Abstract
Background
Small intestinal bacterial overgrowth (SIBO) is a significant and increasingly recognized syndrome. While the development may be multifactorial, impairment of the ileocecal valve (ICV), small bowel motility, and gastric acid secretion have been hypothesized to be risk factors. ICV dysfunction remains largely unexplored using standard technology. The wireless motility capsule (WMC) that evaluates pressure, pH, and temperature throughout the GI tract provides the ability to assess these parameters.
Aims
The primary aims of this study were to assess the relationship of ICV pressures, small bowel transit time (SBTT) and intestinal pH with lactulose hydrogen breath testing (LBT) results in subjects with suspected SIBO.
Methods
We retrospectively studied consecutive patients referred to our institution for WMC and LBT from 2010–2012. Ileocecal junction pressures (IJP), as a surrogate for ICV pressures, were defined as the highest pressure over a 4-min window prior to the characteristic ileocecal pH drop. SBTT and pH were calculated and compared with LBT results.
Results
Twenty-three patients underwent both WMC and LBT, with positive results observed in 15 (65.2 %). IJP were significantly higher in LBT(−) negative vs. LBT(+) (79.9 vs. 45.1, p < 0.01). SBTT was significantly longer in LBT(+) versus LBT(−) (5.82 vs. 3.81 h, p = 0.05). Among LBT(+) subjects, gastric pH was significantly higher versus LBT(−) subjects (2.76 vs. 1.63, p = 0.01). There was poor correlation between IJP and other parameters (SBTT, small bowel pH, and gastric pH).
Conclusions
Low IJP is significantly associated with SIBO. While this is physiologically plausible, to our knowledge, this is the first study to make this connection. Prolonged SBTT and higher pH are also independently associated with SIBO. Our findings add value of the WMC test as a diagnostic tool in patients with functional gastrointestinal complaints and suggest re-focus of attention on the ileocecal valve as a prominent player in intestinal disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Small intestinal bacterial overgrowth (SIBO) is important as it is become an increasingly recognized clinical problem [1–3]. Although the clinical manifestations of malabsorption and nutritional deficiencies may be seen in patients at the most severe end of the spectrum, most often SIBO is suspected in patients presenting with relatively mild and nonspecific symptoms such as abdominal discomfort, bloating, and diarrhea [4, 5]. For this reason, the diagnosis of SIBO may be overlooked or confused with functional bowel disease [6, 7]. Indeed, SIBO has also been attributed as a contributing pathophysiological factor in irritable bowel syndrome (IBS) by some authors [8–10] and this line of thinking has led to numerous trials of antibiotics for this condition, with only modest benefit [11, 12].
Despite its potential importance, the pathogenesis of bacterial overgrowth remains poorly understood in most patients without overt intestinal stasis. Theoretically, SIBO may result from defects in intestinal clearance including immune and nonimmune defensive factors (such as luminal pH) as well as impaired intestinal motility. In addition, reflux from colonic contents resulting from a dysfunctional ileocecal barrier may be a contributing factor, but in the absence of surgical resection of the ileocecal region, this mechanism has yet to be clarified [13–16]. Until recently, the absence of an easily administered test to evaluate these parameters; particularly, the ileocecal valve has been a major limitation to the study of this field. However, with the advent of a commercial wireless motility capsule or WMC (Smartpill®), it is now possible to measure pressure, pH, and temperature throughout the gastrointestinal tract.
The primary aim of this study was to assess the relationship between ileocecal junction pressures (IJP) and SIBO, as indirectly measured by lactulose breath testing (LBT). A secondary aim was to assess the relationship between intestinal pH and small bowel transit time (SBTT) with LBT, in order to further our understanding of the pathophysiological mechanisms underlying SIBO. To this end, we developed a novel noninvasive approach to assess ileocecal valve pressures using information that is readily obtained by the existing WMC, along with standardize measures of intestinal transit time and pH, to test the hypothesis that this and other WMC measurements may predict the results of the lactulose hydrogen breath test.
Methods
Subjects
This was a retrospective study of consecutive patients who were referred for WMC and LBT testing at a single academic tertiary referral center from December 2010 to December 2012. Twenty-three patients were identified as having undergone both tests, and data from these subjects were used for further analysis. Additionally, normative data were developed using the results of WMC testing in healthy controls, as previously published [17–19].
Measurements
Lactulose Hydrogen Breath Testing
Lactulose breath testing was performed using a Quintron BT Digital Microlyzer calibrated with Quingas-5. A 10-g dose of lactulose was administered followed by standard protocol obtaining samples every 20 min for a total duration of 3 h. The test was considered positive if it showed one or more of the following: (a) A baseline breath concentration of >10 parts per million (ppm) for H2 or >7 ppm for CH4 only if patients were compliant with their preparation, (b) an increase within 90 min (small intestine) that was followed by a larger peak (colonic) was indicative of a positive study (with a decrease of at least 5 ppm following the first peak). The first increase must have be one of the following to be considered positive: (1) an increase of at least 12 ppm CH4 over the baseline by 90 min; (2) if producing H2 only: an increase of at least 20 ppm (parts per million) H2 over the baseline by 90 min. Patients were instructed to not take antibiotics for a minimum of 4 weeks prior to the test, to avoid laxatives and any other medications that effect GI motility for a least one week prior to breath testing. On the day prior to the test, patients were instructed to avoid high-fiber foods such as bran, coarse breads, pasta, nuts, beans, and uncooked vegetables. Patients were also instructed to avoid all caffeinated beverages. Twelve hours prior to the test, they were instructed to consume no food except water. All breath tests were evaluated by a single, experienced reader who was blinded to the results of the WMC testing.
Wireless Motility Capsule (Smartpill) Testing
Prior to WMC ingestion, patients were instructed to hold all proton pump inhibitors and H2 receptor blockers for 7–10 days prior to the study (however, this protocol may not have been followed by all patients). Patients were also asked to remain NPO (nothing per os) starting at midnight the evening prior to the ingestion. On the day of the study, all patients were given a standardized SmartBar bar (255 calories, 75 % carbohydrate, 21 % protein, 3 % fat, 3 % fiber) just prior to pill ingestion and were instructed to remain NPO for 6 h with the exception of small quantities of water (up to ½ cup). Participants were also instructed to keep the SmartPill data receiver within 3 feet of themselves at all times during the 5-day study period and were prompted to push the button on their external data receiver whenever they had a bowel movement. Patients were also told to remain on the toilet bowl for a full 2 min after having a bowel movement in order to ensure communication with the data receiver if the pill had passed. At the completion of 5 days, patients were instructed to return their data receivers and diary/log of events. Data from each receiver were subsequently downloaded and analyzed by MotiliGI software.
For the purpose of this study, we developed a novel method to estimate the ileocecal junction (IJ) pressure or IJP, a surrogate marker for ileocecal valve function. This was based on time stamping the characteristic drop in pH as the pill exits the ileum into the cecum and then identifying the highest/peak pressure over a 4-min window prior to this event. This pressure was taken to represent the IJ pressure. All SmartPill readings, including IJ pressure measurements, were interpreted by a single, blinded reader. Small bowel transit times were measured as per standard WMC criteria. Additionally, gastric and small bowel pH measurements were also obtained and compared among patients with and without positive LBT. Patients on chronic acid suppressive therapy (proton pump inhibitors and/or H2 receptor blockers) were excluded from all analyses evaluating gastric and small bowel pH.
Normative Data and Definitions
Based on the wireless capsule motility studies of 47 healthy control patients, normative values were established for ileocecal junction pressures, small bowel transit time, small bowel mean pH, and gastric mean pH. The mean value in the healthy control group plus twice the control group standard deviation was used as the cutoff point for normal values of SBTT. Because control data small bowel mean pH, gastric mean pH, and IJ pressures were skewed, interval estimation using Horn’s method [20] was used to establish a normal cutoff.
Statistical Analysis
Summary statistics (mean, standard deviation, frequencies, and percentages) are presented for demographic variables by lactulose breath test result. Small bowel pH, contractions/minute, amplitude, motility index, and IC valve pressure, gastric pH, and transit times are summarized by lactulose breath test result and in healthy controls. Means values were compared using ANOVA and two-sample t tests. When the variables were extremely skewed, nonparametric Kruskal–Wallis and Wilcoxon’s rank-sum tests were used, and medians were reported. Pearson’s correlation coefficients were calculated to examine the linear association between IJP, SBTT, small bowel pH, and gastric pH. The occurrence of prolonged SBTT was compared between LBT results and in healthy controls using Fisher’s exact test. The association between LBT result and exceeding the normal cutoff based on healthy control data for SB mean pH, gastric pH, SBTT, and IJ pressure was compared using Fisher’s exact test. Receiver operating characteristic (ROC) curves were used to determine the cutoff values of IJP, SBTT, and gastric pH for classifying those with positive versus negative LBT result. The optimal threshold was determined using Youden’s J statistic [21] to maximize sum of sensitivity and specificity. Logistic regression models were used to investigate the association between LBT positivity and SBTT, IC valve pressure, gastric pH, and SB mean pH. Odds ratios and 95 % confidence intervals are presented. All analyses were conducted using SAS v. 9.3 (SAS Institute; Cary, NC). Two-sided p values <0.05 were considered statistically significant.
Results
Patient Characteristics and LBT Results
Twenty-three patients were identified who underwent both WMC and LBT. Fifteen of the 23 patients (65.2 %) had positive LBTs, and 8 (34.8 %) had negative LBTs. The demographic and clinical attributes of patients with and without a positive LBT are illustrated in Table 1. Table 2 represents the summary statistics of healthy control subjects that were used to determine the normal cutoffs for the variables IJ Pressure, SB mean pH, gastric pH, and SBTT.
Results of WMC Testing
Table 3 summarizes the results of the main WMC parameters in patients with and without a positive LBT.
Ileocecal Junctional Pressures
There was a significant difference in IJP among LBT-positive patients, LBT-negative patients, and healthy controls (p < 0.01). IJ pressures were significantly higher in patients with negative breath testing as compared with positive breath testing [79.88 (standard deviation (SD) = 21.46) vs. 45.07 (SD = 20.86) mm Hg, p < 0.01]. IJ pressures were also significantly higher in the historical healthy controls as compared with the positive LBT group [61.47 (SD = 23.29) vs. 45.07 (SD = 20.86), p = 0.02]. Mean IJP were also higher in the negative LBT as compared with healthy controls, with a borderline statistically significant difference between the groups [79.88 (SD = 21.46) vs. 61.47 (SD = 23.29), p = 0.05].
We additionally found that 66.7 % of LBT-positive patients had IJ pressures below the lower limit of normal cutoff, while none of the LBT-negative subjects had IJ pressure below the normal cutoff (p < 0.01) (Table 4).
pH Studies
After excluding all patients with a history of long-term PPI use, gastric pH was found to be significantly higher in the LBT-positive group as compared with the LBT-negative group [2.76 (SD = 0.93) vs. 1.63 (SD = 0.39), p = 0.01]. There was also a significantly higher gastric pH observed in the LBT-positive group as compared with healthy controls [2.76 (SD = 0.93) vs. 1.18 (SD = 0.52), p < 0.01]. There were no statistically significant differences between gastric pH among LBT-negative patients as compared with healthy controls [1.63 (SD = 0.39) vs. 1.18 (SD = 0.52), p = 0.09]. The difference in the proportion of patients exceeding the upper limit of normal for gastric pH in the LBT-positive group (100.0 %) as compared with the LBT-negative group (75.0 %) which was not statistically significant (p = 0.31) (Table 4).
Small bowel pH was additionally found to be higher in the LBT-positive group as compared with the LBT-negative group [7.19 (SD = 0.34) vs. 6.65 (SD = 0.44), p = 0.09], although the difference was not statistically significant. No statistical significance was observed when comparing small bowel pH in LBT-positive patients and healthy controls (7.19 vs. 7.17, p = 0.87).
Small Bowel Transit Time
Small bowel transit time was longer in LBT-positive patients as compared with LBT-negative patients [5.82 (SD = 2.84) vs. 3.81 (SD = 1.84) h, p = 0.05]. Small bowel transit times were also longer in the LBT-positive group as compared with healthy controls [5.82 (SD = 2.84) vs. 4.25 h (SD = 1.52), p = 0.057]. There was no significant difference in SBTT between the negative LBT group and historical healthy controls (p = 0.54). Using the manufacturer’s recommended cutoff (6 h) [16], there was a significantly lower percentage of LBT(+) patients (60 %) with normal SBTT as compared with healthy controls (89.1 %; p = 0.02). However, there was not a significant difference in the proportion of LBT(+) and LBT(−) patients with normal SBTT (p = 0.34). Using the 2 SD cutoff for the normative data, 67 % of patients with positive breath tests had normal small bowel transit time as compared with 100 % in the negative breath test group (p = 0.02).
Overlap of Putative Pathophysiological Abnormalities Among LBT-Positive Patients
There was only minor overlap between LBT-positive patients who had delayed SBTT and low IJP. Only 3 patients with LBT positivity exceeded the upper limit of normal SBTT and also fell below the lower limit of normal for IJ pressure. The same 3 patients were found to have SBTT above the normal cutoff and gastric pH above the normal cutoff along with IJ pressure below the normal cutoff. Further, there was poor correlation between IJP and SBTT (r = −0.13, p value = 0.55), IJP and SB pH (r = −0.20, p value = 0.51), and IJP and gastric pH (r = −0.20, p value = 0.43). There was also poor correlation between SBTT and these variables.
Predictive Value of WMC Parameters for LBT Positivity (ROC)
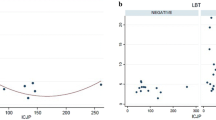
The optimal cutoff point for gastric pH in differentiating between those with positive and negative LBT (excluding PPI users) was 2.05, which yields 66.7 % sensitivity, 100 % specificity, and AUC of 0.86. The optimal cutoff for SBTT was 6.75, which gives 40 % sensitivity, 100 % specificity, and AUC 0.71. The optimal cutoff for IJP was 54.5, which gives an 80 % sensitivity, 100 % specificity, and AUC of 0.90 (Fig. 1).
Based on these ROC cut points, we found that 80 % of LBT-positive patients had IJP below 54.5 versus 0 % among LBT-negative subjects (p < 0.01). In total, 40 % of LBT-positive patients had SBTT above 6.75 versus 0 % of LBT-negative subjects (p = 0.01). In total, 66.7 % of LBT-positive patients had gastric pH above 2.05 versus 0 % of LBT-negative subjects; however, this difference did not reach statistical significance (p = 0.07) (Table 5).
Logistic Regression
Univariate logistic regression analyses showed a significant association between LBT positivity and IJ pressure (OR 0.93, p = 0.01), a borderline significant association between LBT positivity and SB mean pH (OR for 0.1-unit increase 1.45, p = 0.06), and SBTT (OR 1.43, p = 0.06). A multivariate logistic regression model was attempted using all the variables found to be significantly associated with univariate analysis at the α = 0.10-level. Stepwise model selection procedures showed that in the presence of IJ pressure, all other predictors were not significantly associated with positive LBT. Using IJP alone to model positive LBT showed that an increase in IJ pressure by 1 unit reduces the odds of having a positive LBT by 7 % (Table 6).
Discussion
Small intestinal bacterial overgrowth remains a controversial clinical syndrome for many reasons. The tests that are used to establish this diagnosis are imperfect and the relationship between SIBO symptoms and functional bowel disease remains unclear. Furthermore, in most cases, the pathophysiological basis of bacterial overgrowth remains obscure. Although our study has some significant limitations, we believe that it provides important data that SIBO may be a result of multiple potential, non-overlapping mechanisms. First, ICV dysfunction in individuals referred for LBT is associated with a higher likelihood of a positive test congruous with SIBO. Secondly, we have demonstrated a novel application of the WMC to assess ileocecal valve function, using ileocecal junctional pressures. This simple method can easily be performed as part of the routine practice of interpreting a wireless motility study, with little additional time. Our results show that this measurement is one of the most important predictors of a positive breath test and brings fresh attention to the role of the ileocecal valve in neurogastroenterological disorders, an area of investigation that has been generally neglected.
There are several endogenous mechanisms that may protect against bacterial overgrowth in the small bowel, including gastric acid secretion, gastrointestinal motility, as well as an intact ileocecal valve. Of these, intestinal motility and IC valve function remain difficult to evaluate. The IC valve is a relatively unexplored sphincter in the gastrointestinal tract, and its role in health and disease remains largely unknown. It has been hypothesized that the IC valve protects against the development of bacterial overgrowth by preventing reflux of colonic contents into the small intestine. Prior attempts to study this valve have been constrained by technical and methodological issues [13, 22]. This is not surprising given the location of the IC valve, which represents a challenge to both peroral or retrograde catheter-based approaches. In this regard, the WMC offers a relatively simple noninvasive way to directly measure the IC junctional pressure, as this represents the only high-pressure zone in this anatomic region. IJ pressures, therefore, may be used as a surrogate for ileocecal valve competency, in a manner analogous to the lower esophageal or anorectal sphincters.
Our results show that patients with SIBO (as defined by a positive lactulose breath test) had significantly lower IJ pressures as compared to both subjects without SIBO and healthy controls. Nearly two-thirds of patients with SIBO had IJ pressures below the lower limit of normal, and together these findings are strongly suggestive of a pathophysiological role for a dysfunctional IC valve in these patients. This is consistent with the few other studies in this area. An ileocecal nipple structure seems to serve the function of a ceco-ileal anti-reflux barrier in humans [23]. Additionally, surgical resection of the IC valve has also been shown to increase colonic-ileal reflux in humans and subsequently lead to bacterial overgrowth [24], similar to what has been described in animals [25]. In a study of cadavers within 2 h of death, an incompetent ileocecal valve (defined by a IJ pressure of 40 mm Hg or less) was associated with increased colonic-ileal reflux [26]. A more recent study of 19 subjects who underwent colonoscopy with manometric ICV measurements and subsequent lactulose hydrogen breath testing (LBT) found that patients with a positive LBT failed to increase IC valve pressures in response to cecal distention as compared with patients with a negative breath test [14]. However, baseline IC valve pressures were low in both groups and not different from each other. Some of the discrepancies between the results of these studies and ours may be explained by confounding factors such as sedation and the effects of colonic distention. It is also clear that much further work needs to be done to fully elucidate the role of the IC valve in SIBO. We suggest that such research may be greatly facilitated by the approach we have used to measure IJ pressures.
The most well-described factor preventing SIBO in normal subjects is gastric acidity, presumably by its sterilizing effects. Inhibition of acid secretion, by either proton pump inhibitors or histamine type 2 receptor blockers, is therefore considered to be one of the primary risk factors for SIBO as shown by several observational as well as prospective studies [27–29]. However, there is a paucity of the literature on direct measurements of gastric or intestinal pH in patients with SIBO. In the present study, we found that patients with positive LBTs had higher gastric pH as compared with those with negative breath testing, even after excluding those on PPI therapy. The mean difference was approximately 1 pH unit which translates into a 10-fold reduction in acid concentration. Even though a pH of 4 or less has been used to define the gastric bactericidal barrier, there is a several log difference in the rate of killing between a pH of 2 and that of 3 [30]. The difference in gastric acid concentrations between the LBT-negative and LBT-positive patients, therefore, could be pathophysiologically relevant, even though the underlying mechanism remains unknown.
There are several limitations to the present study of which the most significant are the relatively small sample size and the retrospective nature of this study. However, this should be taken into context as there are no published studies on patients undergoing both WMC and testing for SIBO that evaluate the ileocecal valve. Additional limitations that the authors would like to acknowledge include that our patient population comes from a tertiary care/motility referral center; therefore, our findings may not be generalizable to the typical patient population referred for motility testing. Lastly, we acknowledge that there are many imperfections with lactulose breath testing including poor sensitivity, the fact that lactulose itself accelerates small bowel transit, as well as difficulty differentiating detection of two distinguishable hydrogen peaks or double peaks occurring in the cecum, with the potential to yield false positive results [31, 32]. Unfortunately, given the retrospective nature of this study, we were unable to confirm the diagnosis of SIBO with the gold standard of small bowel aspirate and jejunal culture. We acknowledge the need for further prospective studies to validate these initial results before all these potential outcomes can be realized.
Despite these limitations, however, we were able to demonstrate statistically robust differences across a variety of measures, with significant clinical and physiological implications. Perhaps, the most important of these is the ability to stratify patients into three putative etiological groups—those with low IJ pressure (“dysfunctional ICV” group), slow transit (“intestinal dysmotility” group), and high gastric pH (“low acid” group). Interestingly, our analysis suggests that these groups may be distinct with only minimal overlap. Furthermore, there was poor correlation between ileocecal junction pressures with SBTT and both small bowel and gastric pH. This finding suggests that there are distinct mechanisms contributing to the development of bacterial overgrowth. Depending on which etiology is present, different subsets of patients with SIBO may respond to different treatment modalities.
In conclusion, we have demonstrated that the WMC can be used to elucidate pathophysiological mechanisms that can be correlated with the presence or absence of SIBO. This finding has several implications. Firstly, it adds real value of this test as a diagnostic aid in patients with suspected SIBO and/or functional gastrointestinal complaints. Secondly, our study supports re-focus of attention on the ileocecal valve as a potentially prominent player in the pathogenesis of SIBO and potentially other intestinal disorders. Furthermore, we have suggested a simple noninvasive way to study ICV function that can facilitate such research in the future. Lastly, the ability to phenotype patients into categories with potentially distinct etiopathogenesis may represent a major advance in the understanding of this common, but undoubtedly heterogeneous syndrome and point the way for tailored therapies.
References
Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–1166.
Stotzer PO, Bjornsson ES, Abrahamsson H. Interdigestive and postprandial motility in small-intestinal bacterial overgrowth. Scan J Gastroenterol. 1996;31:875–880.
Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. N Engl J Med. 1991;325:1461–1467.
Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth. A comprehensive review. Gastroenterol Hepatol (NY). 2007;3:112–122.
Abu-Shanab AA, Quigley EMM. Diagnosis of small intestinal bacterial overgrowth: the challenges persist! Expert Rev Gastroenterol Hepatol. 2009;3:77–87.
Quera PR, Quigley EM, Madrid SAM. Small intestinal bacterial overgrowth. An update. Rev Med Chil. 2005;133:1361–1370.
Quigley EM, Abu-Shanab A. Small intestinal bacterial overgrowth. Infect Dis Clin North Am. 2010;24:943–959.
Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563.
Pimentel M, Lembo A, Chey WD, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32.
Ford AC, Spiegel BM, Talley NJ, et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286.
Tack J. Antibiotic therapy for the irritable bowel syndrome. N Engl J Med. 2011;364:81–82.
Lauritano EC, Gabrielli M, Scarpellini E. Small intestinal bacterial overgrowth recurrence after antibiotictherapy. Am J Gastroenterol. 2008;103:2031–2035.
Quigley EM, Borody TJ, Phillips SF, Wienbeck M, Tucker RL, Haddad A. Motility of the terminal ileum and ileocecal sphincter in healthy humans. Gastroenterology. 1984;87:857–866.
Miller LS, Vegesna AK, Sampath AM. Ileocecal valve dysfunction in small intestinal bacterial overgrowth: a pilot study. World J Gastroenterol. 2012;18:6801–6808.
Dinning PG, Bampton PA, Kennedy ML, et al. Basal pressure patterns and reflexive motor responses in the human ileocolonic junction. Am J Physiol. 1999;276:G331–G340.
Kajimoto T, Dinning PG, Gibb DB, de Carle DJ, Cook IJ. Neurogenic pathways mediating ascending and descending reflexes at the porcine ileocolonic junction. Neurogastroenterol Motil. 2000;12:125–134.
Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2007;27:186–196.
Rao S, Kuo B, McCallum RW et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544.
Tran K, Brun R, Kuo B. Evaluation of regional and whole gut motility using the wireless motility capsule: relevance in clinical practice. Therap Adv Gastroenterol. 2012;5:249–260.
Horn PS, Pesce AJ, Copeland BE. A robust approach to reference interval estimation and evaluation. Clin Chem. 1998;44:622–631.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35.
Nasmyth DG, Williams NS. Pressure characteristics of the human ileocecal region—a key to its function. Gastroenterology. 1985;89:345–351.
Shafik AA, Ahmed IA, Shafik A, et al. Ileocecal junction: anatomic, histologic, radiologic and endoscopic studies with special reference to its antireflux mechanism. Surg Radiol Anat. 2011;33:249–256.
Gazet RJ, Jarrett J. The ileocecal-colic sphincter. Studies in vitro, in man, monkey, cat, and dog. Br J Surg. 1964;51:368–370.
Kumar D, Phillips SF, Brown ML. Reflux from ileum to colon in the dog. Role of external ligamentous attachments. Dig Dis Sci. 1988;33:345–352.
Kumar D, Phillips SF. The contribution of external ligamentous attachments to function of the ileocecal junction. Dis Colon Rectum. 1987;30:410–416.
Thorens J, Froehlich F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996;39:54–59.
Shindo K, Fukumura M. Effect of H2-receptor antagonists on bile acid metabolism. J Investig Med. 1995;43:170–177.
Lewis SJ, Franco S, Young G, et al. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557–561.
Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13:251–256.
Riordan SM, McIver CJ, Walker BM et al. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–1803.
Corazza GR, Menozzi MG, Strocchi A et al. The diagnosis of small bowel bacterial overgrowth. Reliability of jejunal culture and inadequacy of breath hydrogen testing. Gastroenterology. 1990;98:302–309.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roland, B.C., Ciarleglio, M.M., Clarke, J.O. et al. Low Ileocecal Valve Pressure Is Significantly Associated with Small Intestinal Bacterial Overgrowth (SIBO). Dig Dis Sci 59, 1269–1277 (2014). https://doi.org/10.1007/s10620-014-3166-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3166-7