19]; paradoxically, current smoking was found to have a protective effect [17]. We identified a higher AZA dose as an independent predictive factor of clinical relapse, as defined in the study protocol. This finding is surprising, but similar observations have been previously reported in a retrospective study by Kim et al., who calculated that a 1-mg increase in remission dosage of 6-MP increased the hazard rate of relapse after cessation of the drug by an average of 1.8 % [15]. Speculative explanations for an increased relapse risk after discontinuation of a higher thiopurine dose include that those individuals requiring a higher dose had more aggressive disease that was harder to bring in remission [15] and that a lower AZA dose is less effective [16]. Whatever the reason, our results should be interpreted with caution, because the degree of statistical significance was modest and the effect of AZA dose per body weight did not reach statistical significance.
There are no dose-ranging studies for thiopurine drugs as maintenance therapy in patients with CD. Likewise, there are no studies substantiating the advantage of body weight as a denominator of the daily gram dose, as opposed to a fixed dose [39]. In prospective trials, daily AZA doses between 2 and 2.5 mg/kg proved effective [5, 13], whereas a dose of 1 mg/kg showed no significant benefit [40]. Analysis of pooled maintenance therapy data revealed a dose–effect relationship, as the odds ratio for response increased from 1.2 at 1 mg, to 3.0 at 2 mg, to 4.1 at 2.5 mg/kg [7]. The AZA dose commonly recommended for prevention of relapse is 2–2.5 mg/kg. In clinical practice, however, many patients take less than 2 mg/kg [16, 20, 39], e.g., because myelotoxicity necessitated dose reduction, or because the drug proved effective at a lower dose. In the current trial, the mean daily AZA dose was 1.75 mg/kg, a value virtually identical to that reported in the withdrawal study by Lémann et al. (i.e., 1.7 mg/kg) [18].
Differences in response and toxicity are most likely a consequence of inter-individual variations in thiopurine metabolism that are ignored by traditional dosing based on weight. 6-MP and AZA are both pro-drugs that undergo extensive metabolic transformations resulting in the formation of the active metabolites 6-thioguanine nucleotides (6-TGN) and the potentially hepatotoxic 6-methylmercaptopurine (6-MMP) ribonucleotides [41]. Genetically determined variation in the activity of the thiopurine methyltransferase enzyme (TPMT), which catalyzes the production of 6-MMP, is considered the most important factor responsible for the wide individual differences in metabolite levels. The literature suggests that AZA therapy could be optimized by individualized dosing based on TPMT-activity and concentrations of 6-TGN and 6-MMP [42]. In agreement with this concept, a recent prospective trial designed to test the efficacy of tailored thiopurine treatment allowing dose adjustments based on metabolite concentrations showed trends favoring individualized over weight-based AZA dosing in CD [43]. During the current study, drug metabolites were not determined. Consequently, the influence of differences in AZA metabolism on outcome remains unknown.
The study medication was generally well tolerated by the patients. This is mainly explained by the fact that patients were highly preselected as they had been taking AZA for more than 4 years before entering the trial. Treatment failure due to idiosyncratic drug reaction, which may be a problem when thiopurines are initiated [44], did not occur. Mild leukopenia reverting spontaneously or after dose adjustment was encountered in few cases. Surprisingly, infection due to herpes virus was more frequent on placebo than on AZA, although the contrary would be expected [29]. We have no ready explanation for this observation and think it was by chance. Overall, the rate of infection was similar in the two study groups. One patient, a 58-year-old man who had been on AZA since 5 years, was operated because of early-stage prostate cancer. There is no evidence in the literature that AZA enhances the risk of prostate cancer in IBD patients [45], and we assume this was a spontaneous occurrence.
One strength of our trial was the prospective, double-blind design considering only patients on long-term AZA therapy of 4 years or longer. Among previous studies of thiopurine cessation in patients with CD, only two were double-blind RCTs [13, 18]. O’Donnahue et al. [13] stopped AZA in 27 of 51 patients in remission or stable good health for at least 6 months and showed a significant benefit for continuation of AZA therapy. The withdrawal trial by Lémann et al. [18] included 83 patients with quiescent disease on AZA for ≥42 months without demonstration of a significant reduction of relapse risk for continuation of AZA in standard two-sided tests. A further strength of our study is the long follow-up period of 24 months. The two abovementioned trials had shorter observation periods of 12 [13] and 18 months [18], respectively. We thought that a longer follow-up would notably increase the difference between study groups, but this expectation did not hold. The delta between time-to-relapse curves was greatest after 12 months, without further increase thereafter (Fig. 2). We hypothesize that the effect of AZA withdrawal is greatest during the first year and then decreases. This assumption is supported by data from Bouhnik et al. [14] involving 157 patients who continued and 42 patients who had stopped AZA/6MP therapy. After 1 year, the cumulative probability of relapse was 11 % in patients on AZA/6MP and 38 % in patients off treatment; during the second year, however, relapse rates increased by about 10 % in both groups. It is also interesting to compare our efficacy results with those from the Lémann study [18]. In the latter trial, respective relapse rates after 18 months on and off therapy were 8 and 21 %, compared with 14 and 32 % in our patients. Hence, relapse rates as well as the difference of relapse rates between treatment groups at 18 months (13 vs. 18 %) tended to be higher in the present trial. Our failure to demonstrate a statistical significance of the drug effect may have resulted—in addition to the smaller sample size—from different statistical methods applied, as we used a conventional two-sided design, as opposed to a non-inferiority design with a one-sidedapproach in the Lémann study.
There are several important limitations to our study. First, owing to slow recruitment, the trial was stopped prematurely after inclusion of 52 patients (100 planned) and therefore lacked the statistical power to detect significant differences between study groups, as required by the protocol. Nevertheless, after the study by the French group [18] involving 82 patients, this is the second largest RCT of AZA withdrawal in patients with CD. Second, endoscopy was not performed as part of the study. Retrospective analysis of available endoscopy results obtained from a subgroup of patients who underwent colonoscopy within 12 months (median 10 weeks) before inclusion did not show a significant association between the presence of mucosal ulcers and clinical relapse. Our results are congruent with those of the aforementioned study by Lémann et al. [18] that also failed to demonstrate a predictive value of endoscopic activity on relapse after stopping AZA. Both in that study and in ours, the number of patients who underwent endoscopy was limited, however, so that on the basis of these two studies no final conclusion can be drawn on this issue. A further limitation of our study was the lacking measurement of fecal calprotectin. Analysis of this surrogate marker of endoscopic activity was not available in the study centers when the trial started. For the same reason, drug levels (6-TG, 6-MMP) were not determined. Finally, we did not restrict the study population to patients with chronic active disease as indication for AZA therapy—as reported in some [15, 18], but not all [13, 14, 16, 17] previous major studies of AZA or 6-MP cessation—and also included patients who received AZA for prevention of relapse after bowel resection. We noted some trend toward higher recurrence rates in patients with chronic active disease as indication, but numbers are too small to allow meaningful conclusions.
In summary, the results of this placebo-controlled AZA withdrawal trial with the longest follow-up so far are in line with evidence from existing data and confirm that discontinuation of AZA treatment in patients with CD in stable remission enhances the risk of clinical relapse. Further studies are required to better define subgroups that benefit most from chronic AZA treatment.
References
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794.
Petritsch W, Fuchs S, Berghold A, et al. Incidence of inflammatory bowel disease in the province of Styria, Austria, from 1997 to 2007: a population-based study. J Crohn’s Colitis. 2013;7:58–69.
Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124.
Ardizzone S, Cassinotti A, Manes G, Porro GB. Immunomodulators for all patients with inflammatory bowel disease? Therap Adv Gastroenterol. 2010;3:31–42.
Candy S, Wright J, Gerber M, Adams G, Gerig M, Goodman R. A controlled double blind study of azathioprine in the management of Crohn’s disease. Gut. 1995;37:674–678.
Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn Disease. A meta-analysis. Ann Intern Med. 1995;123:132–142.
Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2009;(1):CD000067. doi:10.1002/14651858.CD000067.pub2.
Peyrin-Biroulet L, Deltrenre P, Ardizzone S, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2009;104:2089–2096.
Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: current management. J Crohn’s Colitis. 2010;4:28–62.
Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–987.
Korelitz BI, Adler DJ, Mendelsohn RA, Sacknoff A. Long-term experience with 6-mercaptopurine in the treatment of Crohn’s disease. Am J Gastroenterol. 1993;88:1198–1205.
Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: special situations. J Crohn’s Colitis. 2010;4:63–101.
O’Donoghue DP, Dawson AM, Powel-Tuck K, Brown RL, Lennard-Jones JE. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet. 1978;2:955–957.
Bouhnik Y, Lémann M, Mary JY, et al. Long-term follow up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet. 1996;347:215–219.
Kim PS, Zlatanic J, Korelitz BI, Gleim GW. Optimum duration of treatment with 6-mercaptopurine for Crohn’s disease. Am J Gastroenterol. 1999;94:3254–3257.
Vilien M, Dahlerup JF, Munck LK, Nørregaard P, Grønbaek K, Fallingborg J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: increased relapse rate the following year. Aliment Pharmacol Therap. 2004;19:1147–1152.
Sokol H, Seksik P, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Current smoking, not duration of remission, delays Crohn’s disease relapse following azathioprine withdrawal. Inflamm Bowel Dis. 2010;16:362–363.
Lémann M, Mary J-Y, Colombel J-F, et al. A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812–1818.
Treton X, Bouhnik Y, Mary JY, et al. Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009;7:80–85.
Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 years review. Gut. 2002;50:485–489.
Mantzaris GJ, Roussos A, Christidou A, et al. The long-term efficacy of azathioprine does not wane after four years of continuous treatment in patients with steroid-dependent luminal Crohn’s disease. J Crohn’s Colitis. 2007;1:28–34.
French H. Mark Dalzell A, Srinivasan R, El-Matary W. Relapse rate following azathioprine withdrawal in maintaining remission for Crohn’s disease: a meta-analysis. Dig Dis Sci. 2011;56:1929–1936.
Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625.
Sokol H, Beaugerie L, Maynadié M, et al. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2063–2071.
Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–1628.
Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.
Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–1085.
Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936.
Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989;111:641–649.
Higgins PD. Who wants to take a thiopurine holiday? Am J Gastroenterol. 2011;106:556–558.
Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018–1024.
Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn’s disease who respond to azathioprine. Clin Gastroenterol Hepatol. 2013;11:389–394.
Holtmann M, Krummenaeur F, Claas C, et al. Long-term effectiveness of azathioprine in IBD beyond 4 years: a European multicenter study in 1176 patients. Dig Dis Sci. 2006;51:1516–1524.
Best WR, Becktel JM, Singelton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology. 1979;77:843–846.
Irvine EJ. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27–32.
Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989;96:804–810.
Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–413.
Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612.
Nyman M, Hansson I, Eriksson S. Long-term immunosuppressive treatment in Crohn’s disease. Scand J Gastroenterol. 1985;20:1197–1203.
Summers RW, Switz DM, Sessions JT, et al. National Cooperative Crohn’s Disease Study: results of drug treatment. Gastroenterology. 1979;77:847–869.
Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713.
Chouchana L, Narjoz C, Beaune P, Loriot MA, Roblin X. Review article: the benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:15–36.
Dassopoulos T, Dubinsky MC, Bentsen JL, et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014;39:163–175.
Reinisch W, Angelberger S, Petritsch W, et al. International AZT-2 Study Group. Azathioprine versus mesalazine for prevention of postoperative clinical recurrence in patients with Crohn’s disease with endoscopic recurrence: efficacy and safety results of a randomized, double-blind, double-dummy, multicentre trial. Gut. 2010;59:752–759.
Smith MA, Irving PM, Marinaki AM, Sanderson JD. Review article: malignancy on thiopurine treatment with special reference to inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:119–130.
Acknowledgments
We are thankful to GlaxoSmithKline, Vienna, Austria, for providing the study medication, case report forms, and patient diaries. Representatives of GlaxoSmithKline did not have any role in study design, protocol development, data analysis, data interpretation, writing of the manuscript, or in the decision to submit the manuscript. We thank Marieluise Harrer and Georgiana Prinz for their help in patient recruitment and data acquisition. There was no funding or other financial support for this work.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wenzl, H.H., Primas, C., Novacek, G. et al. Withdrawal of Long-Term Maintenance Treatment with Azathioprine Tends to Increase Relapse Risk in Patients with Crohn’s Disease. Dig Dis Sci 60, 1414–1423 (2015). https://doi.org/10.1007/s10620-014-3419-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3419-5